Abstract
Objective
To identify the risk of systemic metastases from T1a and T1b N0 breast cancers in patients treated in an academic center, and to seek factors to identify the patients at greatest risk of such failure.
Summary Background Data
With the demonstration that adjuvant chemotherapy reduces the death rate from breast cancer by roughly one quarter across all risk groups, controversy has reigned regarding possible exclusions from therapy. T1a and T1b N0 tumors (1-cm diameter and smaller with negative axillary nodes) have been considered at low risk for metastasis since the report from Memorial Sloan-Kettering Cancer Center of a 90% survival rate at 10 years. Subsequent reports have suggested an even more favorable prognosis for this group. However, consensus statements advise selecting some of these women for treatment, and many do receive adjuvant chemotherapy.
Methods
Sequential patients with breast cancer at the Emory Clinic were prospectively staged and followed up for outcome. The records of patients with T1a and T1b N0 tumors were reviewed for exact tumor diameter, grade, receptor status, adjuvant therapy, and outcome. A corrected data set was stripped of patient identifiers and analyzed by Kaplan-Meier methods. Subgroups were formed based on tumor grade (1 vs. 3), adjuvant chemotherapy (use vs. no use), and adjuvant tamoxifen (use vs. no use) and were compared via log-rank tests.
Results
Two hundred eighty-two women were identified. Two developed metastatic disease and one experienced a local failure after breast-conserving treatment. The estimated disease-free survival rate at 10 years was 98.7%. With only two distant failures and one local failure, there was no significant difference by grade, receptor status, or use of adjuvant chemotherapy or tamoxifen.
Conclusions
The risk of systemic failure from such tumors barely exceeded 1% at 10 years. Unless future studies can identify a subgroup at higher risk, the cognitive changes associated with cytotoxic chemotherapy or the loss of estrogen involved do not appear to have sufficient offsetting benefit to warrant chemotherapy for this group of women.
With results from international overviews of breast cancer adjuvant therapy showing reduced deaths by roughly one quarter across all risk groups, 1–4 controversy has reigned regarding possible exclusions from therapy. T1a and T1b N0 tumors (1-cm diameter and smaller with negative axillary nodal staging) have been known to have a relatively low risk of systemic failure since the report from Memorial Sloan-Kettering Cancer Center in 1989. Rosen et al. 5 cited a 90% 10-year survival rate for this group of patients with mastectomy alone. More recent reports from Europe have suggested much better survival for these patients, 6 yet reviews from patients in cooperative group trials have been conflicting, with higher failure rates. The controversy has been reflected in the recommendations for adjuvant therapy made by consensus development panels. The NIH Consensus Statement of 2000 advocated tamoxifen for this subgroup if they were receptor positive; if receptor negative, “the decision to consider chemotherapy should be individualized.”7 The St. Gallen Consensus Statement of 2001, done 3 months later, advocated chemotherapy for this subgroup if hormone receptor negative, histologic grade 2 to 3, or age younger than 35 years, placing them in the “average/high risk” category. 8
Considerations of adjuvant therapy balance possible risk against possible benefit. The benefit of adjuvant therapy is now understood to be a relative reduction in risk of recurrence that is therapy-specific. This reduction must be related to the absolute risk. We addressed two questions: What is the risk of systemic or local failure with breast cancers 1 cm in diameter or less, and can a higher-risk subgroup be identified among this group?
METHODS
Patients with breast cancer at the Emory Clinic were prospectively staged and followed up for outcome. They were unselected except by referral to the institution. Two hundred eighty-two consecutive women with T1a or T1b breast cancer were pathologically staged as node negative by either axillary dissection or sentinel lymph node biopsy and followed up for outcome. The mean follow-up was 7 years. Forty-eight percent had breast-conserving therapy and 52% had mastectomy. They were reviewed for microscopic diameter of the invasive component of the carcinoma, histologic grade, receptor status, use of adjuvant therapy, and outcome. In accordance with a protocol approved by the University’s Human Investigations Committee, a corrected data set was stripped of patient identifiers and analyzed by Kaplan-Meier methods. Grade 1 versus 3 and the use of adjuvant systemic chemotherapy or adjuvant tamoxifen were assessed via log-rank tests to compare survival curves.
RESULTS
The mean tumor diameter was found to be 0.75 cm, the median 0.8 cm. The percentage distribution is shown in Table 1. Among the women treated with breast conservation, there was one local failure, a less than 1% rate of ipsilateral breast tumor recurrence. Two women developed metastatic breast cancer. The estimated distant disease-free survival rate at 10 years was 98.7%.
