Abstract
Objective
To examine trends in outcomes of patients undergoing resection at a single tertiary care referral center over a 16-year period.
Summary Background Data
Hepatic resection is considered the treatment of choice in selected patients with colorectal metastasis confined to the liver. Although a variety of retrospective studies have demonstrated improvements in short-term outcomes in recent years, changes in long-term survival over time are less well-established.
Methods
Data from 226 consecutive patients undergoing potentially curative liver resection for colorectal metastases between 1984 and 1999 were analyzed. Actuarial survival rates related to prognostic determinants were analyzed using the log-rank test.
Results
The median survival for the entire cohort was 46 months, with 5- and 10-year survival rates of 40% and 26% respectively. Ninety-three patients operated on between 1984 and 1992 were found to have an overall survival of 31% at 5 years, compared to 58% for the 133 patients operated on during the more recent period (1993–1999). Both overall and disease-free survival were significantly better in the recent time period compared with the earlier period on both univariate and multivariate analyses. Other independent factors associated with improved survival included number of metastatic tumors ≤ 3, negative resection margin, and CEA < 100. Comparisons were made between time periods for a variety of patient, tumor and treatment-related factors. Among all parameters studied, only resection type (anatomical versus nonanatomical), use of intraoperative ultrasonography, and perioperative chemotherapy administration differed between the early and recent time periods.
Conclusions
Long-term survival following liver resection for colorectal metastases has improved significantly in recent years at our institution. Although the reasons for this survival trend are not clear, contributing factors may include the use of newer preoperative and intraoperative imaging, increased use of chemotherapy, and salvage surgical therapy.
Hepatic resection has gained acceptance as the most effective therapy for patients with colorectal metastases confined to the liver. Advances in surgical planning, operative technique, and perioperative care have resulted in improved short-term outcomes, with experienced centers now reporting in-hospital mortality rates of less than 5%, even with major resections. 1–5
Long-term survival and potential for cure following surgical resection for hepatic colorectal metastases have been demonstrated in numerous uncontrolled studies. In most series, the overall 5-year survival rate reported following hepatic resection with curative intent ranges from 25%–37%, and with median survival of between 24 and 40 months. 1–3,6–10 As hepatic resections have become safer and indications have broadened, there are increasing expectations regarding assessment of trends in long-term patient outcomes. Although some improvement in survival are being reported in more recent series compared with those reported from earlier decades, survival trends within a given group or institution have not been clearly demonstrated.
The objective of this study was to examine outcomes in patients undergoing hepatic resection for colorectal metastases at our institution during a 16-year period and, in particular, to address temporal trends in long-term survival.
METHODS
From January 1984 to December 1999, inclusive, 226 consecutive patients underwent resection with curative intent at the Johns Hopkins Hospital. Patients undergoing palliative or incomplete resection, or those with combined ablative procedures, were excluded from the analysis. A retrospective analysis of a prospectively collected database was performed.
Patient follow-up was obtained from office records, letter, or telephone contact. Deaths within 30 days of operation were considered perioperative mortality. Patient demographics, operative and pathologic findings, and postoperative course were evaluated both by univariate and multivariate models to determine the impact on overall and disease-free survival, which was calculated from the time of liver resection. Disease-free survival analysis was based on 192 patients as time-to-recurrence was not known in 34 patients. Survival analyses were done by the Kaplan-Meier method. 11 Differences in survival were compared using the log-rank test. Fisher’s exact or the χ-square tests were used for univariate comparisons. Multivariate analysis was performed with the Cox Proportional Hazard Model. 12 Statistical analysis was performed using the SPSS Statistical Software Package (version 9.0, Chicago, IL). Differences were considered significant if P < .05.
Time trends were studied comparing two time periods, 1984 to 1992 and 1993 to 1999. These two periods were chosen in order to achieve a balance of sufficient sample number and adequate follow-up. In 41 patients, the regional nodal status was not available, and in 59 patients preoperative carcinoembryonic antigen (CEA) was not determined. Patients receiving preoperative chemotherapy were defined as those in whom systemic therapy was administered within 6 months of liver resection, either for measurable metastatic disease or in the adjuvant setting following primary resection. Only patients undergoing initial hepatic resection were included, although some of these patients underwent subsequent repeat hepatic resection.
RESULTS
Demographics
In the 16-year period, 226 patients with hepatic metastatic colorectal cancer underwent complete resection with curative intent at Johns Hopkins Hospital. The median age was 62 years (range 32–87). There were 145 men (64%) and 81 women (36%). In the 9-year period between 1984 and 1992, 93 patients underwent resection compared to 133 patients in the 7-year period between 1993 and 1999. Median follow-up of the survivors in the early group (84–92) was 121 months and 22 months for the recent time period (93–99).
