Abstract
Objective
To determine whether patients with tertiary hyperparathyroidism due to single- or two-gland disease undergoing limited resection have similar long-term outcomes compared with patients with hyperplasia undergoing subtotal or total parathyroidectomy.
Summary Background Data
Tertiary hyperparathyroidism occurs in less than 2% of patients after renal transplantation. Approximately 30% of these cases are caused by one or two hyperfunctioning glands. Nevertheless, the standard operation for this disease has been subtotal or total parathyroidectomy with autotransplantation.
Methods
Seventy-one patients underwent surgery for tertiary hyperparathyroidism. At the time of surgery, 19 patients who had a single or double adenoma underwent limited resection of the enlarged glands only (adenoma group). The remaining 52 patients with three- or four-gland hyperplasia had subtotal or total parathyroidectomy with implantation (hyper group). Long-term cure rates between the two groups were compared.
Results
In the adenoma group, 7 patients had a single adenoma and 12 underwent resection of a double adenoma. In the hyper group, 49 patients had subtotal and 3 had total parathyroidectomies. After surgery, 70 of 71 patients (99%) were cured of their hypercalcemia. The incidence of postoperative transient hypocalcemia was significantly higher in the hyper group (27% vs. 5%). No patients in either group had permanent hypocalcemia requiring long-term supplementation. With up to 16 years of follow-up, there have been no recurrences in the adenoma group, whereas three patients (6%) in the hyper group have had recurrent or persistent hyperparathyroidism.
Conclusions
Patients with tertiary hyperparathyroidism who underwent limited resection of a single or double adenoma only had equivalent long-term cure rates compared with patients undergoing more extensive resections. Therefore, the authors recommend in patients with tertiary hyperparathyroidism and enlargement of only one or two parathyroid glands that the resection be limited to these abnormal glands only.
Tertiary hyperparathyroidism occurs in patients with chronic renal failure who have undergone a successful kidney transplant. The development of tertiary hyperparathyroidism is thought to arise from a resetting of the homeostatic response mechanism of the parathyroid tissue secondary to prolonged alterations in serum calcium and phosphate in patients with renal failure.
Although tertiary hyperparathyroidism is not a common disease process in the renal transplant population (1.6–3% in some series), 1–4 it can cause significant problems, including pathologic fractures, joint disease, renal calculi, mental status changes, muscle weakness, and peptic ulcer disease as well as pancreatitis. 4–10 Surgery remains the only curative therapy for tertiary hyperparathyroidism. 1,3,11,12
The standard surgical approach to patients with tertiary hyperparathyroidism has been either subtotal parathyroidectomy or total parathyroidectomy with autotransplantation. This surgical strategy is based on the belief that tertiary hyperparathyroidism is generally the result of hyperplasia of all four (or more) glands. However, several reports indicate that up to 29% of patients with tertiary hyperparathyroidism may have disease limited to one or two glands. 4,13–22 Although some surgeons recommend subtotal or total parathyroidectomy with autotransplantation for fear of recurrent disease in these patients, others propose resection of only the enlarged glands after a formal neck exploration. 1,3,4 To determine whether resection of a single or double adenoma in patients with tertiary hyperparathyroidism results in equivalent long-term cure rates, we reviewed our experience.
METHODS
Between January 1984 and May 2001, 3,995 patients underwent kidney transplantation at the University of Wisconsin Hospitals and Clinics. Of these, 71 were referred for clinically significant post-transplantation hyperparathyroidism requiring parathyroid exploration; these patients form the basis for this report. The criteria for surgical referral were symptomatic hypercalcemia after kidney transplantation, or asymptomatic hypercalcemia 1 or more years after successful renal transplant (Ca++ > 10.5 mg/dL with elevated parathyroid hormone [PTH] levels and a normal serum creatinine level). Patients in whom hyperparathyroidism developed after failure of their transplanted kidney were excluded.
All patients underwent a formal neck exploration through a Kocher incision. All glands were identified and examined. If three or four glands were enlarged, a 3.5-gland (subtotal) or a 4-gland (total) parathyroidectomy with transplantation of the remaining tissue into the brachioradialis muscle was performed. In most instances, if only one or two glands were enlarged and the remaining glands were normal, then only the enlarged glands were resected.
Outcomes were based on chart review. Postoperative hypocalcemia was defined as a serum calcium level of 7.9 mg/dL or less with associated symptoms, including numbness and tingling in the perioral region or in the extremities. Recurrence was defined as a serum calcium level exceeding 10.5 mg/dL in consecutive samples 6 months after surgery. Persistent disease was defined as a serum calcium level greater than 10.5 mg/dL within 6 months of surgery. Data were recorded as mean ± SEM. Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL).
