Abstract
Objective
The aim of this study was to determine if a significant genetic component contributes to the pathogenesis of symptomatic gallstones.
Summary Background Data
Gallstones represent a polygenic disorder that affects more than 30,000,000 Americans and results in more than 750,000 cholecystectomies in the United States annually. Risk factors include age, gender, race, parity, obesity, and diabetes. A family history of gallstones also has been identified as a risk factor suggesting that genetics play a role in gallstone formation. However, the role of genetics in the pathogenesis of gallstone formation has not been determined.
Methods
A gallbladder disease-specific questionnaire was administered to 904 healthy unrelated adult volunteers (association study). The questionnaire ascertained a history of cholecystectomy and gallstone disease in first-degree relatives, as well as medical history, demographic, and anthropometric data. A logistic regression model was used to identify risk factors for symptomatic gallstone disease in a multivariate analysis. A maximum likelihood based variance decomposition approach was then used in 1,038 individuals from 358 families (family study) to estimate the additive genetic heritability of symptomatic gallstone disease.
Results
In the association study significant risk factors for symptomatic gallstone disease were female gender (relative risk 8.8, P < .003), obesity (BMI > 30, relative risk 3.7, P < .001), age > 50 (relative risk 2.5, P < .001), and a positive family history of previous cholecystectomy in a first-degree family member (relative risk 2.2, P < .01). In the family study the additive genetic heritability of symptomatic gallstones was 29% (P < .02), age and gender were significant covariates and explained 9.3% of the phenotypic variation in gallbladder disease.
Conclusions
These data suggest that genetic factors are responsible for at least 30% of symptomatic gallstone disease. However, the true role of heredity in gallstone pathogenesis is probably higher because data based on symptomatic gallbladder disease underestimates the true prevalence in the population.
Gallbladder disease represents a major healthcare problem in the United States. Approximately 12% of the U.S. population, or 30,000,000 Americans have gallstones. More then 750,000 cholecystectomies are performed each year, and the cost of caring for these patients is between 8 and 10 billion dollars annually. 1 Age, gender, race, obesity, diabetes, and parity have all been identified as significant risk factors for the development of gallstones. 2,3 Many of these patients also have a family history of gallstones, but surprisingly little is known about the link between genetics and gallstone disease in humans.
Approximately three-fourths of the patients with gallstones in the United States have stones that are composed primarily of cholesterol. The pathogenesis of cholesterol gallstones is known to be multifactorial, with the key factors including: 1) cholesterol supersaturated bile; 2) nucleation and growth of cholesterol monohydrate crystals; and 3) altered biliary motility. In addition, epidemiologic evidence, particularly ethnic differences in gallstone prevalence, family clustering, and twin studies suggest the importance of genetic factors that affect susceptibility to gallstone formation and gallbladder disease. 4
Gallstones are likely to result from a complex interaction of the environment and the effects of multiple undetermined genes. A possible genetic basis for gallstone disease has been suggested by studies documenting an increase in the incidence of gallstone disease in first-degree relatives of patients relative to controls. Van der Linden and Simonson 5 showed a 2.5:1 ratio favoring a familial occurrence of gallstones in a Swedish population. Gilat et al., 6 in a study of gallstone disease in Israel, demonstrated a nearly 2:1 ratio, while in India gallstones have been shown to be three times more frequent in family members of gallstone patients than controls. 7 In a twin study from Denmark, 14 of 25 monozygotic twins had concordance of clinically symptomatic gallstone disease, compared to 6 of 40 (same sex) and 0 of 36 (different sex) dizygotic twins. 8
In order to better define the role of genetic factors in human gallbladder disease and gallstone pathogenesis we have conducted two studies: 1) an association study on a group of unrelated individuals to determine if family history is a risk factor for gallbladder disease; and 2) a family study on a group of Midwestern white families to quantify the influence of genetic factors on symptomatic gallstone disease.
METHODS
Association Study
Subjects
Subjects for the association study were healthy unrelated adult volunteers. Subjects were asked to complete a gallbladder disease specific questionnaire detailing a history of gallstone disease, prior cholecystectomy, and a history of gallstone disease in first-degree family members. Subjects were considered to be affected if they answered yes to either of the following questions: 1) “have you had your gallbladder removed?”; or 2) “have you ever had a x-ray or ultrasound of your gallbladder that showed stones?” In addition, demographic and medical history data were collected. Trained technicians in all subjects recorded anthropometric data including height, weight, waist circumference, and hip circumference.
A total of 904 study subjects were evaluated between January 1999 and September 2000. The mean age was 52.6 ± 13.5 years (range 18–84 years). The study population was predominantly female and white. 798 (88%) subjects were female, and 106 (12%) were male. Ninety-six percent of the subjects were white. The mean weight was 87.6 ± 26.2 kg, and the mean body mass index (BMI) was 32.2 ± 9.8 kg/m2.
Statistics
Univariate and multivariate statistical analysis was performed using the SPSS statistical package (SPSS Inc, Chicago, IL). Chi Squared and Student t-test were performed as appropriate to determine possible risk factors. A P value of < .05 was considered significant. Variables investigated as possible risk factors for gallstones included age, gender, height, weight, body mass index (BMI), family history of gallbladder disease in a first-degree relative, history of diabetes, hypertension, dyslipidemia, coronary artery disease, cerebrovascular disease, tobacco use, and alcohol use. Variables found to be significant in the univariate analysis were placed into a logistic regression model to identify risk factors of symptomatic gallstone disease or gallbladder dysfunction.