Primary and Metastatic Tumor Characteristics
The primary tumor was in the colon in 160 cases (71%) and in the rectum in 66 cases (29%). Sixty-three percent of the primary tumors had involved regional lymph nodes, and 89% had well- or moderately-differentiated histology. Liver metastases presented synchronously with the primary tumor in 67 patients (30%) and liver resection was performed within 12 months of the primary resection in 107 patients (47%). One hundred and forty-one patients underwent resection for solitary metastases (62%), and 20 patients (9%) had 4 or more resected tumors. The median size of the liver tumors was 3.9 cm with 93 patients (41%) having tumors larger than 5 cm. In 125 patients, the tumor was located only within the right lobe, and 48 were only within the left lobe. Fifty-four patients had bilobar disease resected. Preoperative CEA was above 100 in 23 of 67 patients (14%). For the entire group, systemic chemotherapy was administered preoperatively in 118 patients (52%).
Surgical Therapy
Of the 226 resections, 47% involved resection of at least one lobe. Fifty-five resections were less than lobar anatomical resections (segmentectomy, bisegmentectomy, or caudate resection), whereas 65 patients (29%) underwent nonanatomical or wedge resection. In no cases in this group of patients was a hepatic arterial infusion pump implanted for postoperative adjuvant regional chemotherapy. Although in all cases gross margins were negative, histologic margins were found to be positive in 12 patients (5%). For the group as a whole, there were two perioperative deaths (0.9%) and the median hospital stay was 10 days (range 3–55 days). Postoperative complications occurred in 42 patients (18.6%).
Long-term outcomes
Kaplan-Meier curves for overall and disease-free survival are shown in Figure 1. Actuarial overall survival was 57% at 3 years, 40% at 5 years, and 26% at 10 years, with a median survival of 46 months. Disease-free survival was 63% at 1 year, 28% at 3 years, and 20% at 5 years with a median recurrence-free survival of 16 months.
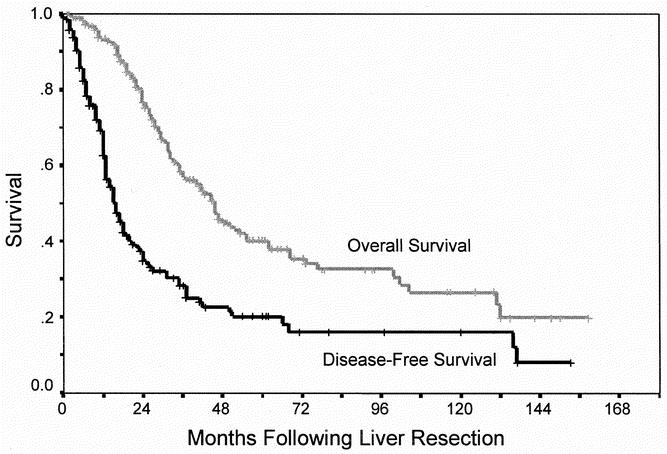
Fig. 1. Actuarial overall and disease-free survival following hepatic resection for colorectal liver metastases.
Predictors of long-term survival
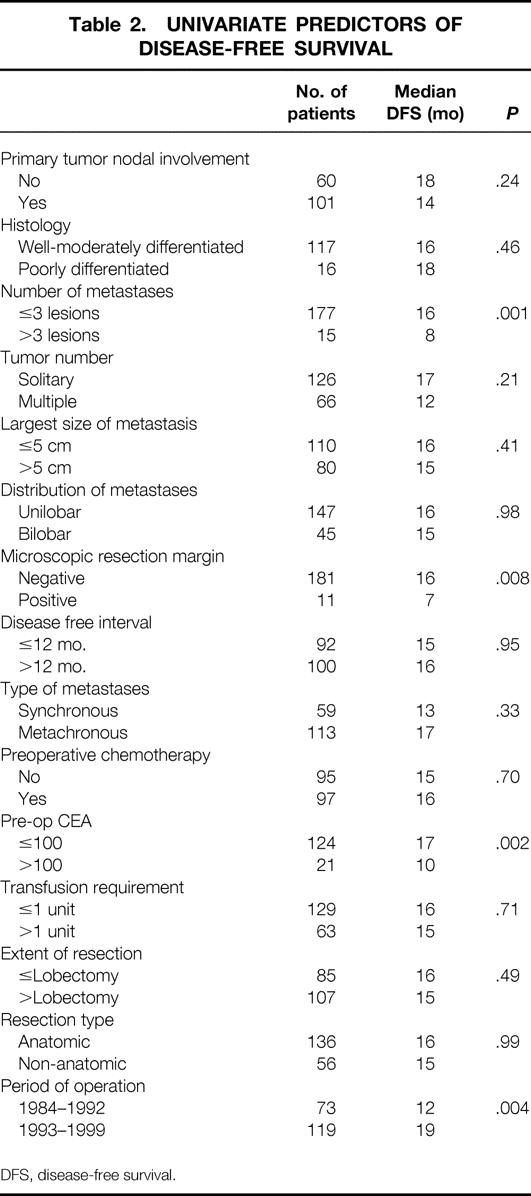
On univariate analysis, differences in overall survival were not seen based on age, gender, transfusion requirement, nodal status of primary tumor, number of metastases, tumor size, or distribution of metastases. Both preoperative CEA greater than 100 and positive microscopic resection margin were significant predictors of overall survival (Table 1). Univariate predictors of recurrence included number of metastases (> 3 lesions), in addition to microscopic margin and preoperative CEA (Table 2). For both overall and disease-free survival, liver resection in the early time period (1984–1992) was associated with worse adverse outcome compared with the most recent time period (1993–1999).