RESULTS
Pretransplant Data
There were 71 patients, 32 men and 39 women. The average age at parathyroidectomy was 43 ± 2 years. The primary renal disease leading to transplantation was chronic glomerulonephritis (n = 10), hypertension (n = 9), polycystic kidney disease (n = 9), diabetes (n = 8), membranoproliferative glomerulonephritis (n = 5), unknown (n = 5), chronic pyelonephritis (n = 4), congenital hypoplasia (n = 4), obstructive uropathy (n = 3), focal segmental glomerular sclerosis (n = 4), Alport’s disease (n = 3), systemic lupus erythematosus (n = 2), amyloidosis (n = 1), renal cell carcinoma (n = 1), lithium toxicity (n = 1), chemotherapy toxicity (n = 1), and IgA nephropathy (n = 1). There were 49 cadaveric transplants, 18 living related transplants, 2 repeat living related transplants, and 2 living unrelated transplants. The average duration of dialysis was 27 ± 4 months. Thirteen patients received a kidney without undergoing dialysis.
Preparathyroidectomy Data
The average preoperative calcium level was 11.0 ± 0.3 mg/dL. Different PTH assays were used during this period to measure PTH levels. The mean preoperative PTH C-terminus level was 1,184.31 pg/mL (reference range 18–120 pg/mL) in 32 patients. In 29 patients the mean preoperative N-terminus level was 98.67 pg/mL (reference range 1.0–6.8 pg/mL). In 14 patients, the mean preoperative intact PTH level was 545.21 pg/mL (reference range 15–65 pg/mL). Preoperative serum phosphate and serum alkaline phosphate levels were 2.14 ± 0.66 mg/dL and 195 ± 171.87 U/L, respectively.
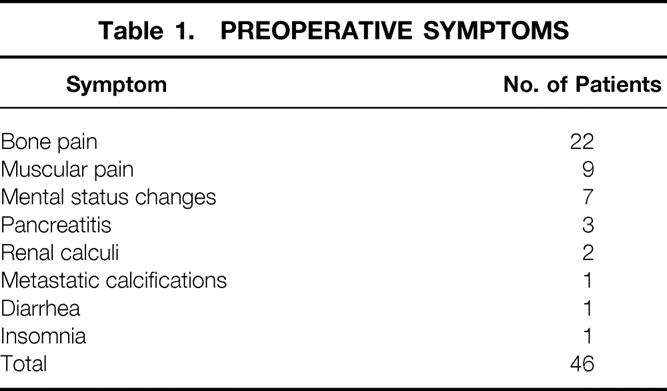
Forty-six patients were symptomatic (66%). Twenty-two presented with bone pain. Nine had musculoskeletal complaints, including myalgias and weakness. Seven patients experienced mental status changes. Three patients presented with pancreatitis and two with renal calculi. Other symptoms on presentation included diarrhea, insomnia, and metastatic calcifications (Table 1). Twenty-five of the patients were asymptomatic at presentation.
Table 1. PREOPERATIVE SYMPTOMS
Surgical Data
The average time between renal transplantation and parathyroidectomy was 4.2 ± 0.7 years. The average duration of parathyroidectomy was 144 ± 5.3 minutes. The average gland weight was 892 ± 174.46 mg. The average length of stay was 3.5 ± 0.3 days. Twenty-one of the patients were found to have either one (n = 9) or two (n = 12) enlarged glands, and 19 of them underwent resection of a single or double adenoma (adenoma group). The remainder of the patients (hyper group) underwent subtotal (n = 48) or total parathyroidectomy (n = 3) with autotransplantation into the brachioradialis muscle. Seven patients (9.8%) were found to have ectopic glands (five at initial neck exploration and two on reexploration for recurrent disease). Four were located in the thymus (5.6%) and three were intrathyroidal (4.2%). Seven of the patients in the hyper group had enlargement of only three glands (13.4%). In a separate patient, only 3 glands were identified and 2.5 glands were removed.
Surgical Outcomes
Sixty-nine of the 70 patients undergoing a first-time exploration for hyperparathyroidism became eucalcemic after surgery. One patient who underwent a 2.5-gland resection required reexploration within 8 days of the initial operation for persistently elevated serum calcium levels. On re-exploration, an ectopic gland was located in the thyroid by intraoperative ultrasound. The patient subsequently became eucalcemic after excision of the ectopic gland. Another patient was referred for persistent hypercalcemia after successful renal transplantation. Six months before transplantation, she had undergone a three-gland parathyroidectomy with autotransplantation into the brachioradialis muscle at another institution. A preoperative nuclear imaging study suggested a parathyroid gland in the substernal region. A gland was identified on reexploration in the thymus. After resection, her serum calcium level returned to normal.