Family Study
Subjects
Subjects for the family study were participants in The Metabolic Risk Complications of Obesity (MRC-OB) Genes project, which was initiated at the Medical College of Wisconsin in 1994 with the formulation of a nine-page questionnaire that collected information on family structure, health and behavior status, and detailed family and personal history of obesity and its health complications. These families were recruited from the TOPS (Take Off Pounds Sensibly, Inc.) membership. TOPS provided mailing material on membership attending its chapters in 10 states (Wisconsin, Illinois, Michigan, Iowa, Minnesota, Ohio, West Virginia, Missouri, Kentucky, and Indiana).
Questionnaire data received from 60,000 respondents were verified and entered into the TOPS Obesity and Metabolic Research Center databases at the Medical College of Wisconsin. Families with at least two obese siblings (body mass index [BMI] > 30 kg/m2), availability of one (preferably both) parents, as well as at least one never-obese sibling and/or parent (BMI < 27 kg/m2) were identified and contacted for ascertainment. Families were scheduled to visit satellites (4–6 per state), where an experienced team undertook the phenotypic procedures. A more detailed questionnaire garnered personal data (date of birth, race, marital status), health history (asthma, kidney or liver disease, hypertension, heart disease, stroke, hyperlipidemia, thyroid disorders, diabetes, medications, menopausal status, and hormonal replacement therapy), weight history (age of onset, maximum and minimum adult weight, dietary and exercise profiles), smoking and alcohol history, as well as family structure data. Exclusion criteria included pregnancy, type 1 diabetes mellitus, history of cancer, renal or hepatic disease, severe coronary artery disease, substance abuse, corticosteroids or thyroid medications above replacement dose, and history of weight loss of more than 10% of body weight in the preceding 12 months.
2,209 individuals distributed across 507 white families have subsequently undergone genotyping using the Weber screening set 9 (Research Genetics, Huntsville, AL). These individuals were subsequently mailed the same gallbladder disease specific questionnaire detailing a history of gallstone disease, prior cholecystectomy, and a history of gallstone disease in first-degree family members as was administered to the subjects in the association study. Forty-seven percent of the subjects responded to the questionnaire. Data for the genetic analyses presented here include 1,038 individuals distributed across 358 white families of predominantly northern European ancestry and residing in Midwestern states. Research protocols were approved by the Institutional Review Board of the Medical College of Wisconsin.
Genotyping
Whole blood was obtained from all consenting family members for DNA extraction. DNA was prepared by using commercial kits (Puregene, Gentra Systems, Minneapolis), which use a nonphenol-based method involving RNase A treatment. The DNA samples were stored in aliquots at 4°C, and back-up DNA samples were stored in ethanol at -70°C. Additional whole blood aliquots were stored at -70°C for further DNA extraction. More than 200 (g genomic DNA with a 260/280 ratio of 1.8 to 2.1 was available from each family member.
Genotyping was performed at the Marshfield (Wisconsin) Medical Research Foundation by using the Weber screening set 9 (Research Genetics, Huntsville AL). This procedure used 387 markers, representing tandem repeat polymorphisms, including 366 autosomal markers, as well as 17 X-linked and 4 Y-linked markers, and yielded an average map density of 10 cM. Initial analyses included validation of the reported relationships between individuals, checked by calculating likelihoods of the relationships based on the autosomal genotype data. The genotypic data also were examined for Mendelian inconsistencies, and those genotypes proven to be inconsistent were removed. The autosomal genotype data were 97.6% complete. The average (±SD) heterozygosity of these markers used was .79 ± .06, and the sex-averaged genetic spacing was 9.1 ± 3.8 cM. DNA was screened by using fluorescently labeled primers from Research Genetics. The PCR assay mixture contained 45 ng DNA, 0.075 (mol/L fluorescently labeled primers, 0.12 units AmpliTaq Polymerase (Sigma), 100 (mol/L each dNTP, 25 mmol/L MgCl2, and buffer. PCR conditions included 27 cycles of denaturation (95°C for 30 seconds), annealing (55°C for 75 seconds), and elongation (72°C for 30 seconds), followed by a final 6-minute elongation period. Samples were analyzed through automated high-throughput scanning fluorescence detectors, each simultaneously detecting three separate dyes.
Statistics
All quantitative genetic analyses were conducted using the maximum likelihood based variance decomposition approach implemented in the computer package SOLAR. 9 This approach, which was developed following methodology originally proposed by Hopper and Mathews, 10 and has been described in detail elsewhere. 9 In short, we used this approach to partition the phenotypic variance (ςP2) into components corresponding to the additive genetic (ςG2) and non-genetic – i.e., environmental –(ςE2) effects. Because these components are additive, such that ςP2 = ςG2 +ςE2, we estimate the heritability, or proportion of the phenotypic variance attributable to additive genetic effects, as h2 =ςG2 /ςP2. We estimate the proportion of the phenotypic variance attributable to nongenetic (environmental) factors as e2 = 1-h 2. In addition to these terms, we also simultaneously estimated the mean effects of sex, sex-specific age and other significant covariates (obesity, menopause, current oral estrogen use, overweight during puberty, overweight during adolescence, smoking, diabetes, and hypertension).
Significance of the maximum likelihood estimates for heritability and other parameters were assessed by means of likelihood ratio tests. The likelihood for the general model in which all parameters were estimated are compared to the likelihood for restricted models in which the value of the parameter to be tested will be held constant at zero. Twice the difference in the natural log (ln) likelihoods of the two models to be compared is distributed approximately as a 1/2:1/2 mixture of χ2 with one degree of freedom and a point mass at zero.
