Abstract
Objective
To determine the safety and efficacy of laparoscopic Roux-en-Y gastric bypass for the treatment of morbid obesity.
Summary Background Data
Laparoscopic Roux-en-Y gastric bypass is a new and technically challenging surgical procedure that requires careful study.
Methods
The authors attempted total laparoscopic Roux-en-Y gastric bypass in 281 consecutive patients. Procedures included 175 proximal bypasses, 12 long-limb bypasses, and 9 revisional procedures from previous bariatric operations. The gastrojejunostomy and jejunojejunostomy were primarily constructed using linear stapling techniques.
Results
Eight patients required conversion to an open procedure (2.8%). The mean age of the patients was 41.6 years (range 15–71) and 87% were female. The mean preoperative body mass index was 48.1 kg/m2. The operative time decreased significantly from 234 ± 77 minutes in the first quartile to 162 ± 42 minutes in the most recent quartile. Postoperative length of stay averaged 4 days (range 2–91), with 75% of patients discharged within 3 days. The median hospital stay was 2 days. No patient died after surgery. Complications included three (1.5%) major wound infections (each followed a reoperation for a complication or open conversion), incisional hernia in 5 patients (1.8%), and anastomotic leak with peritonitis in 14 patients (5.1%). Three gastrojejunal leaks were managed without surgery, four by laparoscopic repair/drainage, and three by open repair/drainage. Only three patients had anastomotic leaks in the most recent 164 procedures (1.8%) since the routine use of a two-layer anastomotic technique. Data at 1 year after surgery were available in 69 of 96 (72%) patients (excludes revisions). Weight loss at one year was 70 ± 5% of excess weight. Most comorbid conditions resolved by 1 year after surgery; notably, 88% of patients with diabetes no longer required medications.
Conclusions
Laparoscopic gastric bypass demonstrates excellent weight loss and resolution of comorbidities with a low complication rate. The learning curve is evident: operative time and leaks decreased with experience and improved techniques. The primary advantage is an extremely low risk of wound complications, including infection and hernia.
Laparoscopic gastric bypass surgery to treat morbid obesity is a promising but technically demanding and complex minimally invasive procedure. To date, several publications have suggested the procedure can be done with safety and potential benefit, particularly in terms of decreased wound-related complications, including infections and hernias, at least with short-term follow-up. 1–5
We have had extensive experience with open gastric bypass surgery and maintain a database with more than 2,500 patients, many followed up to 15 years after surgery. We and others have published outcome data for open gastric bypass surgery with outstanding outcomes. 6–12 Our goal in developing our laparoscopic gastric bypass program was to introduce the procedure while avoiding increased risk for our patients. The current data were gathered to determine whether laparoscopic gastric bypass provided an acceptable complication rate and adequate short-term weight loss and are presented from the very beginning of our program, which includes our learning curve for the procedure. We believe the results to be very encouraging and suggest that traditional open bariatric surgeons should move carefully toward laparoscopy as the future standard of care for the surgical treatment of morbidly obese patients.
METHODS
Data were prospectively collected on 281 consecutive patients undergoing an attempt at total laparoscopic gastric bypass to treat morbid obesity at our institution during the 43-month period between March 1998 and October 2001. Our initial experience with laparoscopic gastric bypass involved a hand-assisted procedure in 25 patients, which aided us in climbing the learning curve;9 however, those procedures are not included in the current report. One surgeon (E.J.D.) performed the majority of the procedures reported herein and initially assisted the other bariatric surgeons in our group (H.J.S., J.M.K.) as they began performing the procedure.
We used a six-port technique to accomplish the procedure. Initial access to the peritoneal cavity was via insertion of a Veress needle in the left subcostal position for insufflation of carbon dioxide gas, followed by insertion of a 12-mm trocar. Additional trocars were then placed in the left supraumbilical area (12 mm) and in the subxiphoid position (5 mm) to allow insertion of a transabdominal Nathanson liver retractor, which was anchored to a rigid arm (Automated Medical Products Corp., Edison, NJ; Iron Intern) to retract the left lateral segment of the liver. Five-mm and 10-mm trocars were placed in the right abdomen for the surgeon and another 5-mm trocar was placed in the left side for the assistant to use. We initially opened the gastrohepatic ligament with ultrasonic dissection (Autosonix, Tyco/US Surgical Inc., Norwalk, CT) to expose the lesser sac. We then transected the lesser curvature mesentery with a vascular staple load (45-mm cartridge length of 2.0-mm staples) on a linear stapler (Endo-GIA II, Tyco/US Surgical). The proximal stomach was transected just below the gastroesophageal fat pad with three or four firings of the 60-mm cartridge of 3.5-mm staples using the same stapling instrument, with completion of the transection at the angle of His. In our first 30 procedures, we used a Baker jejunostomy tube with the balloon inflated to 15 mL, pulled back by the anesthesiologist to lodge at the gastroesophageal junction, to create a pouch volume of 15 to 20 mL. A soft latex-free rubber half-inch-diameter drain was placed in the lesser sac to aid in subsequent identification of the appropriate space for passage of the Roux limb in the retrocolic, retrogastric position after dissection of a 3- to 4-cm opening in the transverse mesocolon. Once the drain was identified via the mesocolic dissection just lateral and superior to the ligament of Treitz, it was pulled through the mesenteric window and left as a marker, which was sutured later to the end of the Roux limb. Alternative methods used included simply opening the mesocolon from below and passing the Roux limb in the retrocolic, retrogastric position or grasping the Roux limb from above with a roticulating grasper.
