Abstract
Objective
To examine the incidence of local recurrence (LR) and factors associated with it in a population of patients who underwent skin-sparing mastectomy (SSM) and immediate reconstruction for invasive carcinoma.
Summary Background Data
The efficacy of SSM has been challenged by concerns about increased risks of LR.
Methods
A consecutive series of 173 patients (176 cancers) with invasive carcinoma underwent SSM and immediate breast reconstruction (June 1986 to December 1997). Data were analyzed by the Kaplan-Meier method, the log-rank statistic test, and the Cox proportional hazards model.
Results
Mean patient age was 47 ± 9 years (27% were 40 or younger). The AJCC stages were 1 = 43%, 2 = 52%, and 3 = 5%. Thirty percent of tumors were poorly differentiated. With a median follow-up of 73 months, the LR rate was 4.5%. The mean local relapse-free interval was 26 months. Seventy-five percent of patients who presented with LR developed distant metastases and died of disease within a mean of 21 months. On univariate analysis, factors associated with higher LR rate were tumor stage 2 or 3, tumor size larger than 2 cm, node-positive disease, and poor tumor differentiation. Actuarial 1-, 3-, and 5-year overall survival rates were 98%, 94%, and 88%, respectively. On multivariate analysis, factors associated with decreased survival were advanced stage, presence of LR, and absence of hormone therapy. LR was a highly significant predictor of tumor-related death.
Conclusions
There is a low incidence of LR after SSM, and it is associated with advanced disease at presentation. LR is an independent risk factor for tumor-related death.
Skin-sparing mastectomy (SSM) has been advocated as an oncologically safe approach for the management of patients with early-stage breast cancer. This operation also minimizes deformity and improves cosmesis through preservation of the skin envelope of the breast. 1–5 Chest wall skin is a common site of local failure after mastectomy, and the incidence of this event after SSM has been reported to be 0%5 to 7%. 6 Although the results of SSM have been analyzed in previous reports, many of these series included patients with invasive and noninvasive breast cancer with variable lengths of follow-up. The objective of this study was to examine survival rates, incidence of local recurrence (LR), and factors associated with it in a population of patients who underwent SSM and immediate breast reconstruction for invasive breast cancer at a single institution.
METHODS
A retrospective review was performed of patients who underwent SSM and immediate breast reconstruction from June 1988 through December 1999 at the University of Alabama at Birmingham. From the 310 patients treated during this period, we selected all those with invasive breast cancer and a potential follow-up greater than 36 months. Records were analyzed for patient, tumor, and treatment characteristics. Young patient age was considered 40 years old or younger. LR was defined as a biopsy-proven cancer in the skin flaps from the mastectomy, the transposed tissues, or over the ipsilateral chest wall. Regional recurrence referred to involvement of ipsilateral axillary or supraclavicular nodes. Ductal carcinoma in situ (DCIS) was considered extensive when it represented greater than 20% of the size of the tumor. Tumor staging was according to the 1997 edition of the AJCC Staging Manual. 7 Follow-up was considered from the time of the SSM. The date of last follow-up was August 30, 2001.
Actuarial curves for local control and survival were calculated using the Kaplan-Meier method, and tests of significance were based on the log-rank statistic. Multivariate analysis was done with the proportional hazards model, using the log-linear hazard function of Cox. The significance of the differences between proportions was tested with the chi-square statistic or with the Fisher exact test, and differences between means were tested with the nonparametric Mann-Whitney test. All the statistics were performed with the aid of the SPSS statistical package. Significance was considered as P < .05.
RESULTS
From June 1, 1986, through December 31, 1997, 173 patients with invasive breast cancer underwent SSM and immediate breast reconstruction at the University of Alabama at Birmingham. Three patients presented with simultaneous bilateral invasive breast cancer; thus, 176 breasts were treated during this period. Seven patients presented with LR after breast-conserving surgery (lumpectomy and radiation therapy) a mean of 74 months after their initial treatment (range 23–120 months).
