Abstract
Objective
To test the hypothesis that pancreatic ductal anatomy may predict the likely success of percutaneous drainage of pseudocysts of the pancreas.
Summary Background Data
Various modalities are currently applied to pseudocysts, with little or no data to aid in the choice of management strategy. Pancreatic ductal anatomy was assessed and a system to categorize ductal changes was established.
Methods
Patients with a diagnosis of pancreatic pseudocyst were evaluated from 1985 to 2000. Two hundred fifty-three patients have been included in this series. Pancreatic ductal anatomy was defined using endoscopic retrograde cholangiopancreatography and categorized as a normal duct, a stricture, or complete cut-off of the pancreatic duct. Communication between the duct and cyst was noted.
Results
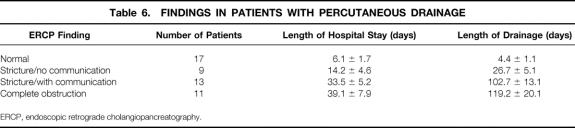
Among the 253 patients, 68 (27%) had spontaneous resolution. Fifty of the remaining 185 had percutaneous drainage and 148 (13 of whom failed to respond to percutaneous drainage) had surgery. There were no deaths in either group. Mean length of time with catheter drainage among all percutaneous drainage patients was 79.2 ± 19.6 days. Patients with normal pancreatic ducts and those with strictures but no communication between the duct and the cyst who had percutaneous drainage had a much shorter length of hospital stay (6.1 ± 4.6 days) than patients with strictures and duct–cyst communication and patients with complete cut-off of the duct (33.5 ± 5.2 days and 39.1 ± 7.9 days, respectively). Length of drainage also correlated with ductal anatomy. All patients with chronic pancreatitis failed to respond to percutaneous drainage.
Conclusions
Pancreatic ductal anatomy provides a clear correlation with the failure and successes of pseudocysts managed by percutaneous drainage as well as predicting the total length of drainage. Percutaneous drainage is best applied to patients with normal ducts and is acceptably applied to patients with stricture but no cyst–duct communication.
With the advent of advanced imaging devices such as spiral computed tomography scanning, magnetic resonance cholangiopancreatography (MRCP), and endoscopic ultrasound, pseudocysts in patients with inflammatory diseases of the pancreas have been recognized with increased frequency. The clinical significance of many of these smaller and often subclinical lesions may be argued. Spontaneous resolution of pseudocysts has also been more commonly recognized, perhaps because of the inclusion of so many early lesions now identified by advanced imaging techniques. The traditional management of pseudocyst emanated for decades from a sentinel report by Bradley et al., 1 who studied 93 patients with ultrasound only. They found spontaneous resolution of pseudocyst in 24 of 54 patients finally studied, but all resolution took place before 6 weeks. They also found that the likelihood of complications increased after 6 weeks of follow-up. Thus, standard therapy dictated treatment after 6 weeks. With improved imaging, the criteria for intervention have been modified depending on imaging confirmation of cyst wall “maturity.” As the basic parameters have been modified, alternative methods for treating pseudocysts have also evolved. Percutaneous drainage (PD) of pseudocysts has been used with success rates ranging from 42% to 92%. 2–6 Because patients with immediate removal of catheters had very high recurrence rates, the duration of catheter drainage has been increased to 2 months or more. With prolonged drainage periods, the frequency of septic complications has also increased. 4–8 The failure rates, unacceptably prolonged drainage periods, and septic complications all have prompted a need for a system by which outcomes might be predicted and patients at high risk for drain failure or complication may be identified. Heider et al. 2 attempted to identify such a factor by multiple logistic regression analysis of 66 patients who underwent surgery and 66 patients treated with PD. The authors could not define a risk factor.
We have previously reported the value in patients with pseudocyst of obtaining preoperative information regarding pancreatic ductal anatomy using endoscopic retrograde cholangiopancreatography (ERCP). 9 We subsequently have continued this protocol and have speculated that pancreatic ductal anatomy may represent a parameter that will correlate with failure and complications associated with PD of pseudocyst. Patients with complete disruption of the main pancreatic duct and consequent isolation of the remainder of the pancreas on the left side of the obstruction might be poorly managed by PD because drainage would not serve to reestablish continuity between the isolated segment of the duct and the intestinal tract, whereas surgical drainage would do so. Anatomy favoring PD may also be defined.