The enteroenterostomy was accomplished using similar techniques in all patients, but with two different measured lengths of intestine depending on whether the patient was undergoing a proximal or long-limb gastric bypass. We performed a long-limb bypass only on super-obese patients (body mass index [BMI] > 50 kg/m2) and often did not perform the long-limb procedure unless patients had a BMI of more than 60kg/m2 and the more distal intestine appeared to be of adequate caliber, because we were concerned about a potential increased risk of obstruction. We measured approximately 30 cm (proximal bypass) or 75 cm (long-limb bypass) distal to the ligament of Treitz, where the small bowel was transected using a 60-mm cartridge of 2.5-mm staples on the linear stapling device. Several centimeters of mesentery were also divided using either a vascular staple load or the ultrasonic dissector. We then measured the Roux limb either 50 cm (proximal bypass) or 150 cm (long-limb bypass) from this transection point. A side-to-side anastomosis was then undertaken between two stay sutures (2-0 Surgidac, Endo-Stitch, Tyco/US Surgical) placed approximately 2 cm apart in the antimesenteric wall of the small intestine. Enterotomies were made with the ultrasonic scalpel and the 60-mm cartridge of 2.5-mm staples was advanced into the openings to create the anastomosis. The enterotomy site was closed with the same-size staple cartridge, with care taken to avoid narrowing the anastomosis. The small bowel mesenteric defect was closed with a running suture.
The gastrojejunal anastomosis was created by various techniques as we modified our approach with experience over time. We performed 13 total laparoscopic procedures in which the anvil of a circular 21-mm stapler was drawn down the esophagus using a percutaneous gastrostomy technique originally described by Wittgrove and Clark. 1,2 In an additional two procedures we inserted the 21-mm anvil transabdominally into the proximal pouch via a gastrotomy. These approaches were used early in our experience, before October 1999. We subsequently began to perform the anastomosis using a linear-stapled technique and have used this approach for the vast majority of our procedures (n = 254). This technique has been described in detail by others. 5 In our first 102 procedures with the linear-stapled technique, we closed the gastrojejunal opening with a second firing of the linear stapler over an adult flexible gastroscope or a 30F rigid bougie. Subsequent to December 2000, we constructed a two-layer gastrojejunal anastomosis in 164 procedures that was completely oversewn with a running nonabsorbable suture (2-0 Surgidac). In 12 of the latter procedures, a two-layer completely hand-sewn anastomosis was created; the linear endo-GIA stapler with 45-mm cartridge of 3.5-mm staples was used in the other 152 procedures to create the internal row. Laparoscopic ultrasound examination of the gallbladder was performed in the last 172 procedures without prior cholecystectomy; if gallstones were found, a laparoscopic cholecystectomy was performed.
Routine management included pre- and postoperative use of subcutaneous enoxaparin (40 mg) and thigh-length intermittent venous compression stockings placed on the patients before the surgical incision. Patients were kept NPO until a contrast barium swallow examination was performed on the first or second postoperative day. Patients were discharged on the second postoperative day if the contrast study revealed no complication and the patient was able to tolerate liquids and/or a puréed diet by mouth. The closed suction drain was usually removed before discharge. Standard discharge medications included a daily multivitamin, vitamin B12 500 micrograms daily, and calcium supplementation. Patients with the gallbladder left in situ were advised to take 300 mg ursodiol twice daily for 6 months after surgery. 10 Menstruating woman were advised to take supplemental iron sulfate 325 mg twice daily. Routine outpatient follow-up was at 2 to 3 weeks and 3, 6, and 12 months after surgery. Annual visits were strongly encouraged beyond 1 year.
Data were prospectively collected and maintained in a computerized database. They are reported as mean ± standard deviation.
RESULTS
Two hundred eighty-one consecutive patients underwent an attempt at laparoscopic gastric bypass between March 1998 and November 1, 2001. Ten patients had undergone a previous failed laparoscopic gastric banding procedure for the treatment of obesity. Patients ranged in age from 15 to 71 years old, with a mean age of 41.6 ± 9.9 years. Eighty-seven percent of patients were female and 82% were white. Preoperative weight ranged from 171 to 446 lb, with a mean of 291 ± 46.6 lb. Preoperative BMI in nonrevisional patients ranged from 40.3 to 71 kg/m2, with a mean of 48.1 ± 6.5 kg/m2. More than half of the patients had undergone previous abdominal surgery. Comorbid medical conditions are listed in Table 1.