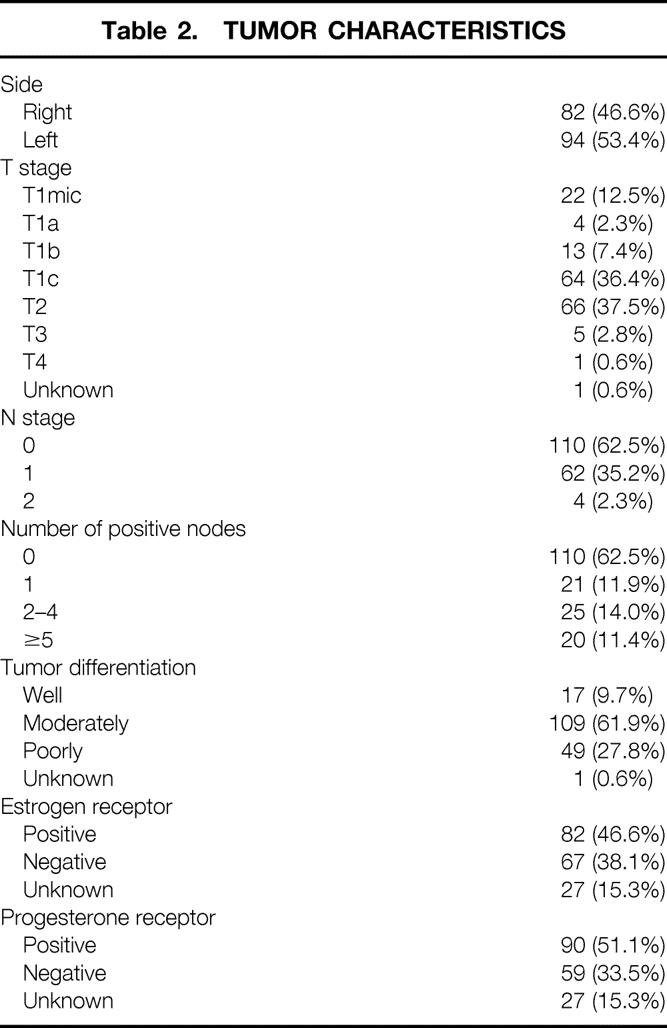
Mean patient age at diagnosis was 47 ± 9 years (range 26–68). The demographic and clinicopathologic characteristics of the patients are shown in Table 1. Most patients were white (92.5%) and premenopausal (60.1%). Pathologic characteristics of the tumor are shown in Table 2. More than 90% of the tumors were infiltrating ductal carcinomas; overall, 55% had extensive DCIS in the surgical specimen. The exact measure of the tumor was known in 153 cases: mean tumor size was 2.08 ± 1.14 cm (range 0.2–6.0).
Table 1. DEMOGRAPHIC AND CLINICOPATHOLOGIC CHARACTERISTICS OF PATIENTS
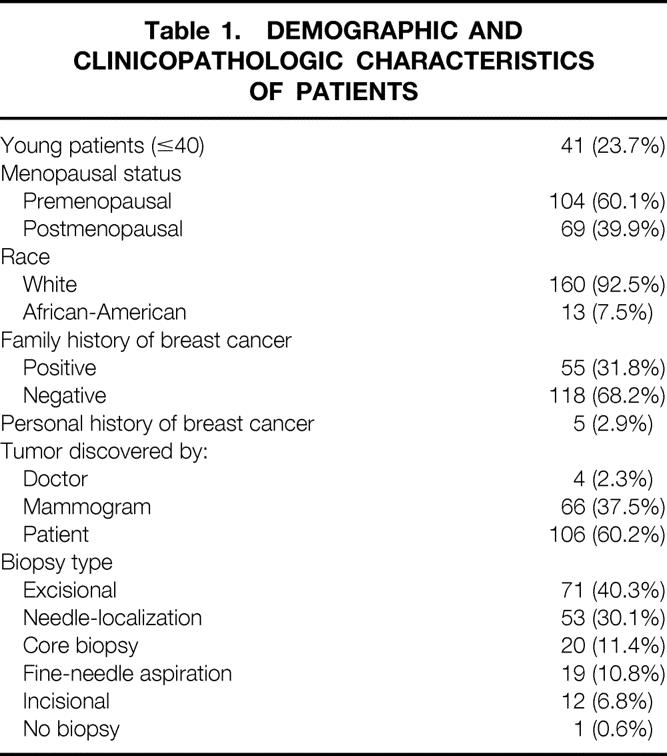
Table 2. TUMOR CHARACTERISTICS
Twenty-seven patients underwent contralateral mastectomy, 3 for contralateral invasive breast cancer and 24 prophylactic. Most patients were reconstructed with a free transverse rectus abdominis musculocutaneous (TRAM) flap using microsurgical technique (67%;Table 3). Thirty-five percent of patients presented with some type of immediate surgical complication. Immediate and delayed complications are also shown in Table 3.