RESULTS
Association Study
Two hundred sixteen subjects (24%) reported a history of prior cholecystectomy or gallstones by radiologic imaging study. The subjects with a history of symptomatic gallstones were significantly older than those subjects with no history of gallstones (58.2 ± 10.1 vs. 50.9 ± 14.0 years, P < .001) and were more obese (BMI 37.6 ± 8.0 vs. 30.5 ± 9.6 kg/m2, P < .001). Three hundred fifty-seven subjects (39%) reported a family history of cholecystectomy in a first degree relative.
Results for a univariate analysis of the risk factors for a history of symptomatic gallstone disease are shown in Table 1 Variables found to be significantly related to a history of cholecystectomy included age, obesity (BMI > 30), female sex, history of gallstone disease in a first degree relative (Fig. 1), and a history of diabetes, dyslipidemia, heart disease, cerebrovascular disease, or hypertension. A history of alcohol consumption showed a decrease in the prevalence of gallbladder disease in this analysis.
Table 1. UNIVARIATE ANALYSIS OF RISK FACTORS FOR SYMPTOMATIC GALLSTONE DISEASE
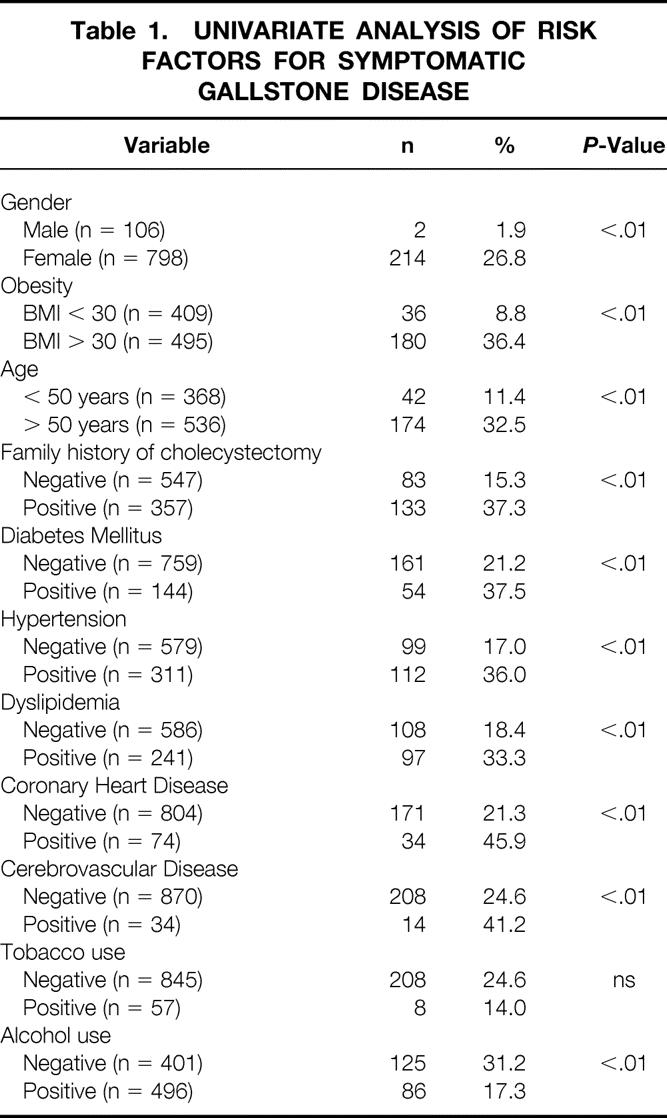
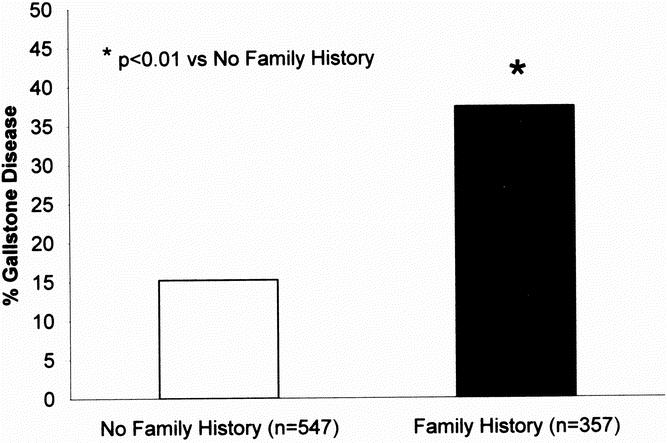
Fig. 1. Prevalence of gallstone by family history in the association study.
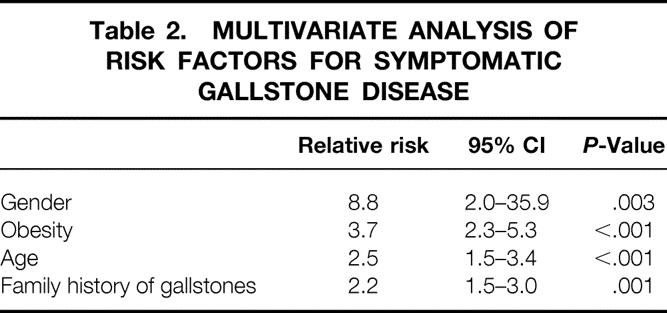
Table 2 shows the results of the multivariate analysis of risk factors for symptomatic gallbladder disease. All risk factors identified in the univariate analysis were entered into a logistic regression model. Female sex was the strongest risk factor with a relative risk approaching 9.0. In addition, obesity (BMI > 30), age (> 50years), and a positive family history of gallbladder disease in a first-degree relative were also found to be significant risk factors.