Comparison of Limited Versus Subtotal or Total Resection
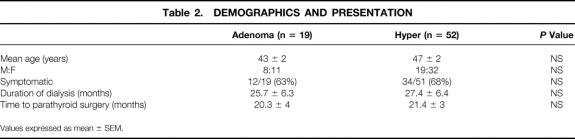
Patients were placed into two groups based on the intraoperative findings and the procedure performed (limited resection vs. subtotal or total parathyroidectomy). The adenoma group consisted of 19 patients who underwent limited resection of one or two adenomas. The hyper group included all patients who underwent subtotal or total parathyroidectomy with autotransplantation. No differences were noted between these two groups with regard to age, gender, symptoms, duration of dialysis, or time between transplantation and parathyroidectomy (Table 2). We also compared the preoperative laboratory values (serum calcium, PTH, creatinine, and phosphate levels) of the two groups; no significant differences in these values were observed (Table 3).
Table 2. DEMOGRAPHICS AND PRESENTATION
Values expressed as mean ± SEM.
Table 3. PREOPERATIVE LABORATORY VALUES
PTH, parathyroid hormone. Values expressed as mean ± SEM.
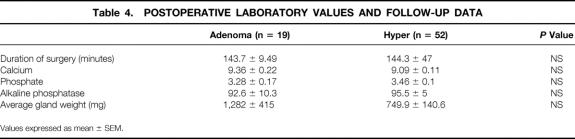
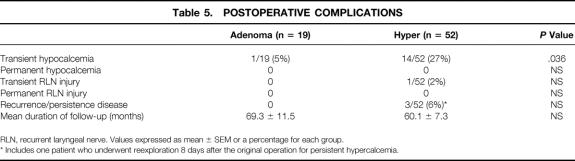
In comparing the postoperative outcomes of the two groups, there was no significant difference in the mean serum calcium, phosphate, or alkaline phosphatase level, gland weight, duration of operation, or follow-up (Table 4). There was a significantly higher rate of transient postoperative hypocalcemia in the hyper group (27%) than in the adenoma group (5%) (P = .036) (Table 5). Importantly, one of the patients in the hyper group with enlargement of a single gland underwent a subtotal parathyroidectomy and developed hypocalcemia. Three of these patients had three-gland hyperplasia and two other patients underwent total parathyroidectomy with autotransplant. All 14 patients who had transient postoperative hypocalcemia required oral calcium and/or vitamin D supplementation lasting 2 to 18 months. Two of the patients required subsequent autotransplantation of cryopreserved tissue. In all patients, hypocalcemia resolved. In contrast, only one of the patients in the adenoma group required oral calcium and vitamin D supplementation for several weeks. Her low serum calcium level coincided with an episode of acute pancreatitis; thus, it is unclear whether the etiology of hypocalcemia was truly secondary to the surgery performed or to the pancreatitis.
Table 4. POSTOPERATIVE LABORATORY VALUES AND FOLLOW-UP DATA
Values expressed as mean ± SEM.
Table 5. POSTOPERATIVE COMPLICATIONS
RLN, recurrent laryngeal nerve. Values expressed as mean ± SEM or a percentage for each group.
* Includes one patient who underwent reexploration 8 days after the original operation for persistent hypercalcemia.
Other surgical complications in either group were rare. There were no postoperative hematomas or infections, and only one patient experienced transient, recurrent laryngeal nerve neuropraxia that resolved within 2 weeks.
Long-Term Follow-Up
The average follow-up was 62.7 months. One patient was lost to follow-up. Thirteen have died. Nine of these patients died with functioning renal allografts. Three patients currently require dialysis. Although there were more instances of recurrence or persistent disease in the hyper group (n = 3 [7.5%]) versus the adenoma group (n = 0), this did not approach statistical significance. Two of the patients in the hyper group have undergone reexploration of the neck. One was found to have an ectopic gland in the thyroid, which was resected 8 days after the original operation when serum calcium levels failed to return to normal (previously referred to). The second patient was found to have an enlarged right inferior parathyroid gland that had been partially resected in the first operation and was re-resected and autotransplanted into the forearm. The third patient is currently asymptomatic and awaiting reexploration.
DISCUSSION
We report our experience and outcomes with surgical management of tertiary hyperparathyroidism. The incidence of this disease is less than 2% in this index population. Nevertheless, it is a disease that produces debilitating symptoms in the majority of patients and can threaten the existing allograft. In our hands, parathyroidectomy in patients with tertiary hyperparathyroidism is associated with a long-term cure rate of 99%.