Table 3. TREATMENT CHARACTERISTICS
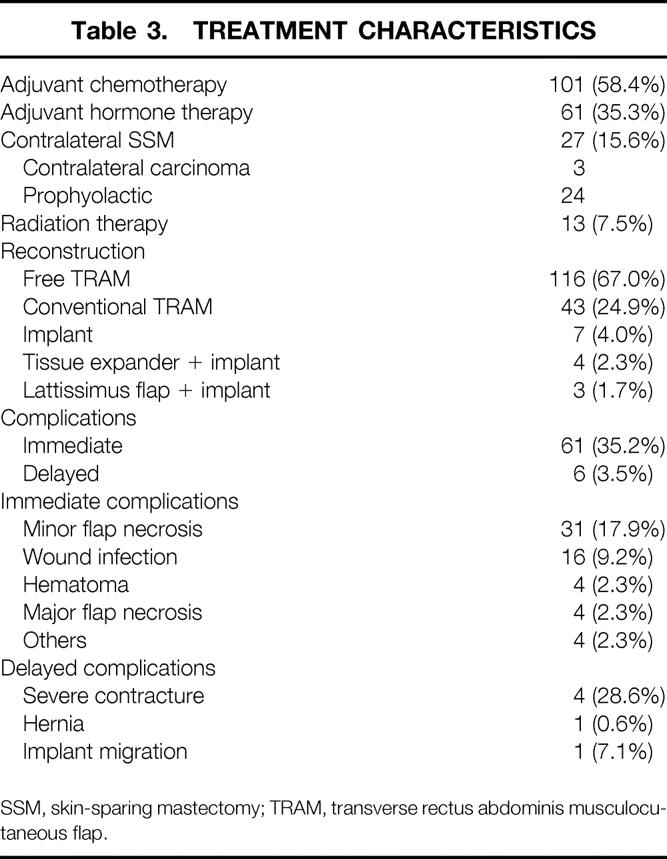
SSM, skin-sparing mastectomy; TRAM, transverse rectus abdominis musculocutaneous flap.
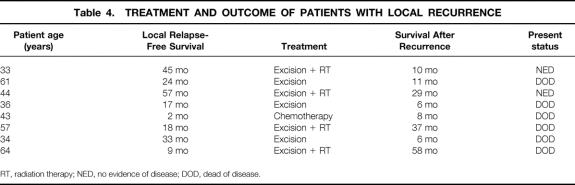
Median follow-up was 73 months (range 37–158). Thirty-one patients (17.9%) presented with distant recurrence, seven (4.0%) with regional recurrence, and eight (4.5%) with LR. The mean time from surgery to LR was 25 months (range 2–57). Seventy-five percent of LRs (6/8) presented within 3 years of treatment. The treatment and outcome of patients who developed LR is shown in Table 4. Seventy-five percent of patients who presented with LR developed distant metastases and died of disease within a mean of 21 months (range 2–57).
Table 4. TREATMENT AND OUTCOME OF PATIENTS WITH LOCAL RECURRENCE
RT, radiation therapy; NED, no evidence of disease; DOD, dead of disease.