Although progress has been made with alternative methods such as endoscopic drainage through the contiguous intestine, 10 through the transpapillary route, 11 or using endoscopic ultrasound, 12 and outcomes at first report have been favorable, with 6% to 23% recurrence rates, the likelihood is great that ductal anatomic abnormalities will similarly apply to these alternate modalities. These modalities use antiquated surgical outcomes for comparison of rates of deaths, complications, and success. 10–12 More recent surgical series suggest a low death rate, and high success rates can be anticipated in the management of pseudocysts with surgery. 2,8,9,13
METHODS
We have adhered to the standard definition of pseudocyst, a peripancreatic fluid collection with a defined wall, developing as a consequence of inflammatory disease of the pancreas (either acute pancreatitis or chronic pancreatitis). The diagnosis of pseudocyst was established in all patients by means of some form of imaging modality, typically ultrasound, computed tomography (CT), or MRCP. Recently endoscopic ultrasound has been used in some. All patients referred to our surgical service with a diagnosis of pseudocyst since 1985 are included in the report. Patients were deemed to be candidates for intervention (surgical or radiologic) when they were diagnosed as having a pseudocyst 4.5 cm or greater in diameter with no sign of spontaneous resolution over a period of evaluation varying in length based on specific clinical criteria. Symptoms referable to pseudocyst were tabulated and represented the basis for intervention. These included pain, weight loss, nausea and vomiting, jaundice, fever, hemorrhage, or acute rupture. Pseudocysts were categorized by size, location in the abdomen, number, and contiguity with other cysts and other structures.
Acute or Chronic Pancreatitis
Patients were categorized as having acute pancreatitis on a clinical basis when a definable recent or temporally remote episode of acute abdominal pain associated with nausea or vomiting occurred with laboratory measures characteristic of that diagnosis. Causative mechanisms such as gallstones, ethanol abuse, or the many less common causes of acute pancreatitis were documented. Prior episodes of abdominal trauma were elicited. It is understood that these patients given a clinical diagnosis of acute pancreatitis lacked any of the classic findings in chronic pancreatitis.
Chronic pancreatitis was assigned as the clinical diagnosis when patients had chronic pain, particularly pain on a daily basis, or pain occurring in the absence of any other signs of acute symptomatology. Other confirmatory evidence for a diagnosis of chronic pancreatitis included structural abnormalities in the pancreas as seen on imaging. Calcification of the pancreas, dominant mass, and a dilated main pancreatic duct by CT scan or MRCP and ductal irregularity, areas of dilation and narrowing (“chain of lakes”), and secondary ductular ectasia by ERCP were all considered confirmatory. Functional derangements of the pancreas were also sought and aided in establishing the diagnosis of chronic pancreatitis. Endocrine insufficiency was manifested by frank diabetes mellitus as well as by elevated fasting blood sugar levels. In some patients, oral glucose tolerance testing was performed. It is necessary to distinguish so-called pancreatogenic diabetes from preexistent primary diabetes mellitus type 1. Evidence for exocrine insufficiency was steatorrhea. In some patients, fecal fat analysis over 72 hours or the now-unavailable bentiromide PABA test was performed. Structural or functional derangements were sufficient to establish the diagnosis of chronic pancreatitis.
On the basis of these criteria, all patients were categorized as possessing pseudocyst as a consequence of either acute or chronic pancreatitis.
Laboratory Analysis and Imaging
All patients were evaluated with a complete blood count (CBC), electrolytes and glucose, liver function tests, and serum amylase and lipase.
Plain abdominal radiographs were obtained in all patients. CT scanning and less frequently MRCP was obtained in most patients. Ultrasound was commonly used as an initial imaging modality, often to assess whether gallstones had played a role in the pancreatitis. When suspicions were raised that the peripancreatic fluid collection actually represented a cystic neoplasm, appropriate measures were taken to confirm or exclude this suspicion. This primarily involved aspiration of fluid to test for the presence of mucin.
Percutaneous Drainage Versus Surgery
The decision to employ PD before or instead of surgical drainage was made on the basis of the circumstances, such as an unacceptably high risk for surgical intervention or the preference of the physician managing the patient before referral to our service. In some, patient preference was the determining factor.
Defining Main Pancreatic Ductal Anatomy
In patients scheduled for elective operation or PD, ERCP was performed 1 day before the intervention. In patients referred to our service having already had PD, ERCP was performed soon after transfer. Any anatomic abnormalities were noted, but specifically ductal anatomy was categorized as normal, normal with stricture, or normal with complete cut-off at some portion of the duct. In this case “normal” is meant to represent a duct with no evidence of chronic pancreatitis. In addition to these previously listed varieties of ductal anatomy seen with acute pancreatitis, we also categorized ducts as chronic pancreatitis. Communication between the duct and the pseudocyst was also noted. We segregated patients for analysis into normal, stricture with communication to the cyst, stricture without communication to the cyst, or complete cut-off at some point in the course of the main pancreatic duct. Any associated abnormalities in the biliary tree were also noted.
Our system for defining the ductal anatomy is shown in Table 1. The scheme for categorizing ductal changes incorporates the system applied to this report but adds the distinction between acute pancreatitis and chronic pancreatitis as separate categories. Thus, we propose type I through type VII ductal categories (Fig. 1).