Numerous studies have reported on the incidence of adenoma as the cause of tertiary hyperparathyroidism. The incidence ranges from 0% to 32%. 4,13–22 The incidence of single or double adenoma in our series was 30%. As with previous series, several of our patients were found to have asymmetric hyperplasia limited to one or two glands, whereas others had true adenomas on pathologic section. 4,15,23 We did not distinguish between these two disease entities at the time of surgery because it would not have altered our surgical approach. Further, patients with either process had similar outcomes. Nevertheless, this issue raises several questions. First, would patients with adenomas have developed primary hyperparathyroidism if they had not developed renal disease? Second, do patients with asymmetric hyperplasia represent a group in which only one or two glands develop a persistent secondary hyperplastic response after the resolution of uremia? There has been at least one report showing that parathyroid adenomas arise in renal transplant recipients in association with the allelic loss of chromosome 11 in the enlarged glands only. 24 In contrast, multiple adenomas and hyperplasia express a high level of EGF receptors but solitary adenomas do not, suggesting further differences in the etiology of these disease processes. 25 However, for the purposes of managing patients with enlargement of only one or two glands, distinguishing between these two potential disease entities appears to be unnecessary.
The incidence of postoperative complications was 22% and consisted of 15 cases of postoperative transient hypocalcemia and 1 case of temporary recurrent laryngeal nerve neuropraxia. This is consistent with previous series. 3,4,14 The incidence of postoperative transient hypocalcemia was significantly higher in the hyper group. Two of these patients underwent total parathyroidectomy with autotransplantation, for which hypocalcemia is a frequent and well-recognized complication. 26,27 However, in reviewing a third subset of patients (those with 3-gland disease who underwent 3.5-gland resection), there was a higher rate of hypocalcemia (3/7 [43%]) compared with patients with 4-gland hyperplasia who underwent an identical operation (8/40 [20%]). Further, of the two patients with adenomas who underwent subtotal parathyroidectomy, significant postoperative hypocalcemia developed in one. These findings are consistent with previous studies and suggest that aggressive resection of parathyroid tissue, particularly in patients who do not have four-gland disease, may not leave adequate parathyroid tissue in situ to prevent postoperative hypocalcemia. 14
We hypothesize that patients with enlargement of only one or two glands have a more limited disease process than those with four-gland hyperplasia and do not require a subtotal or total parathyroidectomy with autotransplantation to achieve similar rates of long-term eucalcemia. The lack of recurrent disease in the adenoma group after limited resection suggests that this hypothesis is correct. Alternatively, a more aggressive resection in this group of patients is not only unnecessary but may also subject them to a greater risk of postoperative hypocalcemia. The data presented here support a surgical strategy of resection limited to enlarged glands so that postoperative complications can be minimized.
There have been numerous other series, including two from our institution, regarding the surgical strategy for tertiary hyperparathyroidism. 4,15,19,22,28–30 Several of these studies describe the results of limited resection for single and double adenoma. 3,23,31 Results from the latter studies have been mixed. Gasparri et al 23 recommended a more aggressive surgical resection because limiting resection to less than a subtotal parathyroidectomy resulted in a very high rate of recurrence of disease. In contrast, Kilgo et al 4 described a limited strategy for single and double adenoma with very favorable outcomes and no recurrent disease at follow-up.
Our data support this more limited strategy for the following reasons. First, there is a high incidence of postoperative hypocalcemia after subtotal parathyroidectomy that is even greater with disease limited to less than four glands. Second, our long-term data indicate that limiting resection to the affected glands only in patients with single or double adenoma results in a cure rate of 100% at 5 years of follow-up. Importantly, there was no significant difference in the rate of recurrence between the adenoma (n = 0) and the hyper (n = 3) groups.
Our surgical strategy is to perform a formal neck exploration in renal transplant recipients with clinically significant tertiary hyperparathyroidism. We wait at least 1 year after the renal transplant to perform a parathyroidectomy in patients with asymptomatic tertiary hyperparathyroidism because most of these patients will have resolution of their hypercalcemia within this period. 3,4,15,32 Thereafter, all patients with hypercalcemia are explored and the size of all four glands is evaluated to determine whether the disease is due to single or double adenoma or hyperplasia. If disease involves only one or two glands, then the enlarged glands are resected. In patients with hyperplasia, we recommend 3.5-gland resection with tagging of the remaining half-gland with a nonabsorbable, colored suture. We prefer to avoid total parathyroidectomy with autotransplantation for hyperplasia because of the high postoperative incidence of hypocalcemia (67%) and the low rate of recurrence after 3.5-gland parathyroidectomy (6%). If all four glands cannot be identified, then a cervical thymectomy should be performed after complete exploration of the neck. With this strategy, we were able to locate seven ectopic glands, including two on reexploration, without having to perform a median sternotomy.