Factors associated with LR after SSM on multivariate analysis were poor tumor differentiation, negative progesterone receptor status, tumor larger than 2 cm, node-positive disease, and advanced AJCC stage (Table 5).
Table 5. FACTORS ASSOCIATED WITH LOCAL RECURRENCE AFTER SKIN-SPARING MASTECTOMY
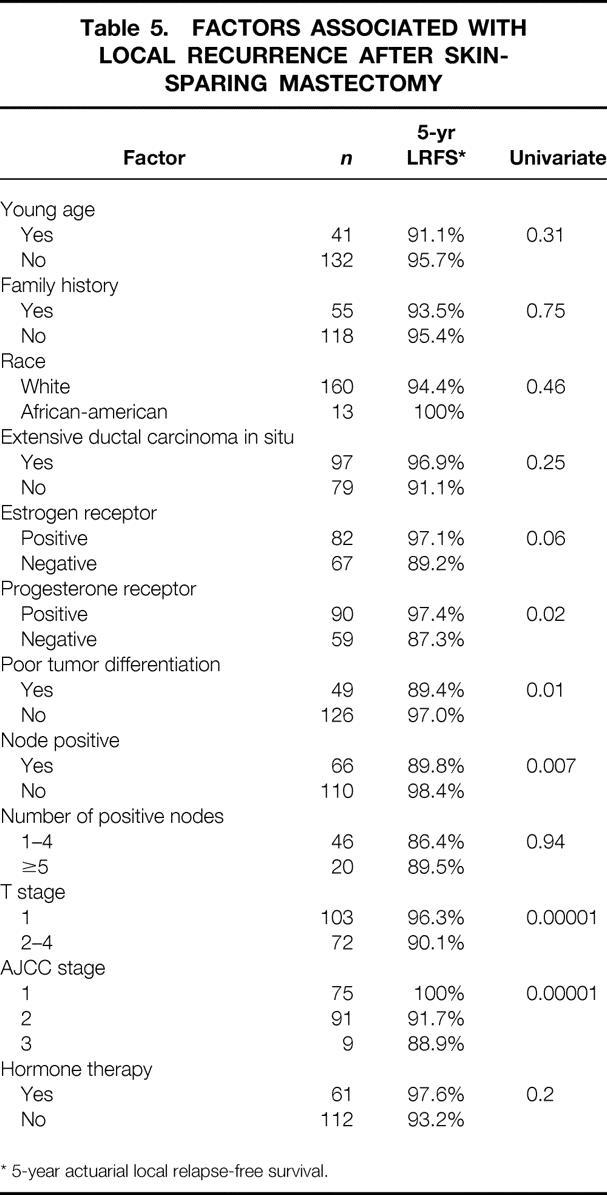
* 5-year actuarial local relapse-free survival.
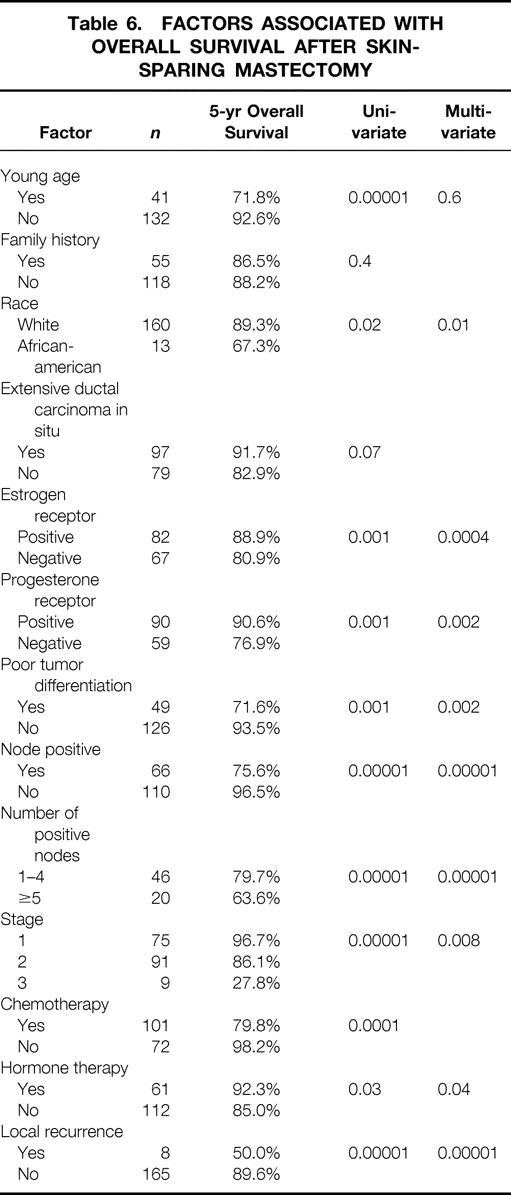
Actuarial 1-, 3-, and 5-year survival rates for the entire population were 98%, 94%, and 88%, respectively. Factors associated with decreased survival were black race, large tumor size, node-positive disease, negative estrogen and progesterone receptor status, poor tumor differentiation, and absence of hormone therapy. LR was found to be highly predictive of tumor-related death (odds ratio 17.2, 95% confidence interval 3.3–90, P = .00001) (Table 6).