Table 1. PREOPERATIVE COMORBIDITIES

Open conversion was required in eight patients (2.8%) due to short instruments (n = 2), short trocar length (n = 1), trocar injury to the colon (n = 1), excessive intraabdominal fat (n = 2), twisted retrocolic limb (n = 1), and persistent gastrojejunal anastomotic leak unable to be controlled laparoscopically (n = 1). These patients are not included in subsequent outcome data because they did not undergo successful completion of the procedure laparoscopically. The hospital length of stay in open conversion patients was 15.8 + 25 days (range 3–76) primarily due to organ failure problems in two patients with hospital stays of 20 and 76 days. The former patient (BMI = 42) had postoperative respiratory failure due to severe obstructive sleep apnea and obesity hypoventilation syndrome requiring tracheostomy. The latter patient (BMI = 52) had severe hemodynamic instability from a postoperative pulmonary embolus causing multisystem organ failure and a prolonged stay in the intensive care unit. A negative abdominal reexploration was performed to exclude a leak as the etiology of cardiovascular collapse, and a gastrostomy tube was placed in the excluded stomach due to severe ileus. A gastric fistula from the gastrostomy tube site and severe wound infection complicated his subsequent management, leading to a prolonged hospital stay, but the problems eventually resolved.
Most patients underwent laparoscopic proximal gastric bypass (94%); the remaining 17 patients (6%) were treated with a long-limb modification. Operative time decreased significantly as we gained experience with the procedure. In the first quartile of procedures, the mean operative time was 234 ± 77 minutes; it decreased to 162 ± 42 minutes in the most recent quartile. Laparoscopic cholecystectomy was performed in eight patients of the past 172 patients in whom we performed intraoperative gallbladder ultrasound.
Hospital length of stay in the 273 remaining laparoscopic bypass patients averaged 4.0 ± 9 days, with a median of 2 days. Seventy-five percent of patients were discharged home within 3 days of surgery, and 90% were discharged within 5 days. There were no early or late deaths in any of the 281 patients. Postoperative complications are shown in Table 2. Each of the wound infections was a result of a reexploration for treatment of a complication, making the wound infection rate of patients not requiring reoperation for complications zero. Similarly, three of five incisional hernias occurred in patients with postoperative open abdominal surgery for complications (one bowel obstruction, one incarcerated internal hernia, one perforated colon 3 months after the bypass), making the incisional hernia rate of patients not requiring reoperation for complications 0.7%. Internal hernia occurring at a mesenteric defect was treated by subsequent surgery in five patients (1.8%).
Table 2. POSTOPERATIVE COMPLICATIONS

An anastomotic leak occurred at the gastrojejunal anastomosis in 12 patients (4.3%) and at the staple line of the excluded stomach in 1 patient. In our most recent 164 procedures in which a two-layer gastrojejunal anastomosis was created, only three leaks have occurred, for a leak rate of 1.8%. Seven leaks occurred in the 102 preceding procedures in which a single-layer linear-stapled anastomosis was created with stapled closure of the gastrojejunal opening, leading us to abandon this technique. One leak occurred at the gastrojejunal anastomosis in the group of patients undergoing revision from a previous banding procedure.
Four of the patients with gastrojejunal anastomotic leaks were clinically stable with leaks shown on a postoperative contrast study that were adequately controlled by the closed suction drain. This subgroup was successfully managed with intravenous nutrition without further surgery. Three patients were unstable and required emergent open laparotomy for treatment. The remaining seven were treated by laparoscopic exploration for repair, drainage, and insertion of a gastrostomy tube into the excluded stomach. Laparoscopic repair of the anastomosis failed in six of the seven patients, but no further surgical treatment was required in five of the seven due to placement of adequate drains to control subsequent leakage. All of the patients with a gastrojejunal anastomotic leak had prolonged hospital stays.
In one patient a leak developed at the jejunojejunostomy anastomosis, requiring reexploration and repair. A second patient with a postoperative obstruction at the small bowel anastomosis underwent laparoscopic enteroenterostomy to bypass this obstruction, which was complicated by a leak mandating open repair. Each of these patients had a prolonged postoperative course, with multiple complications, systemic sepsis, and organ failure, but ultimately survived.
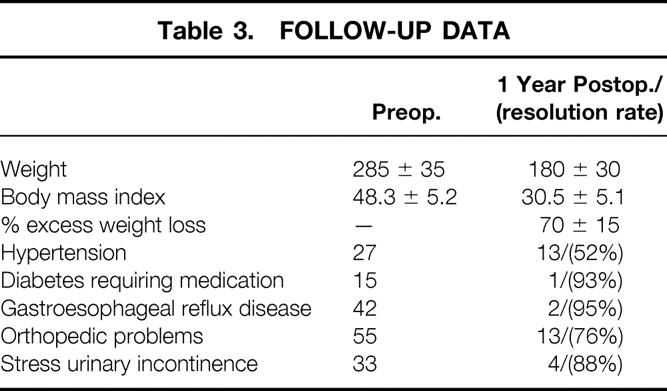
Follow-up was available in 69 of 96 total patients (72%) who were at least 1 year out from their surgery, excluding revisional procedures. Table 3 provides outcome data on these patients at 1 year of follow-up.
