Abstract
Objective
To familiarize surgeons with the specific complications of cutaneous, gastrointestinal, inhalation, and systemic infection with Bacillus Anthracis, which may require surgical treatment.
Summary Background Data
The recent cases of intentional exposure to Bacillus Anthracis in the United States make familiarity with the basic microbiology, clinical manifestations, diagnosis, treatment, and control of this disease essential if mortality and morbidity is to be minimized, particularly following mass exposure. Although the treatment of Bacillus Anthracis infection is primarily medical, there are specific surgical complications with which the surgeon should be familiar.
Methods
A review of the literature was undertaken, utilizing electronic databases on infection with Bacillus Anthracis, as well as consultation with experts in this field. Emphasis was placed on the diagnosis and treatment of complications of infection that might require surgical intervention.
Results
Cutaneous anthrax infection results in eschar formation and massive soft tissue edema. When involving the extremities, increased compartment pressure requiring fasciotomy may result. Primary infection of the gastrointestinal tract may result in oropharyngeal edema and respiratory compromise requiring a surgical airway. Direct involvement of the lower gastrointestinal tract can result in intestinal ulceration, necrosis, bleeding, and perforation, which would require surgical exploration and resection of affected segments. Systemic sepsis, most often associated with inhalation anthrax, can cause massive ascites, electrolyte derangements, and profound shock requiring aggressive fluid resuscitation and careful hemodynamic monitoring and respiratory support. Systemic anthrax infection can also lead to gastrointestinal involvement by hematogenous dissemination, resulting in complications and requiring surgical management similar to direct gastrointestinal infection.
Conclusions
Cutaneous, gastrointestinal, inhalation and systemic infection with Bacillus Anthracis can result in complications which would require familiarity with the pathogenesis and manifestations of this disease in order to recognize and treat promptly and successfully by surgical intervention.
The recent terrorist attacks in New York City and Washington D.C. and the subsequent cases of anthrax exposure and infection throughout the United States make the once-theoretical risk of a bioterrorist attack with aerosolized anthrax very real. It is estimated that 100 kg of anthrax spores released upwind of Washington D.C. would result in 130,000 to 3 million deaths; many of these patients would die undiagnosed and untreated. 1 In a covert, large-scale release of anthrax, the initial deaths would result from a delay in recognizing the clinical manifestations of cutaneous, inhalation, or gastrointestinal disease and instituting appropriate treatment. To reduce the death rate, knowledge of the clinical manifestations, disease course, and medical as well as surgical treatment of anthrax infection is imperative. 2
Data regarding the diagnosis and management of anthrax comes from human case reports, mostly from foreign countries in which the disease is endemic, and experimental animal studies. The closest incident to a large-scale bioterrorist attack with aerosolized anthrax was the accidental release of the agent from a military microbiology facility at Sverdlovsk in 1979, from which limited information is available. There were at least 79 cases of anthrax infection, resulting in 68 deaths. 3,4 With such limited data and individual clinical experience, not all recommendations are rigorously evidence-based but rather have been clinically derived, as well as based on established surgical and medical practices.
The Centers for Disease Control and Prevention (CDC) has begun a process of physician education to increase familiarity with the clinical manifestations and treatment, but there has been little discussion of the surgical aspects of anthrax infection. Although the primary treatment of anthrax infection is medical, surgical complications can occur, particularly if a large number of individuals are infected. Awareness of these complications and knowledge of their treatment are critical if the deaths and complications are to be limited. Aside from the surgical indications, it is likely that in the case of a large-scale anthrax exposure, all physicians, including surgeons, would play a pivotal role in diagnosing and treating these patients. As such, a review of intentional infection with aerosolized anthrax, emphasizing surgical management, seems timely.