Table 6. FACTORS ASSOCIATED WITH OVERALL SURVIVAL AFTER SKIN-SPARING MASTECTOMY
DISCUSSION
Immediate breast reconstruction after SSM has been increasingly used for treatment of early breast carcinoma because of better cosmetic results 1–5 and reduced cost compared with delayed breast reconstruction. 8 From the oncologic point of view, it has been advocated as safe, with LR rates reported between 0%5 and 7%. 6 Previous studies, however, have mixed patients with invasive and noninvasive carcinomas, and the follow-up period has been variable. In the series by Toth et al., 5 there were no LRs reported in 50 consecutive patients with a median follow-up of 51 months; however, almost half of their patients did not have invasive cancer. In the series by Hidalgo et al., 3 there were no LRs reported in 28 patients, but the mean follow-up was 27 months. Carlson et al. 1 reported an LR rate of 4.8% in 327 SSMs with a mean follow-up of 38 months. In that study 42% of the SSMs were performed prophylactically or for stage 0 disease. The present series included only patients with invasive cancer and a minimum follow-up of 36 months. The LR was 4.5%, with a median follow-up of 61 months. The only reported study of invasive cancer with longer follow-up is by Kroll et al. from the M. D. Anderson Cancer Center. 6 In that series, the authors reported an LR rate of 7% in 114 patients who underwent SSM, with a minimum follow-up of 6 years. The mean and median follow-ups were not given. Both Donegan et al. 9 and Crowe et al. 10 have analyzed the time course for LR in a large population of patients with recurrent breast cancer after mastectomy. Their results show that approximately 70% of LRs occurred in the first 3 years.
Factors associated with local recurrence after SSM have not been previously analyzed in detail. In the study by Kroll et al., 6 the incidence of LR was lower (6.2%) in patients with T1 tumors than with T2 tumors (9.3%). The difference, however, was not statistically significant (P = .5). They reported that nuclear grade of the tumors did seem to have an effect on the incidence of LR. When these differences were cross-tabulated, they were found not to be statistically significant (P = .5). In the present series, tumor size was one of the most significant predictors of LR after SSM. The actuarial 5-year local relapse-free survival rate was 96% versus 90% when the tumor was smaller or larger than 2 cm, respectively (P = .0001). Other factors significantly associated with local recurrence in the present study were tumor stage, poor tumor differentiation, and negative progesterone receptor status. All these factors were found to be significant in univariate analysis. Multivariate analysis would require a larger number of patients or LR events. These findings are consistent with other reports of factors associated with LR after conventional mastectomy. 9–11
Factors associated with survival after SSM are the same consistently reported to be significant in breast carcinoma irrespective of treatment type. The most significant and independent predictor of survival after SSM is tumor stage at presentation (P = .0002). Other independent factors associated with survival were poor differentiation of the tumor and absence of treatment with hormone therapy. One of the most striking findings in the present study was that LR is an independent predictor of survival after SSM. The actuarial 5-year survival rate was 90% when no LR was present versus only 50% in patients with LR (P = .03).