Table 1. CLASSIFICATION: ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY
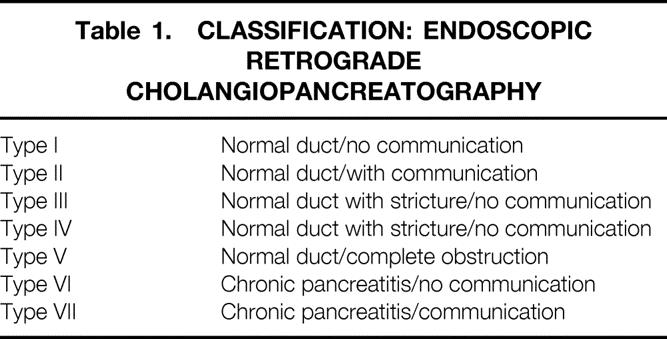
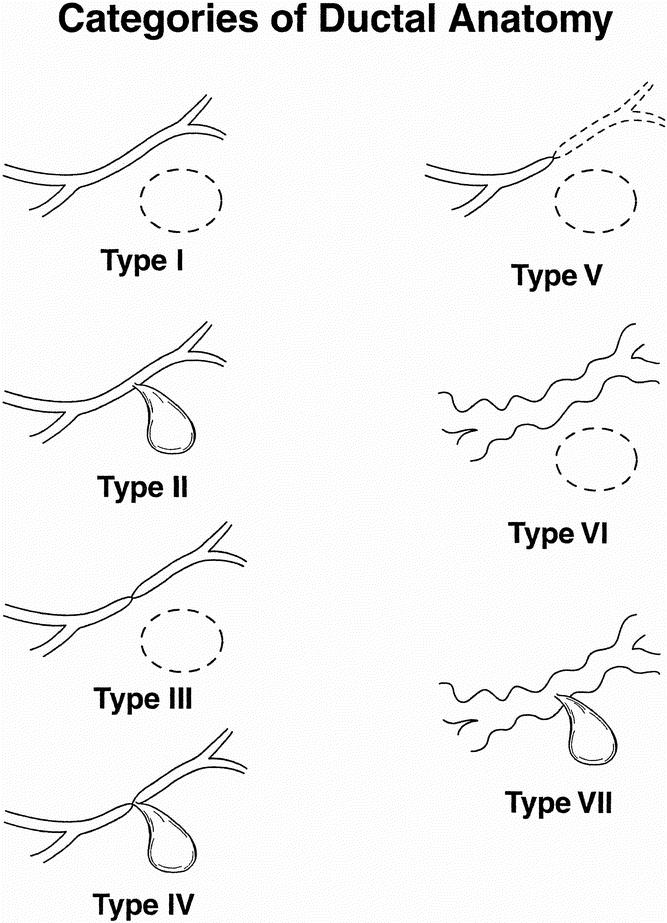
Figure 1. Categories of ductal abnormalities seen in patients with pseudocysts. Type I: normal duct/no communication with cyst. Type II: normal duct with duct–cyst communication. Type III: otherwise normal duct with stricture and no duct–cyst communication. Type IV: otherwise normal duct with stricture and duct–cyst communication. Type V: otherwise normal duct with complete cut-off. Type VI: chronic pancreatitis, no duct–cyst communication. Type VII: chronic pancreatitis with duct–cyst communication.
Postprocedure Data
After surgical management patients were evaluated for complications and length of hospital stay. After PD patients were evaluated for complications, LOS, length of drainage, and need to cross over to surgical management. Where feasible, follow-up CT scans were performed to assess recurrence of pseudocyst.
RESULTS
A total of 253 patients with pseudocyst were evaluated from 1985 to 2000. There were 187 men and 66 women. The mean age among all patients was 46 ± 4.1 years. The cause of pancreatitis was ethanol in 138 and biliary in 80. Twenty-five patients had pseudocyst after abdominal trauma. The remaining 10 patients had a variety of the known less common causes of pancreatitis and consequent pseudocyst formation. The mean interval from initial presentation to intervention or spontaneous resolution was 22.7 ± 5.2 days. Spontaneous resolution was documented in 68 (all with acute pancreatitis), and therefore no ERCP was performed. The mean interval from initial presentation to intervention among the 185 patients who had intervention was 19.6 ± 3.3 days. Fifty patients had PD, 19 of whom had this procedure performed before transfer to our institution. Thirteen patients originally treated with PD failed to respond to this management and required surgery (26%). One hundred forty-eight patients underwent surgery. There were no deaths in either group. ERCP was successfully completed in 179 patients. In the remaining patients ductal anatomy was established either with intraoperative pancreatography or with transcystic injection after PD.