Table 2. MULTIVARIATE ANALYSIS OF RISK FACTORS FOR SYMPTOMATIC GALLSTONE DISEASE
Family Study
Data on prior cholecystectomy for gallbladder disease were available for 1,038 individuals who participated in the MRC-OB genes project. Two hundred and thirteen subjects (21%) were male and 825 subjects (79%) were female. The mean age of the population was 49.8 ± 14.2 years (54.6 ± 15.5 years in males and 48.6 ± 13.5 years in females). As shown in Figure 2, the prevalence of symptomatic gallstone disease in the family study was significantly greater (P < .01) in females as compared to males, 28% versus 6.6% respectively. Table 3 shows the results of gender, age, obesity (BMI), and family history of cholecystectomy in a first-degree relative stratified by gallstone disease status. Subjects with symptomatic gallstones were more likely to be female (P < .05), older (P < .05), heavier (P < .05) and to have a positive family history (P < .05).
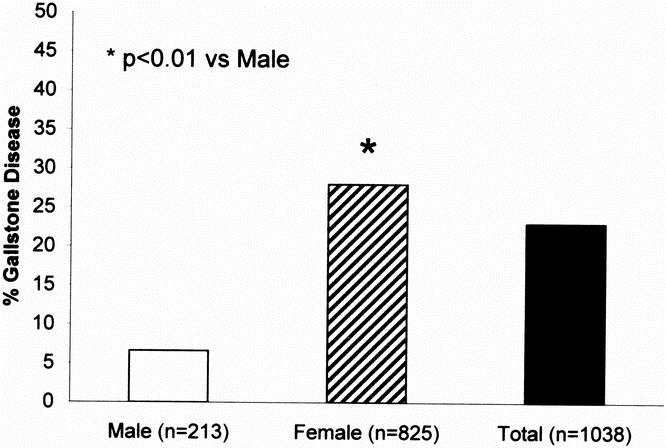
Fig. 2. Prevalence of gallstone disease in the family study.
Table 3. FAMILY STUDY CLINICAL CHARACTERISTICS
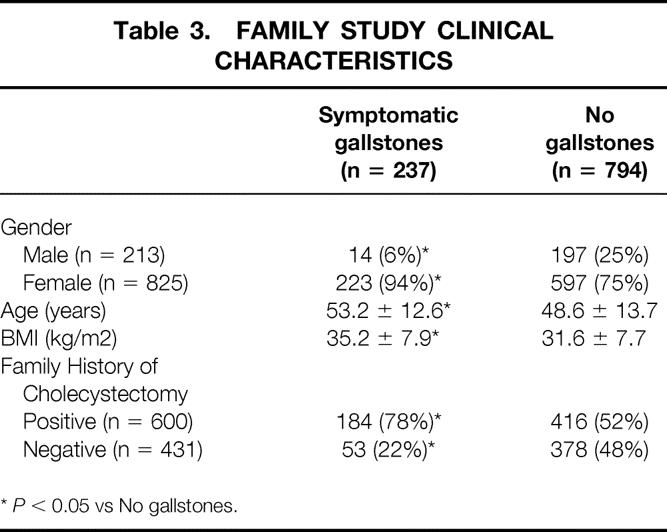
*P < 0.05 vs No gallstones.
The additive genetic heritability (h2) of gallbladder disease in this population is .29 ± .14 (P = .013), indicating that a substantial portion of the variations in gallbladder disease is due to the additive effects of genes. In this model, age and gender were found to be significant covariates and explained approximately 9.3% of the phenotypic variation in symptomatic gallbladder disease.
DISCUSSION
Symptomatic gallbladder disease is common in the United States and results in approximately 750,000 cholecystectomies per year. The vast majority of cholecystectomies are performed for gallstone disease with cholesterol gallstones accounting for nearly 75% of all gallstones. Supersaturation of cholesterol in bile, the nucleation and growth of crystals in the gallbladder, and gallbladder dysmotility are important factors in gallstone pathogenesis. Gallstones are likely to result from a complex interaction of the environment and the effects of multiple undetermined genes. In this study we have investigated potential risk factors for symptomatic gallbladder disease using a gallbladder disease specific questionnaire. In a predominantly white, female population of 904 unrelated adults, 24% of the subjects reported a history of previous cholecystectomy. A multivariate analysis of this population revealed that female gender, obesity (BMI > 30), age, and a positive family history of gallbladder disease in a first-degree relative to be significant risk factors for symptomatic gallstone disease. A subsequent investigation of more than 1,000 individuals from 358 families demonstrated a prevalence of prior cholecystectomy or gallstones of 23%. Using a maximum likelihood based variance decomposition approach, the additive genetic heritability was approximately 30% for symptomatic gallstone disease.