Recently we have started to use intraoperative PTH assays. We find this assay particularly useful in patients with one- or two-gland disease and in patients with ectopic glands because it enables us to determine whether further resection is needed to achieve eucalcemia.
Discussion
Dr. Collin J. Weber (Atlanta, GA): First of all, Dr. Rikkers, I too have successfully treated occasional patients by removal of one or two enlarged parathyroid glands in patients who have had a renal transplant. I must say that I have found it quite useful to be able to analyze intraoperative PTH during those procedures.
A cautionary note. Although I too agree that a patient who has had a renal transplant can develop a parathyroid adenoma like anyone else, the greatest likelihood is that this represents two-gland hyperplasia, as was described so aptly in years past by both Drs. Wells and Dr. McGarity and others. Molecular studies are required to confirm monoclonality of these lesions. Otherwise, they are probably hyperplasia.
I believe it is important to focus our attention on the renal population and how much they can benefit from what I call parathyroid reduction surgery. This is an important study for a number of reasons, but perhaps the most important one is how much these patients benefit from an increase in bone density.
I do have several questions which may help us to further understand whether these lesions were in fact adenomas by the classical definition of hyperplasia.
For example, were the patients who you described with one- or two-gland disease on dialysis for a shorter or longer period prior to their renal transplant than the other patients in your group? Were they noted to be hypercalcemic before their renal transplant? If that was the case, I think the diagnosis of hyperplasia would be favored. Have you utilized intraoperative PTH monitoring in any of these cases? And perhaps more important, have you, as Dr. Udelsman has done, and others I would applaud, measured parathyroid hormone as well as calcium in the postoperative period? I think long-term follow-up in this interesting patient population will be useful.
Dr. Robert Udelsman (New Haven, CT): I would like to thank you for the privilege of the floor and sending the manuscript in a timely fashion.
Dr. Rikkers and his colleagues from the University of Wisconsin have addressed the management of renal allograft recipients who develop autonomous parathyroid hormone secretion that is inappropriate to the serum calcium level, so-called tertiary hyperparathyroidism. They demonstrate that resection limited to macroscopically enlarged parathyroid glands appears to be curative. The manuscript is well written and the data are quite convincing.
Follow-up calcium data are presented, but as Collin Weber mentioned, parathyroid hormone data were not. It would be important to give us some PTH follow-up in these patients as this would help us understand the natural history of these patients.
In the manuscript you described transient hypocalcemia as a complication in 27% of patients who underwent conventional surgery for multigland hyperplasia. I suggest that if some of these patients underwent total cervical parathyroidectomy with immediate heterotopic parathyroid transplant to the forearm as described by Dr. Wells, that in fact hypocalcemia is not a complication, but rather an expected postoperative finding.
Finally, I would also like to suggest that if some of these patients had a hungry bone syndrome, they would be especially likely to develop postoperative hypocalcemia. I am particularly delighted to discuss this manuscript because I had something to do in the role of training Dr. Chen, who was involved in the study, and I am delighted to see the quality of the work being performed at Wisconsin.
Dr. Gerard M. Doherty (St. Louis, MO): The part that I like a lot about this paper is the explanation of the physiology of this disease. When we talk medical students through the understanding of renal osteodystrophy during an operating room session, I always get a little stuck making the transition from secondary hyperparathyroidism to tertiary hyperparathyroidism, on the question of why this should be multiple-gland disease. Why should there be multiple independent glands that remain persistently autonomous after transplantation has fixed the underlying problem of the renal failure?
I think this paper points out that it doesn’t really have to be multiple glands that are abnormal. Just one gland can remain autonomous and still have the same effect. From a physiology standpoint I like this a lot and it will help greatly in our explanations.
I don’t like the paper as much, though, in its implications about the possibility of minimally invasive parathyroidectomy. I think that is going to be some people’s inclination on reading this, to try and apply to the population the operation that we all do now for primary hyperparathyroidism and that Dr. Udelsman has so nicely described. This is a different population, if only because there is a much higher incidence of multiple-gland disease. This is a population in which 70% of the patients have multiple-gland disease, at least by these data, and so we would be much more dependent on our intraoperative PTH values to be able to pick out every one of those patients so that we get this right at least the vast majority of the time.
I like the paper very much overall. The one thing I would like you to describe, though, is how you would progress from here. What is the next step? Will you design a study to help us answer the question of whether we can apply some of these minimally invasive parathyroidectomy techniques to this patient population, or alternatively, how should we pick out the patients in whom that can be applied?