Several series have reported that LR after conventional mastectomy is associated with a worse prognosis than LR after breast-conservation therapy. In the series by Gilliland et al., 11 distant metastases eventually developed in every patient, and all died of breast cancer. Most series with a long-term follow-up report a 10-year disease-free survival rate of 7% to 17% after initial LR. 12–16 In our series, 75% of patients with LR developed distant metastases and died of disease within a relatively short period (mean 21 months, range 6–58). The impact of LR after mastectomy on survival has not been supported by other studies. In the study by Newman et al. 17 from 23 patients with LR, with a median follow-up of 26 months, 61% were alive with no evidence of disease. We agree with Carlson 18 that local recurrence after SSM is a manifestation of tumor biology, so aggressive tumors tend to recur locally and in distant sites with obvious differences in survival.
Because SSM improves the result of breast reconstruction and it is oncologically safe, we conclude that it can be used in the treatment of early breast cancer without compromising local control. However, patients with advanced stage at presentation or poorly differentiated tumors have an increased risk of LR. The presence of LR appears to be an independent adverse prognostic factor for survival, but it is mainly related to the biology of the tumor rather than the surgical modality.
Discussion
Dr. William C. Wood (Atlanta, GA): The challenge to skin-sparing mastectomy does not come from scientific data but from the teachings of our fathers that by taking more skin with a mastectomy we were likely to diminish local failure and ultimately death from breast cancer. This report gives the reassuring finding that only 2 of 173 women with skin-sparing mastectomy for invasive cancer developed isolated local recurrence. Both of those women are now free of apparent disease after excision and radiation. Six other patients developed local failures as part of systemic metastases that were occurring over the same period. With three quarters of the patients who developed local recurrence doing so in the presence of or shortly preceding systemic metastases, it is not surprising that the usual suspects for metastatic disease were those factors that predicted for local failures. The data from this excellent group—and in the absence of our president, I will slip and acknowledge both the excellence of the group and the excellence of the manuscript and that I appreciate the opportunity of reading it in advance, even if those comments are clearly iterative to the fine work of our Program Committee on each and every paper presented here today. These data agree with the roughly 600 patients in the Emory skin-sparing mastectomy series that is very closely monitored by Dr. Grant Carlson, who is here as well. I would like to ask two questions of the authors. First, your data validate very clearly your exclusion criteria for this series. And I wonder if you would tell us what those exclusion criteria are. Second, can you tell us anything about the two women who developed isolated local failures, about their grade, size, stage, and so forth?
Dr. John B. Mccraw (Jackson, MS): Kirby Bland has asked me to speak from the perspective of a plastic surgeon, since he and I have worked on this joint problem for many years. May I congratulate Dr. Urist for acting as the driving force behind this project, which we have all followed with great interest. The authors were kind enough to provide me with a copy of the manuscript. It seems to me that the authors have demonstrated, at least in the early follow-up period, that the skin-sparing mastectomy is a safe method in the majority of the patients that we treat with invasive breast cancer today. This may not have been the case 50 or 100 years ago, but the presentation of the disease has changed, and so the method of surgical treatment should be subject to reevaluation. It is becoming clear through this paper and several other recent papers that, whether the mastectomy skin excision is radical or conservative, wide skin excision cannot be credited with either the success or the failure of surgical treatment of breast cancer. The experience of Dr. Urist’s group, like others, brings a new focus on tumor biology as the fundamental determinant of the outcome. The evolution of mastectomy skin excision has gone from total excision of the breast skin by Halsted, to Patey’s use of skin grafting in half of his cases, to the current primary skin closure popularized by Auchincloss and Madden. The paradigm shift away from the radical mastectomy occurred when Patey suggested that wide skin excision is more important than the excision of the pectoralis major muscle. Forty years later, it is the mainstream of mastectomy surgery to remove as much skin as possible and still get a primary closure. The skin-sparing mastectomy is the first retreat from radical skin excision, and there is reasonable data to support that approach. I have one comment and one question. My comment is to say that we have also used the skin-sparing mastectomy for 15 years, both in patients who are having immediate reconstruction and in patients who are unsure about their decision for reconstruction. As a method of simple mastectomy, the skin-sparing mastectomy creates less skin tightness and less scarring. Patient acceptance is much better because the remaining skin is movable, and because the shape of the inframammary fold is preserved. In the case of a breast reconstruction, the tight skin closure of the modified mastectomy usually commits the patient to skin replacement using the complex TRAM breast reconstruction. The skin-sparing mastectomy affords the patient the option of having a simple method of breast reconstruction, such as a tissue expander or a latissimus flap. My question for Dr. Urist is this: Is there any evidence that demonstrates any curative advantage of wide skin excision over limited skin excision? To phrase it another way, should the skin-sparing mastectomy become the new gold standard mastectomy for the vast majority of patients that we treat today? Perhaps we need to reassess the need for universal wide skin excision in patients who need a mastectomy.