Surgical Management
In the operated group there were 36 patients who had complications (Table 2). Wound infection accounted for 5 of 148 patients (3%). No reoperation was required. No episodes of sepsis or readmission to the hospital occurred. Major postoperative hemorrhage was not encountered. Cyst-jejunostomy alone combined with pancreaticojejunostomy or pancreaticojejunostomy alone (in patients with chronic pancreatitis) was performed in 123, distal pancreatectomy was performed in 10, and cyst-gastrostomy was performed in 15 patients. Drainage with closed suction devices was selectively used in 78 patients. No pancreatic fistulas occurred in the surgical group. CT scans were obtained after surgery in 86 patients, most commonly in patients with ethanol abuse and recurrent episodes of acute pancreatitis or acute exacerbations of chronic pancreatitis or in patients treated with PD. Recurrence of pseudocyst was not seen in any patient who underwent surgery and in 11 of 50 (22%) patients treated with PD. Seven patients had pseudocyst develop remote from the site of the previously surgically drained PS during follow-up evaluation.
Table 2. COMPLICATIONS IN OPERATED PATIENTS

Percutaneous Drainage
Among the 50 patients with PD, 37 experienced complications (74%) (Table 3). The most common complication was sepsis related to catheter obstruction, which occurred in 16 of the 50 (32%). There were 25 episodes of sepsis in the 16 patients. Thirteen of the 50 patients with PD (26%) required surgery. Twelve of the 13 (92%) patients requiring surgery had had episodes of sepsis; the other patient had severe pain and persistence of more than 200 cc3 drainage per 24 hours after 127 days of drainage.
Table 3. COMPLICATIONS IN PATIENTS WITH PERCUTANEOUS DRAINAGE

Main Pancreatic Ductal Anatomy
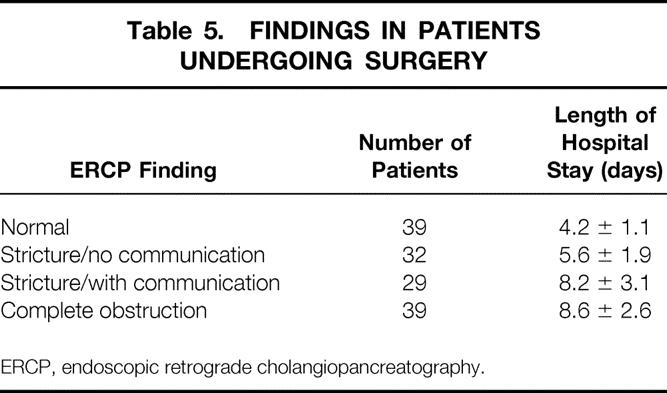
One hundred seventy-nine patients had ERCP. Ductal anatomy was classified as normal in 54 patients, stricture without communication in 42 patients, stricture with communication in 39 patients, and cut-off in 44 patients (Table 4). Among patients who underwent surgery, 39 had ERCP categorized as normal, 32 had stricture without communication, 29 had stricture with communication, and 39 had cut-off. Among patients who had PD, 17 patients had pancreatic ductal anatomy categorized as normal, 9 as stricture without communication, 13 as stricture with communication, and 11 as cut-off. The impact of ductal anatomy on outcome revealed that 10 of the 11 patients with PD (91%) who had cut-off required subsequent surgery. The remaining three patients (23%) who required surgery had stricture with communication. No patients with normal anatomy or stricture without communication with PD required subsequent surgery. Data for LOS for the surgical group is listed in Table 5. Mean LOS was 4 to 8 days. The mean LOS for patients who underwent PD is depicted in Table 6. LOS was as high as 39 days for patients with cut-off. LOS was gradational: it was longest for patients with cut-off and only 6.1 days for patients with normal ductal anatomy. For patients with PD, the length of drainage is also presented in Table 6. Length of drainage was also gradational and dependent on ductal anatomy. Overall mean length of drainage was 79.1 ± 13.1 days. Mean length of drainage was longest (119.2 ± 20.1 days) for patients with ductal anatomy categorized as cut-off.
Table 4. ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY: FINDINGS

Table 5. FINDINGS IN PATIENTS UNDERGOING SURGERY
ERCP, endoscopic retrograde cholangiopancreatography.
Table 6. FINDINGS IN PATIENTS WITH PERCUTANEOUS DRAINAGE
ERCP, endoscopic retrograde cholangiopancreatography.
Acute Pancreatitis Versus Chronic Pancreatitis
Clinical evaluation of all patients yielded a diagnosis of acute pancreatitis in 160 patients and chronic pancreatitis in 86. After ERCP, 143 were confirmed as having acute disease and 17 were reclassified as having chronic disease. Thus, a total of 103 patients were confirmed as having chronic pancreatitis.