Dr. Richard E. Goldstein (Nashville, TN): Dr. Chen, I think that you probably set a record for lead time in sending the article to us, and I would like to thank you very much. It will stand as a record that I think will be very difficult to beat in the future. I will shorten some of my comments because a lot of the topics were touched on by some of the other discussants, but I do want to ask several things.
One, in those patients in the series who had four-gland hyperplasia, clearly the preference at your institution was to perform subtotal parathyroidectomy rather than total parathyroidectomy with autoimplantation. And I would ask you to comment briefly why there is clearly such a preference.
There is also some debate as to whether to routinely resect the upper thymic tissue in this patient population, somewhat similar to some of the issues that pertain to the MEN 1 population. And I wonder whether you might comment on that.
Third, I also wanted to just echo some of the comments by Dr. Udelsman as far as whether some of the postoperative hypocalcemia you are seeing could be due to bone hunger. We have seen several patients that had very substantial bone hunger, and in some of this population that would certainly not be unexpected.
Overall, I thought this paper was excellent, and clearly these patients with single or double adenomas or perhaps single or double hyperplastic glands are being cured. I do want to inject one bit of caution. Clearly, in the hands of surgeons who are very used to dealing with these processes, you might have more experience in recognizing one or two glands that are enlarged versus several that may be fairly normal in size. But as a surgeon at my institution who has the privilege of doing reoperative neck surgery, I can tell you that it can be extremely challenging. And part of my philosophy in dealing with some of these patients is to anticipate what may happen down the road and how I might plan a second operative procedure if it was necessary.
I wanted to thank you very much for asking me to comment on this paper. I really enjoyed it. And I thank the Association for the privilege of the floor.
Dr. Mitchell H. Goldman (Knoxville, TN): I have two questions.
First, I notice that your time of surgery after transplantation was about 4.5 years. That seems a little bit late given the fact that most of us notice that there is a resolution of the secondary hyperthyroidism within 6 to 12 months after transplantation. I was wondering why the delay.
The other part of my question along that line is, was there a difference in the single-adenoma or double-adenoma patients and the patients with “hyperplasia” in that time delay? In other words, were the hyperplastic ones more toward the lower end of the range and the adenoma ones at the upper end of the range?
Finally, were there any patients who were returned to hemo- or peritoneal dialysis in the group, and what was the effect on those patients after they had one or two adenoma resections?
Dr. Herbert Chen (Madison, WI): I would like to start by thanking Dr. Rikkers for presenting the work and the many discussants for their insightful comments and provocative questions.
First of all, the most important point that Dr. Doherty brought up is that we are not advocating minimally invasive parathyroidectomy for patients with tertiary hyperthyroidism. We feel that these patients need a full exploration with identification of all parathyroid glands. However, we believe that limited resection should be done, if possible, when you have a surgeon with significant experience in identifying normal parathyroids from diseased parathyroids. The criterion we used in this study was size. At the time of surgery, if I have any questions if it is a normal or abnormal parathyroid gland I just call Jim Starling, who is the senior surgeon at our institution, and I have him take a look as well.
Where do we go with the next step, Dr. Doherty asked. Well, Dr. Weber alluded to intraoperative PTH, and I think that is going to be the next step or the new technique we use to manage these patients. In this study only about 10% of the patients underwent intraoperative PTH testing. And that is because we have just begun using that technique at our institution this year. We found that assay to be useful in two specific instances.
In one patient which I explored and thought there were double adenomas present, I took out the two enlarged glands and checked the intraoperative PTH level. Indeed, it did fall below 50%, the criterion set with primary hyperparathyroidism. But I caution that we need to do more work with the assay before we can define what the criteria should be for intraoperative parathyroid hormone testing for patients with tertiary hyperparathyroidism.
The second incident in which intraoperative PTH testings is helpful is after a 3.5-gland or subtotal resection. I think that protects you if you have a fifth enlarged gland someplace else that is still hyperfunctioning and you think you are done but you are really not. Fortunately, we haven’t had that situation happen recently since we started using the assay.
Dr. Weber mentioned the terminology difference between adenoma and hyperplasia. And perhaps it was our mistake to call them adenomas in one group and hyperplasia in the other group. You really don’t know until you do the genetic or pathologic studies, and perhaps we should refer to them as the number of diseased glands, one or two diseased glands or three or four diseased glands. We did note that there was no difference in the dialysis time between those with single- or double-gland enlargement versus with multiple-gland enlargement.
Both Dr. Weber and Dr. Udelsman commented on the postoperative PTH levels, which I think are important. Unfortunately, we don’t have long-term follow-up on a lot of the patients with regards to PTH levels. I can tell you in the short term that PTH levels fall. And what our transplantation people do is, once we see normalization of the PTH and calcium levels, they follow calcium. Because even if you had an elevated PTH level with normal calcium long term, would you explore the patient? Probably not. So I submit that although the PTH level is important, the calcium is the important number.