Table 3. FOLLOW-UP DATA
DISCUSSION
Our data show excellent results, particularly during the development of a program in laparoscopic gastric bypass surgery by surgeons experienced primarily in open gastric bypass surgery. The laparoscopic procedure produced the anticipated weight loss with fewer wound-related complications than the open procedure, including fewer wound infections and incisional hernias. Our group has reported a major wound infection rate of 5% and an incisional hernia rate of 20% after open gastric bypass. 6–8 These risks were reduced to 1.1% and 1.8%, respectively, of all patients undergoing the laparoscopic procedure, and most of these complications developed in patients undergoing postoperative open exploration for a complication. The complications of stomal stenosis and marginal ulcer were significantly less (P < .01) than our previous experience with open gastric bypass. 6,7
In five patients an internal hernia developed in one of the three potential mesenteric defects created by the Roux-en-Y gastric bypass procedure: the defect at the small bowel anastomosis, the transverse mesocolic defect with bowel herniating into the lesser sac, or Petersen’s defect posterior to the Roux limb. We have not seen an internal hernia develop at the small bowel mesenteric closure. We believe our early efforts at closure of the defects at the time of laparoscopic gastric bypass were inadequate, because we did not close the mesocolon or Petersen’s defect in our first 30 procedures and closed only the medial side of the defect in our subsequent 100 procedures. Five internal hernias were diagnosed and surgically repaired during this early period. For the past 150 procedures, we closed both the medial and lateral aspects of these defects with running nonabsorbable suture, and we have not seen an internal hernia in our last 150 procedures using this technique.
The dreaded complication of gastric bypass surgery is an anastomotic leak from the gastrojejunal anastomosis, with resulting peritonitis. The overall risk of this complication reported in the current series of 5% is higher than that reported in large series of patients undergoing open gastric bypass. 6–10 However, this complication occurred primarily in the group of patients treated with a single-layer linear-stapled anastomosis, in which the openings for insertion of the stapler were closed with a subsequent firing of the stapler. Improvements in technique, including performance of a two-layer anastomosis on a routine basis, have decreased this complication to an acceptable rate of less than 2% in our most recent 164 procedures. Perhaps, just as importantly, we have found that routine closed suction drainage of the gastrojejunal anastomosis, used throughout our experience with laparoscopic gastric bypass but not in our open bypass patients, allowed us to avoid reoperation for treatment in a significant proportion of patients with a leak. This finding has been previously reported by others. 5 Of note, a leak at the jejunojejunostomy anastomosis may be very difficult to recognize and can cause extensive complications. Our two patients who had this complication suffered significant sepsis and organ failure before their ultimate slow recovery. A low threshold for reoperation is recommended in any patient with a possible jejunojejunostomy leak. A laparoscopic reexploration to rule out this complication is feasible.
It is a tribute to our vigilant staff, outstanding critical care services, and careful decision-making in the care of these complex patients that there were no deaths in our series, even though this report includes our first laparoscopic bypass procedures. Our extensive experience with open bariatric surgery clearly provided us with a tremendous advantage for starting the laparoscopic approach, and we believe it is very important for surgeons contemplating performing the laparoscopic gastric bypass procedure to receive extensive training in both advanced laparoscopic techniques and bariatric surgery/bariatric patient management.
The learning curve for the laparoscopic skills used in this procedure is steep and difficult. Our group climbed the learning curve by concentrating maximal experience in one individual’s hands, including triage of appropriate patients for laparoscopic surgery to one individual with advanced laparoscopic skills. Once the skills and routine for the procedure were developed and improvements in the technique made, the other members of the group embarked on their learning curves with the added benefit of the more experienced surgeon serving as an on-site mentor and surgical assistant.
The laparoscopic approach has also had a profound impact on our general surgery residency training program. Before 1998, third-year general surgery residents would perform 20 or more open gastric bypass procedures during their rotation in general surgery. At that time, although we had witnessed a progressive change toward laparoscopic approaches for many general surgery procedures, such as cholecystectomy, appendectomy, and hernia repair, the gastric bypass remained a major abdominal procedure with gastrointestinal anastomoses that played an increasingly important role in training our residents in gastrointestinal surgery. We had resisted the notion that laparoscopic surgery required additional training beyond the general surgery residency, and our advanced laparoscopic procedures were done with a general surgery chief resident, including Nissen fundoplication, splenectomy, repair of hernias, and so forth. However, the laparoscopic bypass has changed that dramatically. We have recognized that the laparoscopic bypass procedure presents a steep learning curve and both the surgeon and first assistant must have the capability to perform advanced laparoscopy. We do not believe that general surgery residents have enough exposure to advanced laparoscopy during their training in our program (or in most others) to become capable surgeons or even first assistants for this procedure at the current time. We have started a fellowship in advanced laparoscopic surgery with a primary emphasis on laparoscopic gastric bypass for the fellows’ training. We believe there is currently no better laparoscopic procedure for advanced training in this specialty than laparoscopic gastric bypass, which involves the creation of two intestinal anastomoses. We continue to perform open gastric bypass procedures in some of our super-obese (BMI > 50) and most of our super-super-obese patients (BMI > 60), and this continues to provide a significant number of procedures for our third-year general surgery residents. Our ultimate goal is to involve our general surgery residents in the laparoscopic gastric bypass procedure if we can reach a point where they have the needed skills. This likely will require intensive skill training in the dry laboratory as well as revised rotation schedules and an added time commitment during residency to laparoscopy.