Dr. John M. Daly (New York, NY): The skin-sparing mastectomy certainly offers an improved cosmetic result for patients with breast cancer who are not candidates or do not choose lumpectomy and radiation. As pointed out, the question of local recurrence may have tempered enthusiasm for the use of this procedure. In addition, it is technically more difficult than a standard mastectomy, which uses a longer incision or removal of larger amounts of native skin. At Cornell, Dr. Rache Simmons and others have studied about 200 patients with breast cancer who have undergone mastectomy. Local recurrences occurred in about 3% of patients undergoing either procedure. Other investigators have reported slightly higher overall recurrence rates but similar results between either a standard or a skin-sparing procedure. The adequacy of axillary dissection appears to be similar between these groups, and local recurrence seems to be correlated, as said before, with tumor stage and differentiation. I have four questions for Dr. Urist.
First, are there any differences in wound healing and flap complications in either standard or skin-sparing procedures at your institution? Did any patients receive neoadjuvant therapy, and did this affect either flap healing or recurrence? If not, should neoadjuvant therapy be given to stage 2 patients who are poorly differentiated or have these risk factors that you identified in your univariant analysis? Third, what percent of patients at your institution now undergo a skin-sparing mastectomy if you look at the total population undergoing a mastectomy? Lastly, is diabetes or continued smoking a contraindication, and does this seem to affect flap failure or problems with wound healing in your procedure?
This was a terrific presentation, and I compliment all the authors for their work.
Dr. R. Phillip Burns (Chattanooga, TN): I too enjoyed this paper. Maybe I missed it, but would you please comment on how you handle the previous biopsy excision site, either needle biopsy or open excision, at the time of the performance of the mastectomy?
Dr. Marshall M. Urist (Birmingham, AL): I would like to thank the discussants for their insightful comments. Dr. Wood, with regard to the exclusion criteria, the one situation we would like to avoid is the use of postoperative irradiation in patients with reconstructions. A recent large series has shown that radiation compromises the cosmetic result in these patients. Patients who will need postoperative radiation should undergo skin-sparing mastectomy alone and delay the reconstruction for 6-12 months. Our guidelines for radiation include patients with 5 or more positive lymph nodes and tumors 4 cm or greater in diameter. Four of the eight patients who recurred in our series required radiation therapy. As everyone knows, it is not always possible to preoperatively predict tumor size or extent of lymph node involvement.
Dr. McCraw, I do not know of any good evidence to support the idea that the risk of local recurrence is related to the amount of preserved breast skin. We utilize skin-sparing mastectomy in patients who are not certain about having reconstruction and wish to delay the final decision about that procedure. Skin is excised when the tumor is in proximity to the skin surface. The trend in our institution has been toward preserving more skin even when reconstruction is not planned. While many patients are candidates for SSM alone, it does not work well in overweight patients who retain redundant skin folds which interfere with the fit and function of their prostheses.