DISCUSSION
Many of the classical tenets for the management of pseudocyst have been scrutinized in the past years. First, the belief that all pseudocysts that persisted beyond 6 weeks required intervention was questioned, particularly in asymptomatic patients. 13,14 This concept has been subsequently confirmed by Heider et al., 2 who found that expectant management was safe in 41 patients. Methods were developed to treat pseudocyst with endoscopy and with percutaneous drainage. The literature of the past decade has extolled the qualities of each modality in isolation. Comparative studies have been reported, with more favorable outcomes commonly found for surgical management, but reports originating from practitioners of the nonsurgical modalities have often found their methodology to be superior. At least one explanation of these divergent data may be that antiquated surgical outcomes are often used for comparison. In the recent report by Heider et al., 2 comparisons are drawn between surgical or percutaneous drainage. In this report multiple regression analysis failed to confirm any variable that correlated with success in PD. In the present study, we have determined that pancreatic ductal anatomy is useful in predicting the success and length of time required for drainage in patients managed by PD. We evaluated pseudocysts in 253 patients. Pancreatic ductal anatomy correlated well with outcomes in patients treated with PD. PD failed in 13 of 50 patients (26%). Among these 13 failures, either complete cut-off of the pancreatic duct (10/11) or stricture in the pancreatic duct and communication between the duct and the pseudocyst (3/13) was the corresponding ductal anatomy. These data are the first applied to pseudocysts to provide such a clear correlation with outcome. These data thus may be applied to direct the choice of modality for managing pseudocyst.
We have found that patients with chronic pancreatitis are uniquely ill suited to PD. Patients with normal ductal anatomy had the most favorable outcome with PD. None of these patients required surgery. Surgical management had high success rates, with a low recurrence rate, shorter LOS, and fewer complications. PD is effective in a subset of patients. Our data confirm that ductal anatomy provides a framework within which logical strategies may be applied to the management of pseudocysts.
The concept of using ductal anatomy to direct strategies in managing pseudocysts was proposed by us in a 1989 report 9 in which 41 patients, all treated with surgery, had ductal abnormalities that altered the surgical plan. Ahearne et al. in 1992 15 developed an algorithm for the management of pseudocysts using data derived from ERCP. Their series of 103 patients with pseudocyst included 69 who had elective management, 40 of whom had ERCP before treatment. Their algorithm, derived retrospectively, called for surgical intervention when either ductal communications with the pseudocyst or obstruction of the pancreatic duct was documented by ERCP. Although the numbers of patients in subset groups were small, their data found a higher failure rate (43%) in patients managed without observing the directives in the algorithm compared with adverse outcomes in patients managed in accordance with them (12%). D’Egidio and Schein 3 classified pseudocysts as type 1 (“acute postnecrotic pseudocysts” occurring after an episode of acute pancreatitis and associated with normal pancreatic duct), type 2 (also “postnecrotic” but with acute superimposed on chronic pancreatitis and likely cyst–duct communication), and type 3 (“retention” cysts, associated always with chronic pancreatitis and cyst–duct communication). According to their classification, the authors recommended serial CT scan for type 1, surgical drainage for type 2, and surgical procedures directed toward specific lesions in type 3. 3 This system has been criticized for the lack of clarity seen in type 2 patients. 16 Nonetheless, Grace and Williamson 15 and Pitchumoni and Agarwal 7 have advocated its use. Our data add considerable detail to the logical approach to pseudocyst. We have seen patients with complete disruption on an otherwise normal duct. This category is not represented in the D’Egidio classification. In addition, our data confirm that PD outcomes are significantly affected by ductal anatomy.
We share the belief that patients with chronic pancreatitis and pseudocysts should be categorized separately (D’Egidio type 3). We previously reported, 9 and our data confirm, that ERCP will provide confirmation of the diagnosis of chronic pancreatitis in a significant number of patients who have been clinically classified as having acute pancreatitis. In the present study 17 of the 160 patients (11%) originally classified as having acute pancreatitis were reclassified as having chronic disease after ERCP. Only three patients with chronic pancreatitis were managed with PD in our series, because they were candidates for additional simultaneous procedures. All three patients with chronic pancreatitis who were managed by PD required subsequent surgery.
Several series have previously compared PD with surgical management of pseudocyst. Isolated reports on the efficiency of PD alone have quoted success rates of 67% to 90%. 3,7,16 These successes have not been reproduced in comparative studies of surgical and percutaneous management. Spivak et al. 8 reported PD in 27 patients, with complete resolution in 17 (62%). They also reported sepsis requiring urgent salvage surgery in one third of patients treated percutaneously. Heider et al. 2 reported a series of 173 patients, 66 treated with PD and 66 treated with surgical management. The remainder were managed by observation alone. PD was successful in 42% and surgical treatment was successful in 88%. PD was also associated with a high death rate (16% vs. 0%) and a high rate of complications (64% vs. 27%).