Table 1. UNIVARIATE PREDICTORS OF OVERALL SURVIVAL
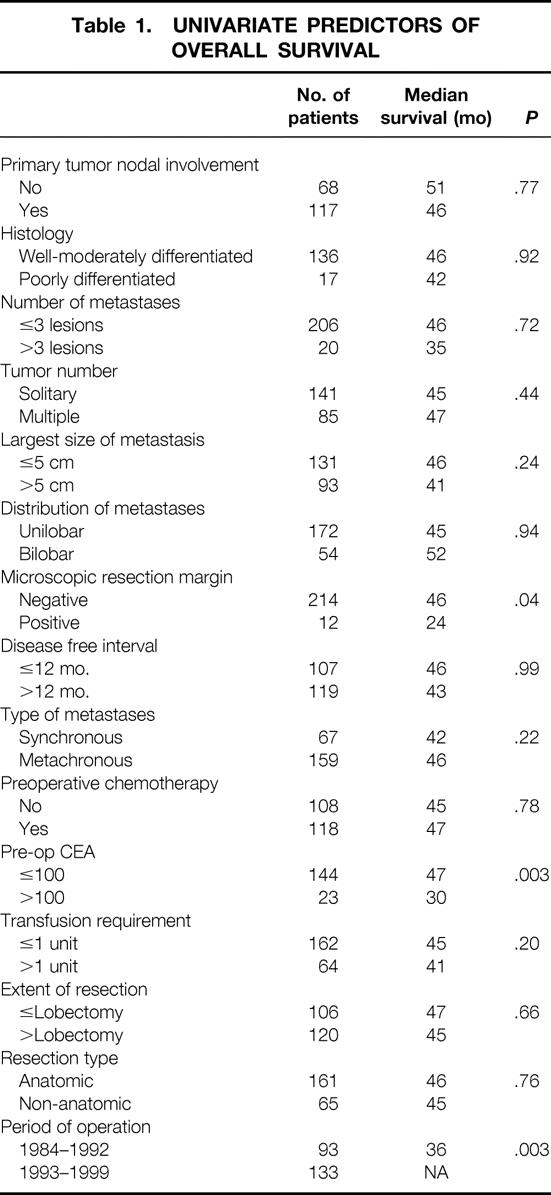
Table 2. UNIVARIATE PREDICTORS OF DISEASE-FREE SURVIVAL
DFS, disease-free survival.
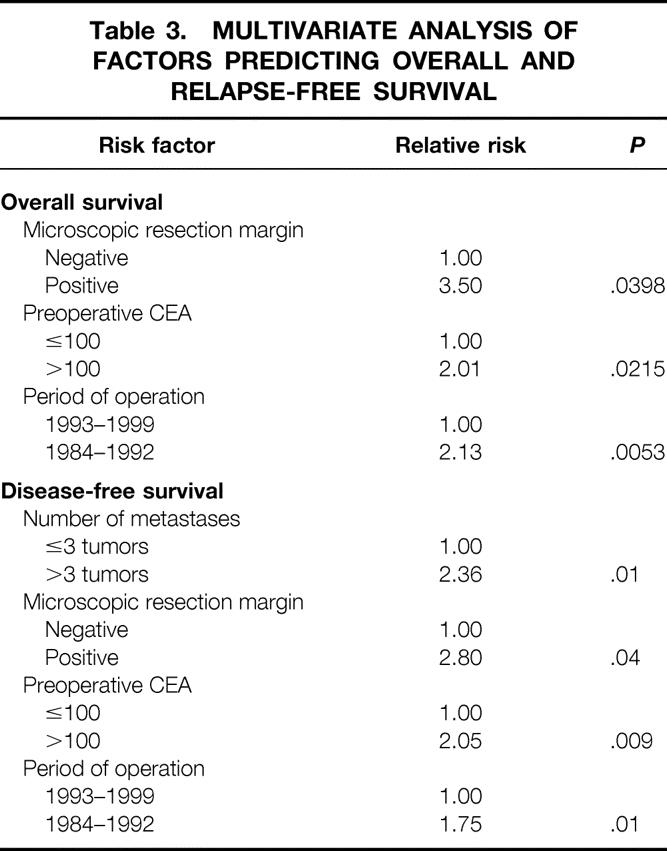
Multivariate analyses of prognostic factors for both overall and disease-free survival are shown in Table 3. Positive resection margin, high preoperative CEA, and early time period of operation (1984–92) were poor prognostic signs. While not predictive of overall survival, a tumor number greater than three lesions was the strongest independent predictor of recurrence.