Dr. Udelsman is correct in saying when you do a total parathyroidectomy with an implant, those patients by definition will have transient hypercalcemia. And in fact 2 of the 14 patients developed hypercalcemia for that reason. But that still leaves 12 patients who developed it who did not have that procedure.
Both Drs. Udelsman and Goldstein alluded to the hungry bones being a possibility. And yes, the hypocalcemia may be due to hungry bones. But I submit to you that the alkaline phosphatase levels both preop and postop in these patient groups were exactly the same.
Dr. Goldstein asked why we do subtotal parathyroidectomies in the patients with multigland disease. And I think that is based on our experience that once you have done a kidney transplantation, the stimulus for parathyroid growth is gone. So as long as the kidney works—and Dr. Udelsman taught me this when I was training—a 3.5-gland resection will be fine, because that remnant will not hypertrophy so long as the kidney is working.
We do not routinely perform a cervical thymectomy. However, if we are missing one of the glands, especially the lower ones, we will go ahead and perform one.
With regard to helpful comments, when you go back for a second operation, Dr. Goldstein, when we have had to, the recurrence has been the remnants, or in the thyroid in one case. We would pursue imaging techniques to localize an abnormal gland before we go in.
Dr. Goldman asked why we wait 4.3 years to operate on these patients. And I think that is more of a historical thing. Initially, when Dr. Starling looked at these patients, he found that a significant number resolve their hyperparathyroidism without surgery within a year, and some patients even did it in 3 or 4 years. When our nephrologists read that paper, they thought that most patients could probably wait and some would get better. But recently we have developed a more aggressive approach, especially knowing that some of these patients will develop decreased bone density because of immunosuppressives. So that is why our current criterion for parathyroid surgery in tertiary hyperparathyroidism is symptoms at any time or asymptomatic disease greater than 1 year after transplantation.
And lastly, Dr. Goldman asked about how many patients have gone back to dialysis; that is, how many of these patients have failed transplantation? Three patients out of 71 are on renal dialysis right now for a failed kidney transplant. None of these patients had recurrent hyperparathyroidism after 3.5-gland resection.
I would like to thank the Association for the honor of discussing this paper.
Footnotes
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Correspondence: Herbert Chen, MD, University of Wisconsin Medical School, Department of Surgery, H4/750 CSC 600 Highland Avenue, Madison, WI 53792.
E-mail: chen@surgery.wisc.edu
Accepted for publication December 2001.
References
- 1.Decker PA, Cohen EP, Doffek KM, et al. Subtotal parathyroidectomy in renal failure: still needed after all these years. World J Surg 2001; 25: 708–712. [DOI] [PubMed] [Google Scholar]
- 2.Diethelm AG, Edwards RP, Whelchel JD. The natural history and surgical treatment of hypercalcemia before and after renal transplantation. Surg Gynecol Obstet 1982; 154: 481–490. [PubMed] [Google Scholar]
- 3.Buchmann P, Keusch G, Ittner J, et al. Tertiary hyperparathyroidism after cadaver-kidney transplantation. Transplant Proc 1984; 16: 1324. [PubMed] [Google Scholar]
- 4.Kilgo MS, Pirsch JD, Warner TF, Starling JR. Tertiary hyperparathyroidism after renal transplantation: surgical strategy. Surgery 1998; 124: 677–683. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Cheigh JS, Ham HW. Urinary stones following renal transplantation. Korean J Intern Med 2001; 16: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbur MA, Kurjak M, Becker K. [Systematic calciphylaxis in chronic renal failure: fulminant course after kidney transplantation]. Pathologe 1997; 18: 453–458. [DOI] [PubMed] [Google Scholar]
- 7.Adler JS, Cameron DC. Erosive spondylo-arthropathy and tertiary hyperparathyroidism. Australas Radiol 1989; 33: 90–92. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt RE, Zeitlin EL. Neuromuscular disease in tertiary hyperparathyroidism. Arch Intern Med 1978; 138: 1013–1015. [PubMed] [Google Scholar]