These data compare favorably with our own. We have had no recurrence of pseudocysts after surgical management and no deaths. Our complication rate of 24% compares favorably with that in prior reports. PD was successful in 74%, although this at times was dependent on very long periods of drainage. These prolonged catheter drainage periods resulted in the anticipated high rate of sepsis (32%) among PD patients. Adams and Anderson 6 also had prolonged drainage (mean 42 days) and consequent “drain track infection” in 50%. Heider et al. 2 also evaluated LOS and the period required for catheter drainage in patients treated with PD versus surgery. They described mean catheter drainage of 27 days for patients successfully drained and 51 days for patients whose pseudocyst failed to resolve using PD. The LOS for patients with successful drains was 31 days and that for patients with unsuccessful drains was 56 days. Patients managed successfully by surgery had an LOS of 17 days.
Our data are the first to define clearly the correlation between pancreatic ductal anatomy and LOS and, more significantly, length of catheter drainage. The overall mean length of drainage for our PD patients was 79 days, with a clear correlation to ductal anatomy. Patients with cut-off, we believe, have an anatomically isolated segment of the pancreas on the far side of the area of ductal obstruction. We thereby speculate that catheter drainage would have a poor likelihood of success because no amount of long-term drainage can be expected to reestablish continuity of the ductal system, and thus no means of resolving the anatomic isolation caused by major ductal disruption. The mean length of drainage for patients with cut-off was 119 days. We also speculated that a patient with ductal anatomy categorized as normal and no communication between the duct and the cyst would have a high likelihood of success when managed with PD because the cyst likely possesses only a minute connection to the pancreatic duct and duct flow would preferentially proceed through the normal anatomic channels to the duodenum. Our mean length of drainage for patients with normal anatomy (4.4 days) supports this speculation. In patients with abnormal but nonobstructed pancreatic ducts (stricture with or without communication), the length of drainage was longest when a communication between the duct and the cyst was demonstrated. This particular finding (communication between duct and cyst) has previously been recognized as a variable that reduced the success of PD.
It has been our bias, and support may be found in the literature, 3,7,16 that patients with chronic pancreatitis and associated pseudocysts are poorly managed by PD. Not only may they be candidates for additional procedures, such as pancreatojejunostomy or resection, but the extensive process of fibrosis in the pancreas would also suggest that abnormal fistulous channels between the pancreatic ductal system and the cyst would be far less likely to permanently seal compared with the abnormal fistulous channels that occur in an otherwise normal, soft pancreas in a patient without chronic changes in the parenchyma. Thus, we believe that our proposed system for defining the categories of ductal abnormalities seen in patients with pseudocysts should include categories specifically denoting the diagnosis of chronic pancreatitis (types 6 and 7).
Discussion
Dr. Anthony a. Meyer (Chapel Hill, North Carolina): I would like to thank the Association for the opportunity to discuss the paper. I thought this was an excellent summary of another large series of pancreatic pseudocysts managed in a slightly different way than we managed ours and studied ours at Chapel Hill. I think it points out some of the more refined and well-defined components of pancreatic ductal anatomy and how they can be utilized in order to plan or individualize care of patients with pancreatic pseudocysts. I have a couple of questions for Dr. Nealon.
Did you notice a change in your treatment over time, this is about a 15-year period of time; did you notice a change in how you utilized management in terms of percutaneous drainage either with better techniques or more skilled ultrasonographers or interventional radiologists?
Then given all your data, other than the people with totally normal anatomy and no duct communication, would you still utilize this type of information in terms of your pancreatic ducts to try percutaneous drainage first?
We all have patients that we would rather never operate on or for whom the operative mortality of a haircut is relatively high, so any type of intraoperative drainage is sort of ferocious to consider, but with the known high likelihood of recurrence or complications and such which would put them at an even greater risk. Would you use any other subgroup of patients other than those with totally normal anatomy to avoid the operative approach?
Dr. Timothy C. Fabian (Memphis, Tennessee): I would like to congratulate the authors on a well-done and important analysis of optimal pseudocyst management. The study is easy even for me to interpret and it contains a couple of unequivocal recommendations. Those are that today patients with pseudocysts should have ERCP prior to definitive management, and then if ERCP demonstrates either duct stricture without communication with the pseudocyst or complete ductal obstruction, then the patient should undergo surgical rather than percutaneous drainage. The data are both clear and compelling.
The two groups which are contraindicated for percutaneous drainage comprise 50% of their entire study population. The length of hospitalization in those patients was 8 to 9 days for surgical drainage versus 34 to 39 days for percutaneous drainage, and the length of the percutaneous drainage was 3.5 to 4 months. It seems pretty clear to me that reprints of this study should be sent to all gastroenterologists, internists, hospital administrators and, very importantly, health care payers.
I really have no criticisms, only a couple of questions. How many of the patients had pseudocyst complications, including rupture, gastric or biliary obstruction, or infection? Were the two populations stratified to make sure you were comparing apples with apples? Secondly, what role do you see for MR pancreatography in pseudocyst management today?
I would like to thank Bill for asking me to discuss this important paper and the Association for the privilege of the floor.