Table 3. MULTIVARIATE ANALYSIS OF FACTORS PREDICTING OVERALL AND RELAPSE-FREE SURVIVAL
Differences between time periods: 1984 to 1992 versus1993 to 1999
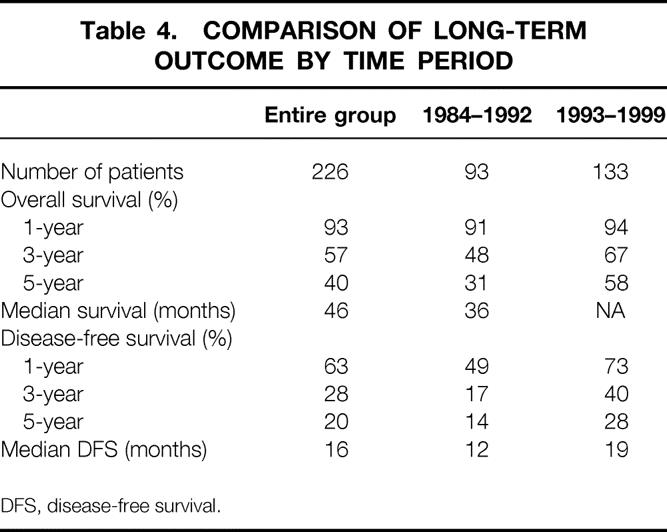
Differences in overall and disease-free survival between time periods is summarized in Table 4. The overall survival was significantly better in patients undergoing liver resection in the recent time period compared those in the early time period (P = .03, Fig. 2). While the 5-year survival for the entire group was 40%, 5-year survival improved from 31% to 58% between time periods. Risk of recurrence was also significantly different between time periods (P = .004, Fig. 3). In the period between 1984 and 1992, 5-year disease-free survival was 14% compared with 28% seen in the period between 1993 and 1999.
Table 4. COMPARISON OF LONG-TERM OUTCOME BY TIME PERIOD
DFS, disease-free survival.
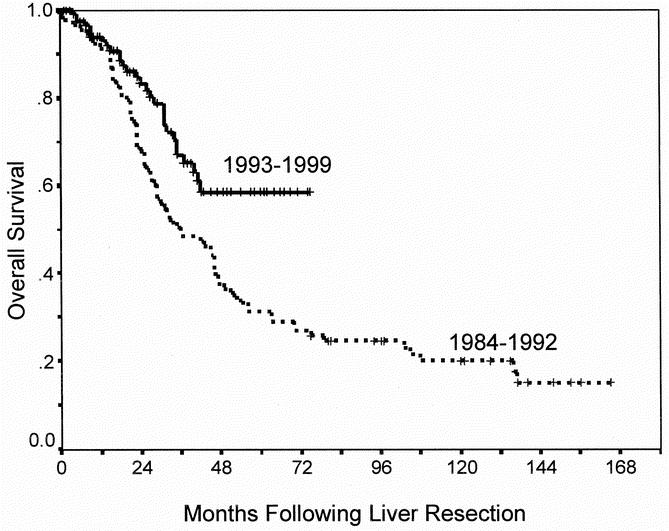
Fig. 2. Overall survival according to time period of resection (P = .003).
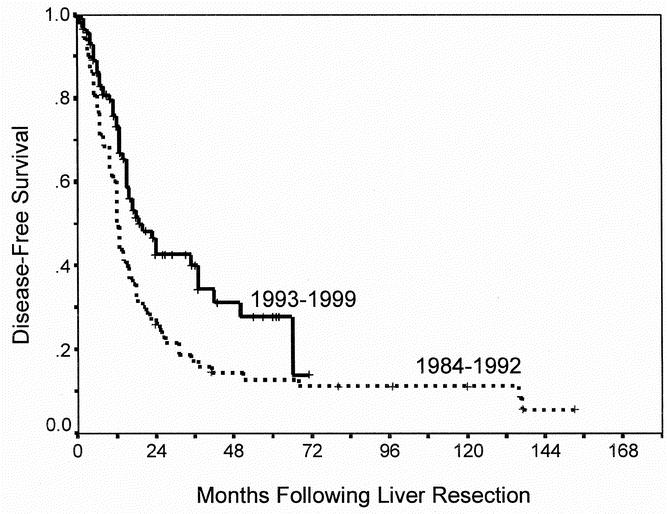
Fig. 3. Recurrence-free survival according to time period of resection (P = .004).
There were some differences seen in short-term outcomes between those patients operated on in the two time periods. Hospital length-of-stay was significantly less in the recent period (13 days vs. 7 days, P = .0001), as was the median perioperative transfusion requirement (2.2 units/patient vs. 1.0 units/patient, P = .003). No significant differences in perioperative mortality (2% vs. 0%) and morbidity (13% vs. 23%) were seen when comparing early versus recent groups. The increase in reported complication rates seen in 1993 to 99 reflects the development of more clearly defined prospectively collected morbidity data in the last decade from our institution.