- 9.Hornum I. Post-transplant hypercalcemia due to mobilization of metastatic calcifications. An alternative to tertiary hyperparathyroidism. Acta Med Scand 1971; 189: 199–205. [DOI] [PubMed] [Google Scholar]
- 10.Dotzenrath C, Goretzki PE, Roher HD. Renal hyperparathyroidism following kidney transplantation. Ann Ital Chir 1993; 64: 381–384. [PubMed] [Google Scholar]
- 11.Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery 2000; 128: 531–539. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher S, Kanagasundaram NS, Rayner HC, et al. Assessment of ultrasound guided percutaneous ethanol injection and parathyroidectomy in patients with tertiary hyperparathyroidism. Nephrol Dial Transplant 1998; 13: 3111–3117. [DOI] [PubMed] [Google Scholar]
- 13.Harach HR, Jasani B. Parathyroid hyperplasia in tertiary hyperpara-thyroidism: a pathological and immunohistochemical reappraisal. Histopathology 1992; 21: 513–519. [DOI] [PubMed] [Google Scholar]
- 14.Demeure MJ, McGee DC, Wilkes W, et al. Results of surgical treatment for hyperparathyroidism associated with renal disease. Am J Surg 1990; 160: 337–340. [DOI] [PubMed] [Google Scholar]
- 15.D’Alessandro AM, Melzer JS, Pirsch JD, et al. Tertiary hyperparathyroidism after renal transplantation: operative indications. Surgery 1989; 106: 1049–1055. [PubMed] [Google Scholar]
- 16.Krause MW, Hedinger CE. Pathologic study of parathyroid glands in tertiary hyperparathyroidism. Hum Pathol 1985; 16: 772–784. [DOI] [PubMed] [Google Scholar]
- 17.Wibell L, Grimelius L, Johansson H. Explorative parathyroidectomy before and after kidney transplantation. Scand J Urol Nephrol 1977; 42: 153–158. [PubMed] [Google Scholar]
- 18.Lundgren G, Asaba M, Magnusson G, et al. Role of parathyroidectomy in the treatment of secondary hyperparathyroidism before and after renal transplantation. Scand J Urol Nephrol 1977; 42: 149–152. [PubMed] [Google Scholar]
- 19.Blake DP, O’Brien TJ, Smith CL, et al. Surgical treatment of renal hyperparathyroidism. Surg Gynecol Obstet 1983; 157: 325–331. [PubMed] [Google Scholar]
- 20.Garvin PJ, Castaneda M, Linderer R, Dickhans M. Management of hypercalcemic hyperparathyroidism after renal transplantation. Arch Surg 1985; 120: 578–583. [DOI] [PubMed] [Google Scholar]
- 21.Hognestad J, Flatmark A. Hyperparathyroidism in uremia and after kidney transplantation. Scand J Urol Nephrol 1977; 42: 137–139. [PubMed] [Google Scholar]
- 22.Christensen MS, Nielsen HE. The clinical significance of hyperparathyroidism after renal transplantation. Scand J Urol Nephrol 1977; 42 (S):134–136. [PubMed] [Google Scholar]
- 23.Gasparri G, Camandona M, Abbona GC, et al. Secondary and tertiary hyperparathyroidism: causes of recurrent disease after 446 parathyroidectomies. Ann Surg 2001; 233: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falchetti A, Bale AE, Amorosi A, et al. Progression of uremic hyperparathyroidism involves allelic loss on chromosome 11. J Clin Endocrinol Metab 1993; 76: 139–144. [DOI] [PubMed] [Google Scholar]
- 25.Duh QY, Gum ET, Sancho JJ, et al. Epidermal growth factor receptors in parathyroid tumors. J Surg Res 1986; 40: 569–573. [DOI] [PubMed] [Google Scholar]
- 26.Brunt LM, Wells SA. Surgical treatment of secondary hyperparathyroidism. Ann Chir Gyn 1983; 72: 139–145. [PubMed] [Google Scholar]
- 27.Calandra D, Paloyan E, Oslapas R, et al. Successful autotransplantation of parathyroid adenomas in seven patients. Am Surg 1983; 49: 324–328. [PubMed] [Google Scholar]
- 28.Alexander PT, Schuman ES, Vetto RM, et al. Repeat parathyroid operation associated with renal disease. Am J Surg 1988; 155: 686–689. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez JM, Mautalen CA, Rodo JE, et al. Tertiary hyperparathyroidism diagnosed after renal homotransplantation. Am J Med 1970; 49: 423–428. [DOI] [PubMed] [Google Scholar]
- 30.Grimelius L, Johansson H, Lindquist B, Wibell L. Tertiary hyperparathyroidism occurring during a renal transplantation programme: report and discussion of three cases. J Pathol 1972; 108: 23–33. [DOI] [PubMed] [Google Scholar]
- 31.Kerby JD, Rue LW, Blair H, et al. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David DS, Sakai S, Brennan L, et al. Hypercalcemia after renal transplantation. N Engl J Med 1973; 289: 389–401. [DOI] [PubMed] [Google Scholar]