Dr. John L. Cameron (Baltimore, Maryland): I have followed Bill Nealon’s work with ERCP and pseudocysts for many years and I have always enjoyed his very logical sequential discussions of his data. I would like to object though, Bill, to one part of your classification. And that is to say “without communication.” Once the communication between a pseudocyst and the pancreatic duct closes, the pseudocyst very rapidly disappears. Cicatrixing of the communication between the cyst and pancreatic duct is what results in a disappearance of the pseudocyst.
As proof of that, all of us have had pancreatic cutaneous fistulas in which we have done five or six sinograms in a row and failed to demonstrate a communication with the pancreatic duct, and then the seventh sinogram clearly shows it. In many of these pseudocysts the communication is small and is difficult to demonstrate. However, they all have communications. Once the communication is gone, the cyst is gone.
I think your classification is fine, but you should modify it so that you say “without identifiable or demonstrable communication,” because it is misleading to say there is a pseudocyst without ductal communication. But that does not detract in the least from the beautiful, logical presentation of Bill Nealon.
Dr. Henry A. Pitt (Milwaukee, Wisconsin): I would like to again congratulate Bill. I agree with his algorithm completely. What I don’t completely understand is that as I view the world literature and current practice in the management of pseudocysts over the last decade, the endoscopists are really the people who are the competition for surgery now, not the interventional radiologists. Therefore, I wonder with the strong endoscopy group at Galveston whether any of your patients are being managed endoscopically and whether this algorithm would apply to those patients as well.
Dr. William O. Richards (Nashville, Tennessee): I too enjoyed the presentation very much. I would like to have Dr. Nealon address the situation of timing. That is, when should the ERCP be done? Is it done immediately before surgical intervention? Secondly, many of our gastroenterologists are very reluctant to do this in patients with chronic pancreatitis for fear of creating acute pancreatitis. I would like you to address whether or not ERCP induced pancreatitis or increased the sepsis rate after the procedure.
Dr. Timothy L. Pruett (Charlottesville, Virginia): Back when percutaneous drainage was first described, Steve Gerzof noted that in the people who had infected pancreatic pseudocysts, recurrence was somewhere around 2%, whereas the uninfected pseudocyst was successfully treated with PCD at a much lower rate. Can you segregate your results of people with colonization of their pseudocysts (which one would expect with scar down and close up reasonably quickly) from those who end up with a catheter drainage that is too small and results in inadequate drainage?
Dr. William H. Nealon (Galveston, TX): Well, we certainly see a subset of patients with pancreas divisum as their mechanism for acute pancreatitis. But I think as far as their pseudocysts being any different, once the pancreatitis has occurred, their cysts behave in a similar way. It would be necessary to visualize the duct through the minor papilla. We would expect the same principles to apply. I would like to thank all the discussants for their interesting comments and kind words. Tony Meyer asked about changes over time, whether with the improved skills of the interventionists and the ability to use CT direction and ultrasound direction, better catheters, et cetera, has improved outcomes in percutaneous drainage. None of these improvements have resulted in improved results for nonoperative techniques. Early on, I will confess that some of my suspicions were raised when, as the last discussant mentioned, people came from outside hospitals with small catheters who had had really fairly benign pseudocysts until someone stuck a straw in there and it got infected. The patients then come to our institution with sepsis. In general, we are finding the same kinds of outcomes even though we have really been blessed at our institution with very skilled interventionists. The question was raised about whether we shouldn’t just operate on all these people. Dr. Walser, my coauthor, is an interventionist. So we are trying to prospectively delineate an algorithm based on ductal anatomy. We are looking at some of the subsets in order to define the outcomes and best management. I am suspicious that operation represents a more favorable outcome in the majority, however. One of my pet peeves, if you read the nonsurgical literature on this disease, is that no one has an agreed-on point at which to determine that percutaneous or endoscopic management has failed and it is time to go to a surgeon. We have decided to at least impose that on ourselves. Nonoperative management which extends beyond 30 days’ duration will be referred to surgical management. It is certainly my bias that operative management is superior in most cases. I would restate that nonoperative measures must be compared to current surgical outcomes. There is even an article in 2001 in GI Endoscopy in which Lehman, a well-recognized endoscopist, mentions surgical series from the 1960s and 1970s in his discussion of pseudocyst management.
Tim Fabian, thank you for your comments. The rate of rupture, infection, and hemorrhage has been comparable to other series. Bleeding has almost always been managed by the interventionists so that our needs for doing emergency operations has been lower, and usually we have been able to get some time to find out what exactly we have as our anatomy before we proceed with any procedure. You mentioned MRCP, and I think that is a good question. You can wonder whether that modality makes ERCP unnecessary in this disease. In my experience, communication between the duct and the cyst is not well defined by MRCP. Certainly, the fine changes of secondary ductular ectasia that we sometimes see in chronic pancreatitis are not well defined. The detail still is somewhat lacking, particularly since we are so interested in the question of communication.