Table 1. DIAMETER OF INVASIVE PORTION OF TUMORS
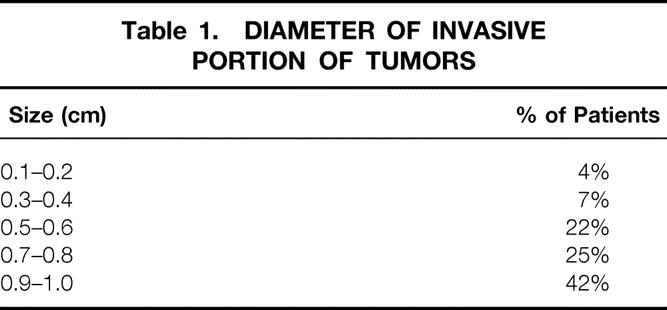
One of the tumors that metastasized was invasive lobular carcinoma, 0.7 cm in diameter, grade 2 of 3, 0 of 26 axillary nodes positive, and estrogen and progesterone receptor positive. This patient was treated with breast-conserving therapy and received adjuvant tamoxifen but no adjuvant cytotoxic chemotherapy. The other tumor was infiltrating ductal carcinoma, 0.7 cm in diameter, histologic grade 2 of 3, and estrogen and progesterone receptor positive. She received adjuvant CMF chemotherapy and tamoxifen. With only two distant failures and one local failure, no significant difference was possible when the data were analyzed for effect of histologic grade, receptor status, age, or the use of adjuvant chemotherapy or tamoxifen.
DISCUSSION
The benefit of adjuvant therapy for breast cancer is often taken for granted today. It was only a few years ago that the benefit of treating systemic metastases at the time of initial therapy, rather than waiting for recurrence, was appreciated to be of sufficient magnitude as to warrant treating an entire population of women at risk, including those who would receive no benefit from the therapy. The entire concept is based on treating populations or groups of patients rather than individuals because we are unable to identify the individuals who would not have the potential for benefit. Initially, only women with multiple lymph node metastases were offered adjuvant chemotherapy, then those with any involved lymph nodes, and finally even those without lymph node involvement. 9–11
Hormone receptor status provides an excellent predictive factor that identifies individuals (receptor negative) who will not benefit from adjuvant tamoxifen and who may be spared the expense and any associated side effects of such therapy. 3 We have not been able to identify reliable predictive factors for benefit or lack thereof for cytotoxic chemotherapy, with the possible exception of the latter in old age. 4
In the absence of predictive factors, the question arises of using prognostic factors to identify a population at so little risk of recurrent breast cancer that the risks associated with treatment would not be supported. An easy example is noninvasive breast cancer (e.g., ductal carcinoma in situ). Many prognostic factors have been shown useful in women with node-negative breast cancer, including grade, mitotic index, S-phase, HER-2 receptor, and size. In retrospective, multivariate analyses some of these are more predictive of prognosis than size in node-negative patients. 12 Multivariate analysis of population effects, however, is not particularly helpful in advising an individual patient. Threshold measurements are helpful; the tumor diameter of 1 cm appears to offer such a threshold measurement.
The SEER data reviewed by Carter et al. 13 in 1989 showed a 5-year overall survival rate exceeding 98% in node-negative patients with tumors less than 1 cm. This was particularly surprising because it reflected population-based data and accepted the diameter measurements of all institutions involved. A European report of tumors 1 cm or less with a 10-year overall survival rate of 98.7% added confidence to the very favorable nature of this subgroup of node-negative patients. 6 Vitucci et al. 14 in the Regina Elena Cancer Institute of Rome have reported a 90% overall survival rate for patients with T1a and T1b tumor sizes, including all nodal states.
These favorable data directly conflict with the concept that “breast cancer is always a systemic disease,” which has become the common wisdom in oncology. Some have suggested that the favorable 5-year survival data for small node-negative tumors simply reflects “lead time bias” and that by 10 years these patients would experience very significant systemic recurrence. The NSABP experience was interpreted as supporting this position. An analysis of the outcome of node-negative patients with T1a and T1b tumors from B-06, B-13, B-14, B-19, and B-20 revealed a relapse-free survival rate of 79% and an overall survival rate of 93% at only 8 years with surgery alone for 61 women who were estrogen receptor negative. For 264 women who were estrogen receptor positive, surgery alone led to a relapse-free survival rate of 86% and an overall survival rate of 90% at 8 years. 15 Although these data are accurate, they reflect a selection of patients for these five national adjuvant trials from a larger population of patients with small, node-negative tumors. The present report is of sequential patients in a single university center.