We have seen a tremendous increase in patient demand for bariatric surgery, which we attribute to the laparoscopic approach to gastric bypass. The Internet has evolved as a tremendous source of information and communication between patients interested in undergoing surgery for obesity. Our practice is currently performing more laparoscopic gastric bypass procedures than open procedures, despite continuing to be selective regarding which patients qualify for a laparoscopic approach in terms of body weight and previous surgery. The increase in interested patients has translated into a 150% increase in the number of bariatric procedures we are performing. Patients appear to believe that the minimally invasive approach is more acceptable, and this influences their decision to consider surgical treatment as an option.
CONCLUSIONS
We were able to establish a successful program in laparoscopic gastric bypass. Significant complications occurred in a few patients, primarily a result of anastomotic leaks. Fortunately, this and other complications have decreased progressively with experience and improved surgical techniques, particularly routine performance of a two-layer gastrojejunal anastomosis and complete closure of all mesenteric defects. Other complications did not occur at an increased rate, and wound-related complications, including infection and hernia, were extremely uncommon. Weight loss was acceptable and resolution of comorbidities occurred as anticipated. The results suggest that surgeons practicing bariatric surgery should make efforts to learn the skills for laparoscopic gastric bypass, because it is likely to become the standard of care for the surgical treatment of obesity.
Discussion
DR. WARD O. GRIFFEN, JR. (Frankfort, MI): As a first comment, I would like to acknowledge that Dr. Sugerman has learned something that we have taught for years. When you create defects in mesentery you should close all of them before you come out of the abdomen, whether you are coming out through a port or through an incision.
Despite a few reports about gastric banding and the vertical banded gastroplasty procedures suggesting that adequate weight reduction has been obtained, I think most bariatric surgeons today would agree that the Roux-en-Y gastric bypass is the standard of care for patients with morbid obesity. It is a big operation on big people. And I think the authors are to be congratulated for having come here with a new technique basically, an old technique but done in a new way, that will be, I think, eventually proven to be a safe procedure once the steep learning curve has been negotiated. I think it depends on us to teach the surgical residents how to do this operation through the laparoscope.
When we first undertook gastric restrictive procedures at Kentucky in 1975, we found that the loop gastrojejunostomy was a very difficult procedure because of the thickness of the mesentery, so we switched to the Roux-en-Y procedure. But we did so with some trepidation because we were concerned about marginal ulcer. However, we found that our marginal ulcer rate was no greater than what the authors have reported today. And they have already answered my first question, which was, how did they treat it? We found it was treatable very easily with H2 receptor blockade.
In the abstract you use the term “chronic heartburn”; in the manuscript, which you very kindly provided me yesterday, you use the term GERD, which is for gastroesophageal reflux disease. Regardless, the fact is that postoperatively you found that your GERD disappeared in a great majority of the patients, about 95%.
In 1981 we presented in the Journal of the Kentucky Medical Association a series of 20 patients who had endoscopically proven reflux. And we were surprised by two things. One, the GERD disappeared practically immediately postoperatively; almost in the recovery room the patient stopped having any kind of heartburn. The other thing we found was that only 1 of the 20 patients, or again 5%, continued to have symptoms. When we reendoscoped that patient we found that the patient had bile reflux, which we thought was contributing to their continued symptoms. And we wondered at that time whether the Roux-en-Y loop we made was too short for that particular patient. I wonder if you would comment on whether you feel that perhaps bile reflux is the reason that some of your patients have not had resolution of their GERD symptoms.
Finally, because I am still concerned about the disruption of the normal physiologic functioning of the gastrointestinal tract with this operation, in your extensive experience with this operation, both open and laparoscopically, I wonder if you have found visceral cancers to occur in these patients postoperatively. In the nearly 1,000 patients that we followed for a number of years, I know of three pancreatic carcinomas and two colon carcinomas that have occurred, and at a relatively young age. And I wondered if you have had any experience with that problem.
I appreciated the paper and I appreciate you sending me the manuscript.
DR. BRUCE D. SCHIRMER (Charlottesville, VA): I also wish to congratulate the authors on an excellent presentation and really superb results with using laparoscopic Roux-en-Y gastric bypass. For those open bariatric surgeons who read this manuscript, the message is clear, and once again I am preempted by Dr. Sugerman, in saying that you can teach an old dog new tricks.
MCV has long been recognized as one of the nation’s leading centers for bariatric surgery, and this paper establishes the fact that a center well established in the performance of open bariatric surgery can improve excellent outcomes for severely obese patients by the appropriate use of a laparoscopic approach. Wound and hernia complications are definitely lower, mortality and severe complications remain low, and weight loss and resolution of comorbidities are excellent.