Most epidemiologic studies in the Western literature have shown that females have a higher frequency of gallbladder disease then males. The female to male ratio of gallstone and gallbladder disease approaches 4:1 in younger subjects (< 40 years of age). 3,11 The female to male ratio decreases to 2:1 in older age groups. 3,12–14 Both the prevalence and incidence of gallstone disease increases with age. 3,12–14 Gallstones are seldom found in prepubertal children and are rare in adolescent whites. Less than 5% of the cholecystectomies performed are in patients less than 20 years of age. 15,16 In the Sirmione study only 1 of 135 in the 18 to 21 year old subgroup had gallstones demonstrated by ultrasonography. Both the GREPCO 12,13 and the Sirmione 14 studies found gallstone prevalence rates that increase linearly with advancing age. An examination of the Third National Health and Nutrition Examination survey (NHANES III) 3 has shown the prevalence of both symptomatic and asymptomatic gallbladder disease in men to increase from 1.3% in the 20–29 year age group to 25.3% in the 60–74 year age group. Similarly, the prevalence of gallbladder disease in females increased from 6.5% in the 20–29 year age group to 33.1% in the 60–74 year age group.
Obesity dramatically increases the risk of gallstone formation, especially in females. 3,17–19 In a large population study, Heaton et al. 20 have shown that a high waist-hip ratio predicts gallstones in men, whereas a high BMI and high weight gain after age 20 do not. In the Framingham study, the clinical risk increased two-fold in those women 20% above the median weight. 11 In the large ultrasonographically surveyed Copenhagen cohort (n = 5581), Jorgensen 21 found that gallstone prevalence correlated positively with body mass index in women but not in men, a feature also seen in the GREPCO 13 study of Roman civil servants and in West Britain. 20 In the Sirmione study, 22 gallstone prevalence was significantly higher in obese subjects of younger age, and incidence rates over 5 years of follow-up were three times higher in obese than in nonobese subjects. In Texas, most Mexican-American teenagers with cholesterol stones were found to be markedly obese. 23 In the large MICOL study of 29,584 individuals carried out on 14 cohorts between 1984 and 1987 in Italy, 17 obesity proved to be one of the most consistent associations with gallstones in both males and females.
A well-established pathophysiologic link between obesity and gallstone formation is cholesterol-supersaturated bile. 24,25 Obese people hypersecrete biliary cholesterol, bile salts, and phospholipids, but the rate of cholesterol secretion supersedes that of the other biliary lipids, leading to cholesterol-supersaturated bile. 26 Weight reduction aggravates this phenomenon, as hepatic stores of cholesterol are mobilized, bile salt synthesis is decreased, and normal gallbladder emptying is interrupted, leading to further supersaturation of bile and rapid gallstone formation. 27,28 In theory, increased flux of cholesterol from the bile into gallbladder muscle cells stiffens the sarcolemmal membranes, decouples signal transduction, and inhibits gallbladder muscle function. 29,30 However, ultrasound data on gallbladder volume and emptying in obese humans are conflicting. 31,32 While most studies suggest increased resting gallbladder volume in obese subjects, some reports demonstrate that these large gallbladders empty normally. 31
The association between gallbladder disease and family history has been hypothesized since the mid 1900s. Jackson and Gay 33 investigated the family members of 100 consecutive white patients undergoing gallbladder surgery utilizing oral cholecystography and cholecystectomy. For 72% of the families, definite or suggestive evidence of gallbladder disease was obtained in a sibling, parent, or offspring. Because of an observed 3:1 female-to-male ratio in the study, they suggested that gallstone disease may be inherited as a sex-linked dominant trait. In a cross-sectional ultrasonographic study of a large cohort in Copenhagen (n = 4,581), Jorgensen 34 found a 2:1 positive association between gallstone disease in first degree relatives of subjects with gallstones, irrespective of the symptom status of the patients. In a 430 pair subset of the Sirmione study, 14,22,35 the relative risk of gallstones was 3.3-fold higher in sons and daughters of parents with gallstones when controlled for age and other confounding variables.
Nürnberg et al. 36 examined by ultrasonography 1,616 symptom-free patients, mostly female, aged 12 to 93 years with gallstones in Northern Germany. They found a threefold increased family prevalence of gallstones in the younger age groups, compared with stone-free controls. Recently, a similar familial frequency of gallstones was found by Kratzer et al. 37 among 1,116 young-to-middle-aged healthy blood donors who were mostly men. In the Italian MICOL study, 17 subjects with a history of gallstones in either parent had an increased risk of having gallstones; the risk was significantly higher when both parents had gallstone disease. The present analysis is consistent with these earlier studies in that a 2.5:1 incidence of symptomatic gallstones was found in both the association and family studies.
Willem van der Linden in Northern Sweden did very useful work on the familial occurrence of gallstones during the 1960s and 70s. He attempted to sort out diagnostic and ascertainment bias with respect to control groups, as well as verifying the presence of stones by oral cholecystography or cholecystectomy. Van der Linden and Lindelof 5 restricted their investigation to proven gallstone patients who were married and who had siblings of the opposite sex. If the patient was a man, the occurrence of gallstones in his wife was compared with that of his sister nearest in age to his wife. If the patient was a woman, her husband was compared with her brother nearest in age to her husband. Employing this approach, 263 individuals (87 males, 176 females—mean ages 49 and 51 years, respectively) were evaluated rigorously for gallstones. The data showed a clear 2:1 ratio in favor of a familial occurrence for gallstone disease. Because familial occurrence does not necessarily reflect genetic factors, van der Linden and Westlin 38 then studied the occurrence of gallstone disease in spouses who had lived together continuously and presumably shared the same environment, especially diet, during adult life. They used similar methodology and found that women married to patients with gallstone disease do not suffer more often from the disease than do other women.
The impact of heredity has been corroborated in three large, carefully controlled prevalence studies (by cholecystography/surgery/ultrasonography) from Sweden, 39 Israel 6 and North India. 7 In each case, the data showed significantly higher, (≥2:1) prevalence rates of gallstones in the first-degree relatives of the probands compared with controls. Hanis et al. 23 also established in Mexican-Americans that the probability of an individual with gallbladder disease having a first degree relative similarly affected was 1.8 times the random probability.