It is of interest that 93 patients in our series received tamoxifen and 20 of them chemotherapy. The protocol in this unit was for this group of women to be spared adjuvant systemic therapy. Exceptions were clearly made by some Emory physicians; in other instances, patients elected to be seen by medical oncologists in the community. With only two systemic failures, no benefit can be evaluated, but each of the patients who failed had received adjuvant systemic therapy.
Some favor adjuvant therapy for these women based on the reduction of the death rate for the small group who would fail without it. The feeling is that any benefit is worth the treatment of all the other women who would not benefit. Such a hypothetical benefit must be weighed against potential risk. The evidence of neurocognitive defects in women who receive adjuvant chemotherapy continues to mount. 16 This has not been ideally studied and is clearly related to the dose intensity of chemotherapy delivered. 17 It is clear, however, that it is a real phenomenon. Some cognitive functional impairment has even been reported from the use of 5 years of tamoxifen. 18 As studies continue to define more precisely the extent of such impairment and the degree to which it resolves over time, it seems essential that women with T1a and T1b node-negative breast cancer, who have a roughly 1% likelihood of systemic recurrence, be informed of the downside of adjuvant systemic therapy as a means of attempting to improve the lot of 1 in 100 of this group of women. It would seem difficult to justify adjuvant chemotherapy for such patients outside controlled clinical trials.
The estimated risk of recurrence in women with 1-cm-in-diameter or less, node-negative breast cancer in this institution was 1.3% at 10 years. No features could be identified to define the individuals at particular risk.
Discussion
Dr. Samuel W. Beenken (Birmingham, AL): I very much appreciate this opportunity to discuss an excellent paper. The authors reviewed their experience with T1a and T1b N0 breast cancer to identify those who were at highest risk for recurrence. They found that systemic failure was too rare to define a high-risk group in their study population and concluded that hormone or chemotherapy was not warranted. This paper addresses an area of high importance because we are seeing more and more patients with early breast cancer. In a series from the New England Deaconess Hospital and others hospitals, Dr. Blake Cady reported the mean maximum diameter of newly diagnosed breast cancers to be 1.5 cm and declining. He predicted that the mean diameter would be 1 cm in the near future. The authors of this paper studied 282 consecutive T1a and T1b N0 patients who were prospectively staged and followed for outcome. What percentage of the total breast cancer population seen at their institution did this group comprise? It has been proposed that we are now detecting many breast cancers before clonal subselection results in a more aggressive phenotype. What percentage of the patients studied had grade 1 histology? One hundred thirteen of the patients studied received either adjuvant tamoxifen or chemotherapy. Both recurrences occurred in this group. How can we be sure that there wasn’t a selection bias resulting in higher-risk women receiving adjuvant therapy? Is it possible that the 111 women who did not recur received some benefit from their adjuvant therapy? Finally, would the authors offer adjuvant hormone or chemotherapy to a 30-year-old woman with T1b N0 breast cancer who had grade 3 histology, overexpressed Ki67, was HER2-neu positive and had a high S-phase fraction? Again, I appreciate the opportunity to discuss this excellent paper.
Dr. J. Dirk Iglehart (Boston, MA): This was an excellent paper: 282 patients, virtually 99% overall survival. The conclusions seem incontrovertible. I have a couple of questions. I was interested in the overall group that this cohort was culled from. How many people with T1a and b tumors had positive nodes? If you have information, it would be interesting. I worry about the T1b ER-negative breast cancer and again, like Dr. Beenken, wonder whether some of those patients were the ones who got the adjuvant chemotherapy, or the 35 or so that got adjuvant tamoxifen, and whether this could have had an effect. You report a 1% failure rate. That compares with a 10% failure rate reported in the cumulative experience of the NSABP and also in Rosen’s work from Memorial Sloan-Kettering. If you think about it, you have 10-fold better results than they reported. Do you have any idea why that might be? I very much enjoyed the paper. I think this is an important topic, and it seems like your data has really nailed it.