My questions for Dr. DeMaria are numerous, and I will start by asking Eric what is the best way to pass the Roux limb—sort of a technical question. We don’t have the right answer, and I wonder if you do.
Second, we have also experienced a high incidence of the Roux limb herniating behind the stomach from sutures being pulled loose. Will the running permanent suture be the answer to this, or should we perhaps consider going to an antegastric placement of the Roux limb?
Regarding the proximal anastomosis: I agree that sewing the stapler defect closed is best; we have not experienced any leaks from this area of the anastomosis since converting to the linear proximal stapled anastomosis and by using a double oversewing of the stapler defect. Clearly, stapling it closed was prone to leaks. But I question your continued use of closed suction drains. We do not routinely place them for open procedures. Leaks may well occur after the second day, when you routinely remove them. We have placed drains in this area when an intraoperative leak laparoscopically was assessed or an anastomotic difficulty was encountered, but we don’t do it routinely. I wonder why you are routinely doing it and how long you will continue to do that.
I also disagree with your choice of using intraoperative ultrasound for assessing gallstones. It would seem an unnecessary and time-consuming step in an already long operation. And your yield of 5% to 6% is surprisingly low in this patient population. Do you have any explanation for that? We prefer to do preoperative ultrasounds, plan to remove diseased gallbladders, counsel patients with normal gallbladders preoperatively about the potential for gallstone formation with rapid weight loss, and then let them decide between the choice of Actigall or prophylactic cholecystectomy.
Next, what are your current criteria for weight limit and BMI limit to attempt the operation laparoscopically?
Finally, I agree with your assessment in the manuscript that this procedure is to initially be done only by two well-trained laparoscopic surgeons (i.e., read that, attendings or fellows). However, now that I have performed over 75 of these personally, I am comfortable with a skilled senior-level surgery resident helping me do them. I disagree that we should forever restrict this operation to one that is performed really only by attendings and fellows, and I feel that with experience and efforts to improve our general surgery residents’ laparoscopic skills, this will eventually become an operation in which skilled residents may routinely participate. Do you foresee this happening at MCV?
I wish to thank the Association for the privilege of the floor and thank the authors for the manuscript.
Dr. J. Patrick O’Leary (New Orleans, LA): Drs. DeMaria, Kellum, and Sugerman should be complimented because they have brought serious obesity to this forum on a number of occasions and actually have presented their data here more than any other group in the SSA.
Bariatric surgery, laparoscopically performed, is the rage sweeping the country these days. It has become the tour de force. Any laparoscopic surgeon who has one bit of gumption now is taking on these seriously obese patients and proving that they can, in fact, do this complex operation.
The results that Drs. Sugerman and DeMaria are sharing with us today are quite similar to what we have been able to achieve in our own practice at LSU in New Orleans, with Louis Martin providing the leadership. He has done about 400 laparoscopic gastric bypasses to this point. The weight loss, the complication rate, and the operative time are quite similar to what has been reported here.
What I have been most impressed with is the rapid return to normal life we have seen in these patients. If you do a laparoscopic gastric bypass procedure, the patient is discharged on postoperative day number 3 or 4. If the procedure has been done open, they may be discharged by day 4, but they are still not well 6 weeks later. In patients done laparoscopically, 10 days later these patients look absolutely normal.
I do have a concern. Many years ago those of us who were involved in treating morbid obesity (before it became a “fad”) imposed on ourselves a long-term follow-up for these patients, perhaps for life, to determine what the effect of serious weight loss and serious malabsorption was going to be on these patients as they matured. What is going to happen to the alimentary tract? My concern now is that this operation is being done reasonably frequently and by people who do not have a commitment or an understanding of the management of seriously obese people throughout their life. My question for the authors: What type of long-term management scenario do you believe is important for patients who have had gastric bypass performed laparoscopically?
DR. Kenneth G. MacDonald, JR. (Greenville, NC): This is a commendable series of patients, particularly in view of the fact that it included the learning curve portion of their experience. I completely agree with the comments regarding establishment of a laparoscopic gastric bypass program: that you need both extensive bariatric and laparoscopic experience, and it seems like the best results or best series have been coming from centers which can combine those two. I would like to bring up a few points for discussion.
The leak rate in your series of 5% was very similar to that which Shauer (Pittsburgh) reported at American Surgical last year and seems higher than in the open operation experience. Although you mention technical factors in performing the gastrojejunostomy as a possible explanation, I wonder about the contribution of routine use of drains in both your and the Pittsburgh series. Two things which perhaps support this hypothesis are that in our own experience at East Carolina, leaks are usually catastrophic events. They are not something that can be managed nonoperatively, as many of these laparoscopic leaks are. Also, in our own experience at ECU with laparoscopic bypass, we don’t routinely leave drains, and we have been very fortunate in our early experience of 50 cases to have no leaks yet. I wonder therefore if drains detect subclinical leaks.
The second comment pertains to your limb length. I know your numbers probably are not sufficient to be statistically significant, but can you comment on the weight loss that you have seen with 150-cm limb versus the 50-cm proximal gastric bypass in your series? I know you have additional experience with varying limb lengths with the open operation.