Several anecdotal reports of concordance of cholesterol gallstones in small numbers of monozygous twins have been published. In a large Danish study, 8 1,900 unselected twin pairs born between 1870 and 1910 were sent a questionnaire, which requested that they describe “any and all admissions to hospital, where, when and for what condition.” Diagnosis of gallstones was obtained from the chart descriptions of the hospitalized cases. In the entire cohort, the crude incidence of cholelithiasis was 2.6%. Of a total of 101 twin pairs with a hospital diagnosis of cholelithiasis, concordance for the disease was found among 14/25 monozygotic pairs compared with 6/40 (same sex) and 0/36 (different sex) dizygotic pairs. Zero concordance among white twins of different sex most likely reflects the different frequencies of symptomatic cholelithiasis between men and women. Further, if silent gallstone cases were ascertained, the actual frequency and concordance of gallstones might have been much more impressive. Kesaniemi et al. 40 randomly selected male twins (17 monozygotic and 18 dizygotic pairs) from the Finnish Twin Cohort. The males were living apart but residing in the Helsinki area. Their ages ranged from 43 to 58 years (mean 50) and mean body weight was 78 kg. Employing oral cholecystography and history of cholecystectomy, gallstones were ascertained in seven monozygotic and three dizygotic subjects, with two monozygotic and none of the dizygotic twin pairs being concordant for gallstones giving 40% pairwise concordance for the former and 0% for the latter. These studies, albeit imperfect, constitute the best information on familial occurrence and monozygotic twin concordance for gallstones.
While epidemiologic studies and the increased risk of a positive family history of gallbladder disease in a first degree relative suggest a role of genetic factors for gallbladder disease, direct evidence for the genetic determination of gallbladder disease is limited. In a recent study by Duggirala et al. 41 of 579 individuals from 32 Mexican-American families, questionnaire data on prior cholecystectomy was used to determine gallbladder disease status. Using a maximum likelihood-based variance decomposition approach the heritability of symptomatic gallbladder disease was estimated to be 44% ± 18%. This heritability is similar to the 29% ± 14% we have reported in our family study of midwestern white families. Similar to our model, age and gender were also found to be significant covariates in this Mexican–American study.
Our findings should be interpreted with caution for the following reasons. First, because obesity is a significant risk factor for gallbladder disease and the subjects in our family study have been ascertained by an obese proband, the prevalence of gallbladder disease in the study population may be higher than the general population. Conversely, it is likely that some of the genetic factors responsible for obesity may also play a significant role in gallbladder disease. Second, gallbladder disease status was defined by a self-reported clinical history of cholecystectomy, rather than an ultrasound examination. Therefore, the reported prevalence of gallbladder disease is underestimated as two thirds of patients with gallstones are asymptomatic. Our estimate of heritability may be low because it is based on symptomatic gallstone disease. Thus, the potential overestimate due to obesity may have been balanced by an underestimate of studying only symptomatic gallstone disease. Because our model did not account for any shared environmental (non-genetic factors) among family members such as diet, our estimates of heritability may be elevated. Moreover, heredity has recently been shown to contribute to approximately one-third of the pathogenesis of other polygenic disorders such as obesity, hypertension and diabetes. As a result, our estimate that genetic factors are responsible for approximately 30% of symptomatic gallstones is consistent with current theories on the pathogenesis of polygenic diseases.
Discussion
Dr. Keith D. Lillemoe (Baltimore, Maryland): I would like to congratulate Drs. Pitt, Nakeeb, and their co-authors for an excellent analysis and fine presentation. It appears that Dr. Pitt and his co-workers have added a fifth ‘F‘ to the four ‘Fs‘ that we learned in medical school as risk factors for gallstone disease. To ‘fat, forty, female, and fertile‘ they have now added ‘family history.‘ Clearly their analysis of these two groups of patients has provided strong evidence that having a first-degree relative with gallstone disease is a significant risk factor to develop with this problem in the patients.
I would like to ask a few questions, however, that may require Dr. Nakeeb to do some speculation. Recognizing the strong influence of female gender as a risk factor as well as the strong preponderance of females in their study population, I would like to ask if they think that the familial risk factor for gallstone disease is gender specific; that is, are males at as much risk for the development of gallstones due to familial factors as are females?
Second, is it really possible even with your sophisticated analysis and razzle-dazzle statistical techniques to separate the familial risk factors from those of obesity reflecting similar eating patterns in the homes of patients shared with first-degree relatives with gallstone disease?
Finally, although I know it is impossible to tell from your data analysis, is it your best guess that this reflects primarily cholesterol gallstone disease, or do you think this is likely through the entire spectrum of different types of gallstones?
Thank you again for letting me review the manuscript and thank you to the Society for the opportunity to discuss the paper.
Dr. J. Patrick O’leary (New Orleans, Louisiana): I am concerned that certain risk factors such as pregnancy, certainly specific to the female gender, may not have been appropriately evaluated in this study. Being with child and the hormonal changes that go along with pregnancy predisposes the woman to the development of a nidus in the gallbladder that ultimately may become a stone. We weren’t appraised of any evaluation with regard to pregnancy or the number of pregnancies being addressed. I wonder if that might have impacted on the data.