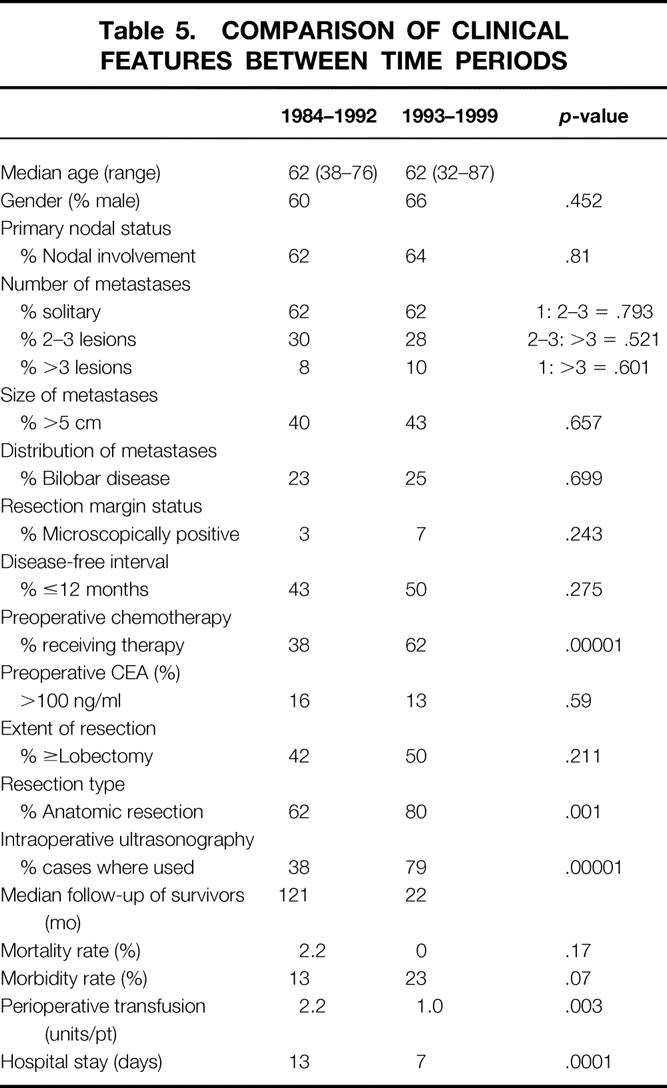
Comparisons were made in demographics, patient selection, and treatment of the two time period groups in order to determine potential reasons for long-term outcome differences (Table 5). No differences were seen in age, gender, stage, or site of primary tumor. Moreover, there were no significant differences between time periods in the number of metastases, size of tumors, distribution, preoperative CEA, disease-free interval, or microscopic margin status. Patients were significantly more likely to have undergone an anatomical resection in the 1993 to 99 period (80% vs. 62%, P = .001), but the percent of lobectomy or greater was not significantly different between groups.
Table 5. COMPARISON OF CLINICAL FEATURES BETWEEN TIME PERIODS
Because improved patient selection is likely a contributing factor to the improved outcome seen in the recent group, an attempt at assessing preoperative and intraoperative evaluation was made. More than 90% of patients had cross-sectional imaging, either computed tomography or magnetic resonance imaging, before resection in both groups. However, details regarding the scanning methodology, contrast technique, and timing relative to resection were not available for analysis in many cases. Positron emission tomography (PET) was performed in only 12 patients, all in the 1993 to 99 group, and staging laparoscopy was not used in any patient.
Intraoperative ultrasonography (IOUS) was used significantly more often in the recent period compared to the early group (79% vs. 38%, P = .00001). While one might predict increased use of IOUS would contribute specifically to an improvement in hepatic disease-free survival, no significant differences in the site or pattern of recurrence were seen between time periods.
The use of systemic chemotherapy was also used more frequently in patients undergoing resection between 1993 and 1999, compared to the 1984 to 92 group. Preoperative chemotherapy was more common in the recent group as was postoperative adjuvant systemic chemotherapy (data not shown). In addition, surgical therapy for recurrences was more common after 1992 than in the early period. Of the 76 patients who developed recurrence in the 1993 to 99 group, 27% underwent repeat hepatic resection or ablation compared to only 12% of the 65 recurrences in the 1984 to 92 group (P < .05).
DISCUSSION
The benefit of liver resection for patients with isolated hepatic colorectal metastases is recognized. Numerous retrospective and prospective series with large number of patients demonstrate a long-term survival benefit. 1–3,6–10 This report, from a single tertiary-care referral center over a 16-year period, concurs with the body of literature that hepatic resection is a safe and effective therapy, with an overall survival rate of 40%. These data justify the optimism regarding the increasingly aggressive approach being offered to many of patients with liver metastases from colorectal cancer.
This study also demonstrates a favorable trend in improved long-term outcome over time. Although significant advances in early detection, patient selection, and operative technique have occurred in recent years, such a trend in survival has not been well established in the literature. Several institutions, however, have published their experience of hepatic resection for colorectal metastases on more than one occasion, including patients from different time periods. Scheele et al. reported their experience in two studies. 13,14 In both series (one of 434 patients from 1960 to 1992 13 and another of 516 patients from 1960 to 1998), 14 the reported 5-year survival rates were similar (39% and 38% respectively). The experience from Memorial Sloan-Kettering Cancer Center was reported on 577 patients between 1985 and 1994, with a 5-year survival rate of 35% and a median survival of 40 months. 15 In a more recent study of 1,001 patients up to 1998, the 5-year survival rate was 37% with a median survival of 42 months. 3 Variability in inclusion criteria, overlap of patient populations, and differences in analytic methods between reports limit the ability to draw conclusions regarding long-term outcome trends in from comparisons of these studies.