Dr. William C. Wood (Atlanta, GA): Both of the questioners put me on the spot. And I am not able to come up with as precise an answer as I would like to without access to the database. I apologize. Dr. Beenken asked what percent of the total population was this subgroup in our institution. It was just under 10%. More recently, it is growing as a percentage and appears the last few years to be closing in on 20%. But it is less than 10% over this 15-year period from which we culled these patients. What percent had grade 1 histology? It was less than 20%. I don’t recall the exact number. But over 80% had grade 2 or grade 3 histology. Is it possible that there is a selection bias and that the people who most needed the chemotherapy got it? I would certainly think there is and I hope that is the case. I have great confidence in the intuition of doctors and patients, and I think if guardian angels encouraged them to get chemotherapy despite our telling them they didn’t need to, they probably were the group who would benefit from it. But that is the problem with nonrandomized, controlled data, which these clearly are. You asked about a 30-year-old T1b N0 grade 3 Her2-positive, high S-phase patient, would I recommend adjuvant chemotherapy for that group. No, I stick by my guns and I would not. We could not find any subgroup in our study, nor, I would add, can Kent Osborne in the large, large database that they keep of referred patients at the former San Antonio database, pick out a subgroup that is at higher risk within these 1-cm-or-less tumors. Dr. Iglehart asks about the T1a, b, what was the incidence of positive nodes. Dirk, sorry, I can’t give that to you exactly. It was well under 10%, however—which was surprising, because their data suggested it is 20%. Those data were almost-1-cm nodules. This did have a distribution of 60% less-than-9-mm tumors. Why 1% versus 10%? Clearly we are just better! No, I clearly can’t explain that. I think we are seeing increasingly mammographically detected smaller tumors. And although I would like to believe the former, we all know that is not true.
Footnotes
Supported by grants from The Elkin Foundation and from “Tournament for the Cure.”
Correspondence: William C. Wood, MD, Emory University Hospital, Suite B206, 1364 Clifton Road, NE, Atlanta, GA 30322.
E-mail: william_wood@emoryhealthcare.org
Presented at the 113th Annual Session of the Southern Surgical Association, December 3–5, 2001, Hot Springs, Virginia.
Accepted for publication December 2001.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med 1988; 319: 1681–1692. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992; 339:1-15:71–85. [PubMed]
- 3.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet 1998; 351: 1451–1467. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet 1998; 352: 930–942. [PubMed] [Google Scholar]
- 5.Rosen PP, Groshen S, Saigl PE, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol 1989; 7: 355–366. [DOI] [PubMed] [Google Scholar]
- 6.Arnesson L-G, Smeds S, Fagerberg G. Recurrence-free survival in patients with small breast cancers. Eur J Surg 1994; 160: 271–276. [PubMed] [Google Scholar]
- 7.Adjuvant therapy for breast cancer. NIH Consensus Development Conference, November 1–3, 2000, Program and Abstracts, Office of the Director, National Institutes of Health.
- 8.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the treatment of primary breast cancer. J Clin Oncol 2001; 19: 3817–3827. [DOI] [PubMed] [Google Scholar]
- 9.Adjuvant chemotherapy of breast cancer. NIH Consensus Development Conference Summary. 1980; 3(3). [PubMed]
- 10.NIH Consensus Development Conference Statement. Adjuvant chemotherapy for breast cancer. September 9–11, 1985. CA Cancer J Clin 1986; 36:42. [DOI] [PubMed]
- 11.Consensus Statement: Treatment of early-stage breast cancer. JNCI Monographs 1992; 11:1. [PubMed]
- 12.Volpi A, DePaola F, Nanni O, et al. Prognostic significance of biologic markers in node-negative breast cancer patients: a prospective study. Breast Cancer Res Treat 2000; 63: 181–192. [DOI] [PubMed] [Google Scholar]
- 13.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status and survival in 24,740 breast cancer cases. The Surveillance, Epidemiology, End Result (SEER) Program of the National Cancer Institute. Cancer 1989; 63: 181–187. [DOI] [PubMed] [Google Scholar]
- 14.Vitucci C, Tirelli C, Graziano F, et al. Results of conservative surgery for limited-sized infiltrating breast cancer: analysis of 962 tested patients: 24 years of experience. J Surg Oncol 2000; 74: 108–115. [DOI] [PubMed] [Google Scholar]
- 15.Tan-Chiu E, Dignam J, Fisher B, et al. Prognosis of node-negative breast cancer patients with small (≤1 cm) tumors: The NSABP experience from protocols B-06, B-13, B-14, B-19 and B-20. Abstract, 22nd Annual San Antonio Breast Cancer Symposium, Dec. 8–11, 1999.
- 16.VanDam FSAM, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 1998; 90: 210–218. [DOI] [PubMed] [Google Scholar]
- 17.Brezden CB, Phillips K-A, Abdolell M, et al. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 2000; 18: 2695–2701. [DOI] [PubMed] [Google Scholar]
- 18.Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat 2000; 64: 165–176. [DOI] [PubMed] [Google Scholar]