Dr. Susan Galandiuk (Louisville, Kentucky): I have a question regarding a similar comment of Dr. Lillemoe. With Association studies there is always a significant risk of bias due to population substructure. It seems as if you are selectively analyzing a group of patients from T.O.P. centers where patients were trying to lose weight and were more frequently women. Do you think that this may have biased your results? And have there been any genetic studies, linkage studies or any kind of transmission disequilibrium studies on these patients?
Dr. Bruce D. Schirmer (Charlottesville, Virginia): One question about the obesity aspect with gallstone formation. We know with weight reduction surgery and with severe diets that if patients lose weight rapidly they will form gallstones. I wonder if the questionnaire in any way looked at whether or not patients had lost weight as part of their obesity. It has been my impression in dealing with obese patients over the years that the ones that have lost weight in a yo-yo fashion have a higher chance of having gallstones than the ones that didn’t.
Dr. Attila Nakeeb (Milwaukee, Wisconsin): Thank you, Dr. Carey and Dr. Townsend and the Association, for the opportunity to present our work. I would like to thank all of the discussants for their insightful comments.
I’ll address Dr. Schirmer’s question first in terms of the procedural aspect of our study. We excluded all patients who lost greater than 10% of their weight in the last year in order to eliminate that bias in both the family and the Association study. In terms of Dr. Lillemoe’s question, is the phenomenon gender specific? The answer is, no, it is not. We have seen similar increases in family history prevalence data in both the males and the females. Unfortunately our population again was heavily skewed to females because they were the ones who responded to our survey studies and would come in for the testing that was required. However, in the percentage of males who did, we were able to investigate that there was similar family history data in terms of prevalence rates.
In sorting out the genetic versus environmental factors, it is a little bit more complicated. In this kind of statistical analysis, the premise is based on having good family structure data, being able to do genotyping in the patients to look at the alleles that are brought down from parents throughout the generations, and to actually track the alleles that get transmitted. The analysis basically lumps the study genetic alleles and factors. Those are the groups that are characterized as the heritable component. The environmental component really gives a much broader sense and really is more of a nongenetic component and really takes into account environmental factors as well as unstudied genetic factors as well. Unfortunately, we don’t have a lot of data on diets and things like that that are shared within the families.
Again, one of the limitations of the study is that it is survey data basically looking at symptomatic gallbladder disease. There is no way to differentiate between cholesterol and pigment gallstones. We know at least in the obese population that cholesterol gallstones are more common. They account for 75% of all the gallbladder disease in this country. So we think that most of these stones are likely to be cholesterol stones.
For Dr. O’Leary, again a very excellent question. Again, parity is going to be – it has been known as one of the four risk factors for gallbladder disease. Unfortunately, in our Association study, we were not set up to look at parity issues, so we have limited data on that. In the family study, however, we do have good data on parity, as well as supplemental estrogen and hormonal replacement therapies. Those were taken into the genetic models and were not found to be significant covariants for this.
In terms of looking at other different deliberium and genetic methods for looking at the study, there has not been any real work done in looking at different deliberium studies in gallstone disease particularly. Some of this has been done in the obesity population, and our co-workers have actually identified four different genes that are associated with obesity.
The long-term goal of this project is to investigate and identify those genetic factors. This population is ideal for studying the genetics of gallstone disease in that it is a population that we have been able to characterize and do genotyping on 2,000 of these individuals. We have good family structure data. And, we are currently going back to look at some of the pre-gallstone phenotypes, such as gallbladder ejection faction, number of stones, to further dissect out which genes are actually involved in this process.
Footnotes
Supported by a grant from the Medical College of Wisconsin Digestive Disease Center.
Correspondence: Attila Nakeeb, MD, Department of Surgery, 9200 W. Wisconsin Ave., Milwaukee, WI 53226.
E-mail: anakeeb@mcw.edu
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Accepted for publication December 2001.
References
- 1.Graves EJ, Owings MF. 1995 Summary: National Hospital Discharge Survey. Advance data from vital and health statistics; no. 291. National Center of Health Statistics, 1997. [PubMed]
- 2.Paigen B, Carey MC. Gallstones. In: The Genetic Basis of Common Diseases, King, Rotter, Motulsky, eds. 2nd Edition. 2001.