A variety of possible explanations for the observed trend in improved outcome can be considered. Preoperative imaging studies play a critical role in improving patient selection. In addition to reducing the incidence of unnecessary surgical exploration, many believe that advances in preoperative imaging contribute to improvement in long-term outcome. 16–18 One recent report addressed the potential role of improved patient selection through better preoperative staging by evaluating the role of preoperative PET scan for improving prognosis. 17 In this study, 35 patients staged with PET were found to have improved survival compared to historic controls, with overall and disease-free 3-year survival of 77% and 40%, respectively. As we did not utilize PET for staging in most patients reported here, its impact on outcome could not be addressed. However, because improved survival trends were seen in our group of patients without the benefit of PET in most cases, other factors must be implicated. Therefore, when determining the impact of newer staging techniques such as PET on long-term outcomes, carefully controlled trials should be considered.
Improvements in intraoperative staging are also a likely to contribute to improved long-term survival. Application of IOUS, in particular, can provide a more accurate identification of otherwise occult intrahepatic tumors, which were then either resected or excluded as a complete resection. 9,18,19 In this study, IOUS was performed to a significantly greater extent in the recent time period. We could not determine, however, based on IOUS findings or the pattern of recurrence between groups, to what extent IOUS may have contributed to the observed outcome trend.
In the last decade, most experienced hepatic surgeons have implemented significant changes in operative technique for liver resection. Innovations in surgical technology, such as mechanical staplers and hemostatic devices, and the use of intraoperative strategies using vascular control and low central venous pressure, have resulted in clear improvement in short-term outcomes. Several studies have reported trends in decreasing mortality and shorter hospital stay despite the increasing extent of liver resections. 3,14,20 Whether advances in surgical technique and operative management contribute to improved long-term outcome is not clear. Although some identifiable technical factors differed between time periods, such as the transfusion requirement and the type of resection, no difference was seen in parameters that are more likely to contribute to long-term survival, such as the incidence of microscopically positive margin.
Systemic chemotherapy is being used with increasing frequency in combination with surgical resection, either following resection or as neoadjuvant therapy. Newer agents and drug combinations demonstrating increasing response rates in advanced disease and increasing survival in the adjuvant setting has fueled this enthusiasm. In this series, the number of patients receiving chemotherapy was significantly higher in the more recent time period. This difference may perhaps be a contributing factor to the improved outcomes observed. The uncontrolled nature of this study, however, limits the ability to draw conclusions regarding the beneficial role of systemic chemotherapy. Indications for chemotherapy, and the choice of agents, and the timing of therapy relative to the liver resection varied considerably in our patients.
The increasingly aggressive surgical approach we have undertaken at our institution in recent years when confronted with recurrent disease after initial resection may also have contributed to the observed improvement in overall survival. A significantly greater proportion of recurrences from the recent group were treated with repeat surgical therapy compared to the early period. Many studies have documented favorable long-term survival in highly selected patients undergoing second resection for recurrent colorectal liver metastases, with 5-year survival rates of up to 41%. 21–24
In summary, these data provide further support for long-term benefit of liver resection of hepatic colorectal metastases. Based on our experience, overall and disease-free survival is improving over time. Factors that may play a role in this trend include improved patient selection through better preoperative and intraoperative imaging, improved surgical technique, more use of chemotherapy, and/or an increasing aggressive use of salvage surgical therapy following recurrence.
Discussion
Dr. John M. Daly (New York, New York): The use of surgical approaches for the management of colorectal hepatic metastasis has clearly increased dramatically in recent decades. As Dr. Choti indicated, as perioperative morbidity and mortality have become more acceptable and the training of surgeons in liver operations has increased, more aggressive surgery with combinations of resection and ablation, the use of regional arterial infusion chemotherapy, and repeated hepatic resections as indicated here in this presentation are commonplace now.
There is a strong sense that long-term outcome is improving. However, reviews of surgical management over time may involve, as Dr. Choti pointed out, patient selection bias and observation time bias. These issues are amongst the most interesting in Dr. Choti’s presentation, and that is what I will ask about.
The overall 5-year survival increased from 31% in the early time period, which is what the standard quoted number is for the decade of the ’80s, to 58% in the most recent time. While CT was used for preoperative staging to the same degree in both of these time period groups, intraoperative ultrasound, as he said, was used much more commonly recently.
The first question then, how often did intraoperative ultrasound change the procedure for you, and did this change the operation? How did this change over time after 1993? What happened as CT protocols improved? Were the changes that occurred in your operative procedure with the use of intraoperative ultrasound? Was it less because the CT improved or did it remain about the same?