The third point deals with the stenoses. This problem seems to be highly variable from center to center and, in my opinion, more common with the laparoscopic than the open operation. I wonder if you observed any difference in stenosis rate between your smaller EEA series versus your linear stapler groups, and with your growing experience is this incidence decreasing?
Finally, while you are not doing it currently, do you have any opinion about the long-term weight loss with the EEA stapler? With our experience at least, I am developing a bias that this is a more reproducible technique and perhaps less prone to dilation. Everybody has experienced the rectal anastomoses that you wish would dilate a little bit. So I am wondering if EEA isn’t perhaps going to maintain a better degree of restriction than the linear anastomosis, but that is going to take an awful long time to prove.
In summary, this is an important and timely report, as usual, from the MCV group. It is well presented, well analyzed, and I do appreciate your ongoing contributions to this field.
Dr. Henry L. Laws (Birmingham, AL): I apologize for getting up again, but I am real interested in the subject, like anybody that gets involved in it even a little bit. I would certainly congratulate Drs. Sugerman and DeMaria on their excellent and extensive experience, which they have looked at in a scientific way. I really appreciate their candid report. We definitely have had some trouble with leakage at the gastrojejunostomy. Our experience is about like theirs in their latter group.
I could not emphasize too much that I believe you need two experienced operators. I believe, after a period, those can be residents. Certainly, our residents do a lot of these operations now.
One of the spin-offs from this operation is that it empowers the laparoscopic surgeon. You have to sew so much on a regular basis, so regularly inside, that you soon become much more skilled with knot tying and suturing, which really makes you stronger as a surgeon. On the other hand, I believe you should work very diligently ahead of time to be ready to take that step before you embark on this procedure.
I can’t help but get up and follow Dr. Griffen. He pointed out in 1981, as he says, that this operation is effective for gastroesophageal reflux. We will use a gastric bypass more than once a year to manage people who have failed previous antireflux procedures. Even though they are not morbidly obese, doing gastric bypass with a Roux limb is sometimes a safer and more effective operation than trying to redo something at the esophageal hiatus itself.
We have embarked, following the lead of Dr. Ken Champion from Marietta, in doing a number of these operations recently in an antecolic, antegastric fashion for the gastrojejunostomy. And frankly, I believe it can be done in most people. On the other hand, everybody should prepared to do a retrogastric, retrocolic anastomosis if that is the only way to get the small intestine up there.
I notice, by the way, that none of the patients in this particular series had pseudotumor cerebri. Did you just not have any here, or do you treat those otherwise? Utilizing gastric bypass for patients with pseudotumor cerebri is a great advance, pointed out by Dr. Sugerman. The operation remedies this disorder.
Dr. F. Charles Brunicardi (Houston, TX): I would like to congratulate Dr. Sugerman and his colleagues on an outstanding series. Dr. Walter Pories showed that an interesting side effect of the Roux-en-Y bypass is resolution of diabetes. And I saw in Dr. Sugerman’s series that 93% of their diabetic patients had resolution of their diabetes. I was wondering if he could please comment on that, and if he had any ideas as to the mechanism involved.
DR. JAMES R. STARLING (Madison, WI): I am exaggerating a little bit, but back home a BMI of 45 to 48 is sort of the norm; 35% to 45% of the patients I operate on weigh between 450 to 550 pounds. I have continued to do those open. Technically I’m not comfortable doing them through the laparoscope.
The second question is calling your gastrojejunostomy anastomosis linear. But as I looked at your one slide, it looked sort of vertical. Is this the same type of technique that Rutledge has on his Web page? A vertical lesser curvature gastrojejunostomy.
Dr. Eric j. DeMaria (Richmond, VA): First I have to reject any impression that somehow I am Dr. Sugerman’s mentor. Dr. Sugerman needs no mentor in the field of bariatric surgery; he is clearly an international expert in this field. I will try to cover some of the topics that multiple discussants had mentioned.
One talked about gastroesophageal reflux. We believe that gastric bypass is the most appropriate surgical intervention for the obese patient with gastroesophageal reflux. The 5% of patients with GERD treatment failure were patients continued on acid-reducing medications long term. We did not confirm that these individuals had true acid reflux; however, we have found that some people simply symptomatically feel better with those medications and continue them, often at the behest of their internist or primary care physician.
In the case of what several individuals have mentioned, the loop gastric bypass or mini-gastric bypass procedure that Dr. Rutledge has tried to popularize in North Carolina, this is in fact not a loop gastric bypass procedure. We have had the opportunity now to handle a number of cases from that part of the country doing Roux revisions on people who have very significant bile reflux symptomatology, and we are concerned about the long-term consequences of this loop bypass procedure.
I agree with those individuals who pointed out that closure of mesenteric defects is a basic surgical principle; however, we were simply inadequately skilled at the time of the beginning of this procedure to accomplish that, and we had to develop mechanisms to do so. And over time, we actually learned that you had to close them more thoroughly than we do in our open surgical procedures.