- 3.Everhart JE, Khare M, Hill M, et al. Prevalence and ethic differences in gallbladder disease in the United States. Gastorenterology 1999; 117: 632–639. [DOI] [PubMed] [Google Scholar]
- 4.Lammert F, Carey MC, Paigen B. Chromosomal organization of candidate genes involved in cholesterol gallstone formation: a murine gallstone map. Gastroenterology 2001; 120: 221–238. [DOI] [PubMed] [Google Scholar]
- 5.Van der Linden W, Lindelof G. The familial occurrence of gallstone disease. Acta Genet Basel 1965; 15: 159–164. [PubMed] [Google Scholar]
- 6.Gilat T, Feldman C, Halpern Z, et al. An increased familial frequency of gallstones. Gastroenterology 1983; 84: 242–246. [PubMed] [Google Scholar]
- 7.Sarin SK, Negi VS, Dewan R, et al. High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology 1995; 22: 138–141. [PubMed] [Google Scholar]
- 8.Harvald B, Hauge M. A catamnestic investigation of Danish twins: a preliminary report. Danish Med Bull 1956; 3: 151–158. [PubMed] [Google Scholar]
- 9.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62: 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet 1982; 46: 373–383. [DOI] [PubMed] [Google Scholar]
- 11.Friedman GD, Kamel WB, Dawber TR. The epidemiology of gallbladder disease: Observations in the Framingham Study. J Chron Dis 1966; 19: 273–292. [DOI] [PubMed] [Google Scholar]
- 12.GREPCO (Rome Group for the Epidemiology and Prevention of Cholelithiasis). Prevalence of gallstone disease in in an Italian adult female population. Am J Epidemiol 1984; 119: 796–805. [PubMed] [Google Scholar]
- 13.GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part I Prevalence data in men. Part II The epidemiology of gallstone disease in Rome, Italy. Hepatology 1988; 8: 904–913. [PubMed] [Google Scholar]
- 14.Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: The Sirmione study. Hepatology 1987; 7: 913–917. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese C, Pearlman DM. Gallbladder disease below the age of 21 years. Surgery 1971; 70: 413–417. [PubMed] [Google Scholar]
- 16.Honore LH. Cholesterol cholelithiasis in adolescent females. Arch Surg 1980; 115: 62–64. [DOI] [PubMed] [Google Scholar]
- 17.Attili AF, Capocaccia R, Carulli N, et al. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian study on epidemiology of cholelithiasis. Hepatology; 1997; 26: 809–818. [DOI] [PubMed] [Google Scholar]
- 18.Heaton KW, Braddon FEM, Mountford RA, et al. Symptomatic and silent gallstones in the community. Gut 1991; 32: 326–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diehl AK. Epidemiology and natural history of gallstone disease. Gastro Clin N Am 1991; 20: 1–19. [PubMed] [Google Scholar]
- 20.Heaton KW, Braddon FEM, Emmett PM, et al. Why do men get gallstones? Roles of abdominal fat and hyperinsulinemia. Eur J Gastroenterol Hepatol 1991; 3: 745–751. [Google Scholar]
- 21.Jorgensen T. Gallstones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption and diabetes mellitus. Gut 1989; 30: 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sama C, Morselli-Labate AM, Taroni F, et al. Epidemiology and natural history of gallstone disease. Semin Liver Dis 1990; 10: 149–158. [DOI] [PubMed] [Google Scholar]
- 23.Hanis CL, Ferrell RE, Tulloch BR, et al. Gallbladder disease epidemiology in Mexican Americans in Starr County, Texas. Am J Epidemiol 1985; 122: 820–829. [DOI] [PubMed] [Google Scholar]
- 24.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease: The effect of cholecystectomy and obesity. J Clin Invest 1977; 59: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaral JF, Thompson WR. Gallbladder disease in the morbidly obese. Am J Surg 1985; 149: 551–557. [DOI] [PubMed] [Google Scholar]
- 26.Carey MC, Small DM. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest 1978; 61: 998–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiffman ML, Sugerman HJ, Kellum JM, et al. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol 1991; 86: 1000–1005. [PubMed] [Google Scholar]
- 28.Shiffman ML, Sugerman HJ, Kellum JM, et al. Changes in gallbladder bile composition following gallstone formation and weight reduction. Gastroenterology 1992; 103: 214–221. [DOI] [PubMed] [Google Scholar]
- 29.Yu P, Chen Q, Harnett KM, et al. Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol 1995; 269: G659-G665. [DOI] [PubMed] [Google Scholar]
- 30.Behar J, Lee KY, Thompson WR, et al. Gallbladder contraction in patients with pigment and cholesterol stones. Gastroenterology 1989; 97: 1470–1484. [DOI] [PubMed] [Google Scholar]
- 31.Vezina WC, Paradis RL, et al. Increased volume and decreased emptying of the gallbladder in large (morbidly obese, tall normal, and muscular normal) people. Gastroenterology 1990; 98: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 32.Acalovschi M, Badea R. Ultrasonographic study of gallbladder emptying in obese patients. Int. J. Obesity 1992; 16: 313–315. [PubMed] [Google Scholar]
- 33.Jackson CE, Gay BC. Inheritence of gallbladder disease. Surgery 1959; 40: 853–857. [PubMed] [Google Scholar]
- 34.Jorgensen T. Gallstones In a Danish population: Familial occurrence and social factors. J Biosoc Sci 1988; 20: 111–120. [DOI] [PubMed] [Google Scholar]
- 35.Progetto S, Barbara L, Festi D, et al. The Sirmone Study: Familial frequency of gallstone disease. Hepatology 1984; 4: 1086. [Google Scholar]
- 36.Nurnberg D, Berndt H, Pannewitz H. Familare Haufung von Gallensteinen. Dtsch Med Wschr 1989; 114: 1059–1063. [DOI] [PubMed] [Google Scholar]
- 37.Kratzer W, Kachele V, Mason RA, et al. Gallstone prevalence in Germany: the Ulm Gallbladder Stone Study. Dig Dis Sci 1998; 43: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 38.Van der Linden W, Westlin N. The familial occurrence of gallstone disease II. Occurrence in husbands and wives. Acta Genet Basel 1966; 16: 377–382. [DOI] [PubMed] [Google Scholar]
- 39.Van der Linden W, Simonson N. Familial occurrence of gallstone disease: Incidence in parents of young patients. Hum Hered 1973; 23: 123–127. [DOI] [PubMed] [Google Scholar]
- 40.Kesaniemi YA, Koskenvuo M, Vuoristo M, et al. Biliary lipids composition in monozygotic and dizygotic pairs of twins. Gut 1989; 30: 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duggirala R, Mitchell BD, Blangero J, et al. Genetic determinants of variation in gallbladder disease in Mexican-American population. Genetic Epidemiol. 1999; 16: 191–204. [DOI] [PubMed] [Google Scholar]