Dr. Daly, I would estimate that 20% of patients who would be eligible for immediate reconstruction choose to undergo the operation. At least 80% of patients who have a consultation with a plastic surgeon decide to go forward with reconstruction. Although diabetes and smoking are associated with an increased risk of skin flap necrosis, we do not consider these factors to be a contraindication to reconstruction. We have not seen increased complications after neoadjuvant chemotherapy.
Dr. Burns, with regard to prior biopsy sites, we excise an ellipse of skin to incorporate site of potential tumor contamination. If a sentinel lymph node biopsy is being performed, a separate axillary incision is commonly utilized. This does not compromise cosmesis and may facilitate exposure for microvascular anastomoses.
Footnotes
Correspondence: Marshall M. Urist, MD, Suite 321 Kracke Building, 1922 Seventh Avenue South, Birmingham, AL 35294.
E-mail: mmurist@ccc.uab.edu.
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Accepted for publication December 2001.
References
- 1.Carlson GW, Bostwick J III, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997; 225: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroll SS, Schusterman MA, Tadjalli HE, et al. Risk of recurrence after treatment of early breast cancer with skin-sparing mastectomy. Ann Surg Oncol 1997; 4: 193–197. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo DA, Borgen PJ, Petrek JA, et al. Immediate reconstruction after complete skin-sparing mastectomy with autologous tissue. J Am Coll Surg 1998; 187: 17–21. [DOI] [PubMed] [Google Scholar]
- 4.Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol 1999; 6: 676–681. [DOI] [PubMed] [Google Scholar]
- 5.Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg 1999; 104: 77–84. [PubMed] [Google Scholar]
- 6.Kroll SS, Khoo A, Singletary SE, et al. Local recurrence after skin-sparing and conventional mastectomy: A 6-year follow-up. Plast Reconstr Surg 1999; 104: 421–425. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society, American Joint Committee on Cancer. AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott-Raven, 1997.
- 8.Khoo A, Kroll SS, Reece GP, et al. Comparison of resource cost of immediate and delayed breast reconstruction. Plast Reconstr Surg 1998; 101: 964–968. [DOI] [PubMed] [Google Scholar]
- 9.Donegan W, Perez-Mesa C, Watson FR. A biostatistical study of locally recurrent breast carcinoma. Surg Gynecol Obstet 1966; 122: 529–540. [PubMed] [Google Scholar]
- 10.Crowe JP Jr, Gordon NH, Antunez AR, et al. Local-regional breast cancer recurrence following mastectomy. Arch Surg 1991; 126: 429–432. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland NM, Barton RM, Copeland EM. The implications of local recurrence of breast cancer as the first site of therapeutic failure. Ann Surg 1983; 197: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberisk WJ, Silver B, Henderson IC, et al. The use of radiotherapy for treatment of isolated local-regional recurrence of breast carcinoma after mastectomy. Cancer 1986; 58: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 13.Halverson KJ, Perez CA, Kuske RR. Survival following loco-regional recurrence of breast cancer: Univariate and multivariate analysis. Int J Radiat Oncol Biol Phys 1992; 23: 285–291. [DOI] [PubMed] [Google Scholar]
- 14.Chen KK-Y, Montague ED, Oswald MJ. Results of irradiation in the treatment of loco-regional breast cancer recurrence. Cancer 1985; 56: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 15.Mendenhall NP, Devine JW, Mendenhall WM. Isolated local-regional recurrence following mastectomy for adenocarcinoma of the breast treated with radiation therapy alone or combined with surgery and/or chemotherapy. Radiother Oncol 1988; 12: 177–185. [DOI] [PubMed] [Google Scholar]
- 16.Toonken LM, Fix I, Jacobson LH. The significance of local recurrence of carcinoma of the breast. Int J Radiat Oncol Biol Phys 1983; 9: 33–39. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 1998; 5: 620–626. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GW. Local recurrence after skin-sparing mastectomy: A manifestation of tumor biology or surgical conservatism? Ann Surg Oncol 1998; 5: 571–572. [DOI] [PubMed] [Google Scholar]