We have developed an underlying hypothesis that, perhaps because of laparoscopic surgery and less intraabdominal adhesions, we might actually be more likely to see internal hernias because of mobility of the bowel.
One way to avoid mesenteric hernias would be the antecolic, antegastric path for the Roux limb. We have had very limited exposure to this and only in the past month or so have we undertaken some of those procedures, so I can’t really comment on that. But I will tell you that the retrocolic, retrogastric Roux is a very short distance for the Roux to travel; it is a very nice tension-free procedure and therefore very appealing.
We have continued to routinely drain the gastrojejunal anastomosis in these patients, mostly because we have been on our learning curve and have been unwilling to change things until we absolutely knew that it was reasonable to change. I don’t think we are causing leaks, because most of these leaks are not subclinical; most of them show up on contrast studies. So I don’t think that is what we are doing. And we do remove our drains fairly early, within 2 or 3 days in most patients.
In terms of laparoscopic ultrasound, this was based on historical precedence at our institution, where we taught intraoperative ultrasound techniques during open gastric bypass, and we continue to do the same with laparoscopy for both training our physicians and our staff.
I think that our experience with the EEA technique is really inadequate to make any great conclusions about it. We did perform our 26 hand-assisted laparoscopic procedures with the EEA. So in total we have about 40 or 50 EEA anastomoses out there, and we haven’t seen any impact on long-term results as far as better or worse with that technique.
Finally, as far as diabetes, we believe that gastric bypass surgery is a superior approach than restrictive procedures such as the vertical banded gastroplasty and more currently the lap-band procedure. In our experience with the lap-band we only resolved 40% of our diabetics. Here, with gastric bypass, we routinely see resolution in 90%. We believe that is a major benefit of gastric bypass. The mechanism is unclear but may involve gut hormone changes.
Finally, what are the limits of laparoscopic gastric bypass in terms of patient body weight and so forth? My practice is now limited to laparoscopic gastric bypass. I have given up doing open bariatric surgery because my colleagues are very good at it. And I have the luxury of the large number of patients. Our volume has increased about 250% in the last few years with laparoscopic bypass surgery, and we have done body mass indices well up into the 60s and in the low 70s as of today. I think our limitations are only the limitations of our technology with this procedure. I think as we develop longer instruments and better trocar access systems and so forth that we will be able to tackle any patient laparoscopically.
Regarding gastrointestinal malignancies, we have seen several cases of colon cancer in long-term follow-up. We have had one patient develop cancer of the stomach and are aware of a case reported in the literature. Our opinion is that visceral cancers do not occur at increased frequency after gastric bypass.
Finally, we agree with Dr. Schirmer that ideally this complex procedure would provide advanced laparoscopic training for general surgery residents. However, despite an organized training program for laparoscopic skill development, we have not been comfortable teaching the majority of our residents how to do this operation. Furthermore, the surgical assistant’s role is equally difficult, and few residents have been able to do this well. Therefore, currently we believe the skills required for this procedure must be attained during a dedicated period of intensive laparoscopy training, such as a fellowship.
Footnotes
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Correspondence: Eric J. DeMaria, MD, Director, MIS Center, Medical College of Virginia, 1200 West Broad Street, P.O. Box 980519, Richmond, VA 23298.
E-mail: edemaria@hsc.vcu.edu
Accepted for publication December 2001.
References
- 1.Wittgrove A, Clark G, Tremblay L. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obesity Surg 1994; 4: 353–357. [DOI] [PubMed] [Google Scholar]
- 2.Wittgrove A, Clark G. Laparoscopic gastric bypass, Roux en-Y: 500 patients: technique and results, with 3–60-month follow-up. Obesity Surg 2000; 10: 233–239. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen N, Ho H, Palmer L, Wolfe B. A comparison study of laparoscopic versus open gastric bypass for morbid obesity. J Am Coll Surg 2000; 191: 149–157. [DOI] [PubMed] [Google Scholar]
- 4.Higa K, Boone K, Ho T, Davies O. Laparoscopic Roux-en-Y gastric bypass for morbid obesity. Arch Surg 2000; 135: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 5.Schauer P, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic gastric bypass for morbid obesity. Ann Surg 2000; 232: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugerman H, Starkey J, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 1987; 205: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugerman H, Londrey G, Kellum J, et al. Weight loss with vertical banded gastroplasty and Roux-en-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg 1989; 157: 93–102. [DOI] [PubMed] [Google Scholar]
- 8.Sugerman H, Kellum J, Engle K, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr 1992; 55: 560S–566S. [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-year follow-up. Am J Clin Nutr 1992; 55: 582S–585S. [DOI] [PubMed] [Google Scholar]
- 10.Brolin R. Gastric bypass. Surg Clin North Am 2001; 81: 1077–1095. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria EJ, Schweitzer MA, Broderick TJ, et al. Hand-assisted laparoscopic gastric bypass. Surg Endosc 2002 (in press). [DOI] [PubMed]
- 12.Sugerman HJ, Brewer WH, Shiffman MH, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg 1995; 169: 91–97. [DOI] [PubMed] [Google Scholar]