John Cameron talked to me about this question of communication. He appropriately observes that some communication must exist, and I agree. Our term “communication” simply denotes communication that is sufficiently large that it can be demonstrated on ERCP. I have a sentence in my manuscript specifically predicated on John’s comments in which I say it is likely that there are minuscule connections between the ducts and the cyst. My terminology of noncommunication means that it is not demonstrated on imaging studies.
Henry Pitt’s comments are absolutely accurate. Really there has been a biphasic activity of endoscopic intervention. Endoscopic management was proposed but was minimally utilized until relatively recently. There has been more enthusiasm both for transenteric drainage using EUS guidance, and for endoluminal drainage, where a stent is placed in the pancreatic duct as a means of decompressing pseudocysts. It continues to be my belief that the basically amputated part of the pancreas when you have a complete obstruction of the duct is a group that is going to be poorly managed even if a straw is placed in the isolated cyst through the stomach, just as it seems to work poorly when one places a straw through the skin into the cyst. My belief is that the success rates will be low by any small tube drainage. We all know that the failure rates for endoscopic management are still in the range of 30%. I believe we have a subset that is going to be poorly managed even by those techniques.
Dr. Richards, I mentioned that we try to do the ERCPs within 12 hours of operation or drainage. We do them the day before operation. There is a study from the 1980s showing that sepsis rates are much higher when ERCP is done any more remote from the operation from that. I actually perform ERCP, which may make it logistically easier to apply this practice. I understand that for some people, negotiating with the GI person might be tougher. Our episodes of pancreatitis are below 1%, which is what they should be for any experienced endoscopist.
Dr. Pruett asked about the effects of the infection on outcomes. We obtain various tests in the cyst fluid including cultures, and colonization is quite common. Infections in operated patients are infrequent. There certainly are patients who had infections related to the external drainage. Overall, we have not been able to subset out the patients with infectious complications as having any different outcome after operative management.
Footnotes
Correspondence: William H. Nealon, MD, Department of Surgery, The University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-0544.
E-mail: wnealon@utmb.edu
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Accepted for publication December 2001.
References
- 1.Bradley E, Clements JL Jr, Gonzales AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg 1979; 137: 135–141. [DOI] [PubMed] [Google Scholar]
- 2.Heider R, Meyer AA, Galanko JA, et al. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg 1999; 6: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Egidio A, Schein, M. Pancreatic pseudocysts: a Proposed classification and its management implications. Br J Surg 1999; 78: 981–984. [DOI] [PubMed] [Google Scholar]
- 4.Van Sonnenberg E, Wittich GR, Casola G, et al. Percutaneous drainage of infected and noninfected pancreatic pseudocysts: experience in 101 cases. Radiology 1989; 170: 757–761. [DOI] [PubMed] [Google Scholar]
- 5.Criadoe Desterano AA, Weiner TM, Jacques PF. Long-term results of percutaneous catheter drainage of pancreatic pseudocysts. Surg Gynecol Obstet 1992; 175: 293–297. [PubMed] [Google Scholar]
- 6.Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg 1992; 215: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitchumoni CA, Agarwal N. Pancreatic pseudocysts: When and how should drainage be performed? Gastroenterol Clin 1999; 28: 615–639. [DOI] [PubMed] [Google Scholar]
- 8.Spivak H, Galloway JR, Amerson JR, et al. Management of pancreatic pseudocysts. Am Coll Surg 1998; 168: 507–511. [DOI] [PubMed] [Google Scholar]
- 9.Nealon WH, Townsend CM Jr>, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg 1989; 209: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman G III. Chronic pancreatitis disease: role of endoscopy. Gastrointest Endosc 1999; 49: S81–84.10049456 [Google Scholar]
- 11.Kozarek RA, Ball TJ, Patterson DJ, et al. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology 1991; 100: 1364–1370. [PubMed] [Google Scholar]
- 12.Chak A. Recent advances in endoscopic ultrasonography. Endosonographic-guided therapy of pancreatic pseudocysts. Gastrointest Endosc 2000; 52: S23–S27. [DOI] [PubMed] [Google Scholar]
- 13.Yeo C, Pastidas J, Lynch-Nuhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990; 170: 411–417. [PubMed] [Google Scholar]
- 14.Vitas G, Sarr M. Selected management of pancreatic pseudocysts: operative vs. expectant management. Surgery 1992; 111: 123–130. [PubMed] [Google Scholar]
- 15.Ahearne PM, Baillie JM, Cotton PB, et al. An endoscopic retrograde cholangiopancreatography (ERCP)-based algorithm for the management of pancreatic pseudocysts. Am J Surg 1992; 163: 111–116. [DOI] [PubMed] [Google Scholar]
- 16.Grace PA, Williamson RCN. Modern management of pancreatic pseudocysts. Br J Surg 1993; 80: 573–581. [DOI] [PubMed] [Google Scholar]