The time periods were chosen based on patient numbers. Can you analyze your data completely differently using time as a continual parameter rather than setting specific time periods? And can a statistician help with that and look to see if time really is a major factor?
Finally, could the differences in median follow-up, which were substantial, explain some of the overall survival result differences?
I enjoyed your presentation immensely. I compliment you on the terrific results of you and your co-authors.
Dr. John S. Bolton (New Orleans, Louisiana): This paper highlights a recent trend toward improved survival after hepatic resection for metastatic colorectal cancer, and I appreciate the authors bringing this information to us. Indirect confirmation of their observation is provided by three recently reported randomized control trials evaluating post-hepatic resection chemotherapy. Two of these have only been published in abstract form in the Proceedings of ASCO, one an ECOG and SWOG trial published in the 1999 Proceedings of ASCO, and the second is a North Central Cancer Treatment Group trial published in the 2001 Proceedings of ASCO. The third is available in manuscript form and is the Memorial Sloan-Kettering prospective, randomized trial reported in the New England Journal of Medicine in 1999. All three of these studies documented 5-year survivals greater than 55% for patients receiving post-hepatic resection chemotherapy. Different regimens were used in all three studies, but in all three studies the five-year survival was greater than 55%. I submit that if over time this finding is validated, that this is a remarkable accomplishment. The achievement of this survival rate for a metastatic solid tumor certainly represents a major advance. Unfortunately, the paper presented today doesn’t bring into focus, as Dr. Choti acknowledged, the reasons for the improved survival. I suspect people will conclude that the improved survival is mostly a function of better patient selection alone, because of better preoperative imaging, perhaps more rigorous abdominal exploration, and evaluation of porta-hepatis lymph nodes and the use of intraoperative ultrasound. I would challenge the authors to dig deeper and to try to help us understand this observed phenomenon better. In that spirit, I have several questions.
One, did you consider using one of the available and validated prognostic scoring systems, such as the Memorial Sloan-Kettering system or the Nordlinger system? These have been validated in much larger databases than your own and certainly have shown that a combination of prognostic variables is a much better discriminant of prognosis than a single variable.
Two, like Dr. Daly, I worry about the median follow-up for your recent group at 22 months. Could you give us the confidence intervals around your 5-year survival rates for the recent group?
Third, one difference in the two groups was that preoperative chemotherapy was used more frequently in the recent group. But it wasn’t clear to me if this is preoperative chemotherapy for the metastatic disease or postoperative adjuvant chemotherapy for patients after resection of the colorectal primary. Also, how many of the recent era patients had postoperative – that is, post-hepatic resection – chemotherapy? I would recommend that this information be added to the manuscript.
Finally, could you tell us the current position at the Johns Hopkins Hospital regarding hepatic artery infusion treatment? Do you use it or not?
Dr. J. Nicolas Vauthey (Houston, Texas): I would like to congratulate Dr. Choti and the Johns Hopkins group for an excellent paper. Dr. Bolton has asked most of the questions I intended to ask. I would simply ask Dr. Choti about his practice regarding adjuvant chemotherapy. If one of his patients comes unreferred, that means unreferred, no oncologist bias, what is his recommendation today regarding adjuvant chemotherapy?
Dr. L. Michael Brunt (St. Louis, Missouri): My question is with regard to the pattern of recurrences in the patients who failed hepatic resections. That is, how many patients had isolated liver recurrences as opposed to extrahepatic disease? In that regard, are you beginning to use modalities such as FDG PET now on a routine basis to identify patients who have extrahepatic disease, and thereby preclude hepatic resection?
Dr. Michael A. Choti (Baltimore, Maryland): Thank you very much for your questions and comments. Due to constraints in time, let me summarize a response to some of the questions as follows.
First let me address the observation time bias. Clearly that is a problem in that the difference in follow-up limits the ability to confidently conclude the impact of time as a variable. We do not have the sufficient numbers in order to evaluate time as a continuous variable. So our statisticians suggested dividing time period into two groups in order to achieve sufficient power. I would suggest to those that have larger series to try to address this same question in order to demonstrate the same current trend.
As Dr. Bolton comments, there are studies, including recent randomized trials, suggesting outcomes are better in more contemporary reports. In fact, one needs to be cautious when comparing new methods to historic controls for those reasons. As mentioned, it may not only be the addition of FDG PET or a new adjuvant therapy that is the reason for improved outcomes in uncontrolled studies. Our study suggests that there is a trend of improved survival independent of any one specific intervention. New methods of patient selection or therapy following liver resection needs to be evaluated in a controlled study to be able to draw conclusions.
Regarding Dr. Brunt’s question about FDG PET, we currently use PET prior to liver resection in most cases. In fact, we are finding in 15-20% of cases that resection with curative intent is not being performed.
There are false positives and false negatives, and at times it is difficult to interpret the results of the PET confidently enough to deny a patient from a potentially curative resection. On occasion a biopsy may be required or exploration by laparoscopy or laparotomy is directed first to a finding on PET scam. This is something that needs to be worked out. I do believe, however, that there is a role for screening or staging these patients with PET scan prior to resection.
Dr. Daly asked how often did intraoperative ultrasonography change the operation, and did more current cross-sectional imaging modalities, have an impact on the changes in intraoperative ultrasonography. With our available data, we cannot answer this question. It is my impression that improvements in preoperative imagine has diminished the benefit of intraoperative ultrasonography at identifying occult disease within the liver. The real way to address this question is to see if there is a difference in the pattern of recurrence following liver resection. That is, if intraoperative ultrasonography is needed identifying significant otherwise occult disease within the liver, one may expect to see a higher number of intrahepatic recurrences in patients who did not undergo intraoperative ultrasonography. We did not find such a difference.
Several discussants asked about the definition of chemotherapy, including the type and duration, both before and after surgery. Answering this question is difficult from our dataset as the diversity of regimens and timing relative to resection is great. Preoperative chemotherapy was defined in this study as any chemotherapy received within six months prior to liver resection. Indications included both patients receiving therapy for their metastatic disease and those undergoing adjuvant therapy following primary tumor resection.
Determining the effect of postoperative adjuvant chemotherapy following liver resection is a difficult parameter to unravel in an uncontrolled study such as this one. Many of these patients received peoperative chemotherapy and then received a different salvage postoperatively. Others were chemo-naïve or received the systemic chemotherapy in the adjuvant setting preoperatively remotely – sometimes years – prior to liver resection.
Regarding our current management regarding the use of chemotherapy, it seems to be highly variable and based on multiple factors, of course, including the desires of the patient, surgeon, and medical oncologist. In a patient who has just undergone, for example, resection of their primary tumor and who perhaps has multiple or bilobar hepatic metastases, we may be more inclined to offer systemic chemotherapy initially for a period of time, two or three cycles, and then proceed with resection if still resectable. This has the advantage that perhaps we can use the measurable disease in the liver as an in vivo gauge of chemo-sensitivity, following with the same regimen postoperatively if they responded. In addition, patients who go on to progress with unresectable disease during preoperative therapy may be saved from an exploration or resection without benefit. Finally, metastases that have a significant response may be easier to resect.
We must keep in mind, however, that resection is the best option in resectable patients and should be advocated when possible, with or without chemotherapy.
Footnotes
Correspondence: Michael A. Choti, MD, Department of Surgery, Johns Hopkins Hospital, 600 N. Wolfe Street, Halsted 614, Baltimore, MD 21287.
E-mail: mchoti@jhmi.edu
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Accepted for publication December 2001.
References
- 1.Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of long-term survivors. Dis Colon Rectum 1988; 31: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheele J, Rudroff C, Altendorf-Hofman A. Resection of colorectal liver metastases revisited. J Gastrointest Surg 1997; 1: 408–422. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 5.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992; 216: 492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119: 647–651. [DOI] [PubMed] [Google Scholar]
- 7.Schlag P, Hohenberger P, Herfath C. Resection of liver metastases in colorectal cancer: competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 1990; 16: 360–365. [PubMed] [Google Scholar]
- 8.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997; 132: 505–510. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg 1997; 63: 605–610. [PubMed] [Google Scholar]
- 10.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994; 116: 703–710. [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 12.Cox DR. Regression models and life tables. J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- 13.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 14.Scheele J, Altendorf-Hofmann A, Grube T, et al. Resection of colorectal liver metastases. What prognostic factors determine patient selection? Chirurg 2001; 72 (5): 547–60. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Blumgart LH, Fortner JG, et al. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg 1995; 222: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valls C, Andia E, Sanchez A, et al. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology 2001; 218: 55–60. [DOI] [PubMed] [Google Scholar]
- 17.Strasberg SM, Dehdashti F, Siegel BA, et al. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg 2001; 233: 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound (IOUS) is essential in the management of metastatic colorectal liver lesions. Am Surg 2000; 66: 611–615. [PubMed] [Google Scholar]
- 19.Paul M, Mulder S, Cuesta M, et al. Impact of intraoperative ultrasonography on treatment strategy for colorectal cancer. Br J Surg 1994; 81: 1660–1663. [DOI] [PubMed] [Google Scholar]
- 20.Malafosse R, Penna C, Sa CA, et al. Surgical management of hepatic metastases from colorectal malignancies. Ann Oncol 2001; 12: 887–894. [DOI] [PubMed] [Google Scholar]
- 21.Tuttle TM, Curley SA, Roh MS. Repeat hepatic resection as effective treatment of recurrent colorectal liver metastases. Ann Surg Oncol 1997; 4: 125–130. [DOI] [PubMed] [Google Scholar]
- 22.Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg 1997; 225: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Trigo V, Shamsa F, Aldrete J, et al. Repeat liver resections from colorectal metastasis. Repeat Hepatic Resection Registry. Cancer Treat Res 1994; 69: 185–196. [DOI] [PubMed] [Google Scholar]
- 24.Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Francaise de Chirurgie. J Clin Oncol 1994; 12: 1491–1496. [DOI] [PubMed] [Google Scholar]