MICROBIOLOGY
Bacillus anthracis is a gram-positive aerobic bacillus, with both a spore-forming and vegetative form. Anthrax spores can survive in their dormant state, typically in soil, for decades until they come into contact with a host through cutaneous breaks, inhalation, or ingestion. After the experimental release of anthrax spores by the British military on Gruinard Island during World War II, spores persisted and remained viable for 36 years until decontamination began in 1979. 5,6 Anthrax spores are resistant to drying, heat, ultraviolet light, gamma radiation, and many disinfectants. 7 Spores are induced to germinate into their vegetative form when they contact an environment rich in amino acids, nucleosides, and glucose, with a temperature at least 37°C and a CO2 concentration of at least 5%, as is found in the host animal or human. 8,9 When nutrients are exhausted or the environment is unfavorable, sporulation occurs. 10,11
The pathogenicity of anthrax is due both to the exotoxins it secretes and the capsule it forms, each encoded by a separate plasmid (pX01 and pX02, respectively). PX01 codes for three toxin elements (protective antigen, lethal factor, and edema factor), which combine to form two binary toxins, edema toxin (edema factor + protective antigen) and lethal toxin (lethal factor + protective antigen). 12 Edema toxin acts to upset cellular water homeostasis, which results in the massive edema characteristic of the disease; lethal toxin induces macrophage secretion of tumor necrosis factor-alpha and interleukin-1-beta, which mediate the systemic shock state. 13–17 The vegetative anthrax capsule, encoded by pX02, inhibits immune cell phagocytosis and is itself weakly immunogenic, thus allowing the bacillus to circumvent the natural immune response. Both factors are necessary for full virulence. 18–21 In addition, given the capacity for genetic manipulation of strains that may be used in bioterrorist attacks, antibiotic resistance may contribute to its virulence. 22 As such, the Working Group on Civilian Biodefense recommended that when used as an agent of bioterrorism, anthrax strains should be presumed resistant to tetracycline and penicillin, and patients should be treated with ciprofloxacin until antibiotic sensitivity can be determined. The CDC, however, currently recommends either ciprofloxacin or doxycycline as first-line treatment. 2,23
CLINICAL MANIFESTATIONS
Cutaneous Anthrax
Cutaneous anthrax is the most common form of anthrax infection, accounting for 90% to 95% of the cases worldwide and all of the prior cases reported in the United States. Many of the exposures have been occupational and resulted from the handling of infected animals or laboratory materials. 24–27 Spores are introduced through breaks in the skin; within 3 to 5 days, the skin bears the primary lesion marked by a painless, pruritic papule that 24 to 36 hours later forms an ulcer with accompanying vesicles. The area goes on to become necrotic, forming a black eschar resembling coal (anthracos in Greek). Characteristic of anthrax, the involved soft tissue becomes markedly edematous and indurated, and regional lymphadenopathy may be noted 28 (Fig. 1). The skin lesion may become secondarily infected, typically with staphylococci or streptococci, leading to purulence and cellulitis. 29 Diagnosis in the setting of bioterrorism would rely on a high index of suspicion as well as the characteristic clinical presentation, with Gram stain and culture of fluid from the vesicle providing confirmation.
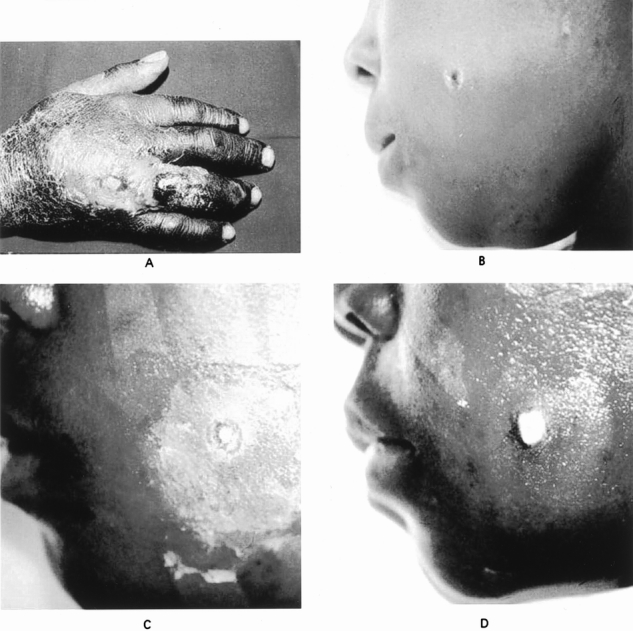
Figure 1. (A) Cutaneous anthrax affecting the hand with characteristic black eschar and extensive edema. (B–D) Progression in a cutaneous anthrax lesion on the cheek over a 7-day period. (B) Eschar formation and edema is evident initially on day 0. (C) Ulceration of the lesion with ongoing edema on day 2. (D) Healing of the ulcerated lesion and resolution of the edema on day 7. (Dixon TC, Meselson M, Guillemin J, et al. Anthrax. N Engl J Med 1999; 341:815–826. Used with permission.)
Medical treatment of localized cutaneous anthrax in the setting of bioterrorism, as recommended by the CDC, is oral ciprofloxacin or doxycycline. The Working Group on Civilian Biodefense recommends initial treatment with ciprofloxacin until sensitivity to doxycycline is established, at which time therapy can be adjusted. The recommended course is 60 days to prevent presumed inhalation of contacted spores, which appear to have prolonged latency in the respiratory tract. Steroids may be used as adjunctive therapy in patients with severe edema, especially in the head and neck region. 2,23
Although 80% to 90% of the cases of cutaneous anthrax are self-limited, associated death results primarily from systemic dissemination and elaboration of exotoxins. 30,31 In cases of cutaneous anthrax with signs of systemic dissemination, extensive edema, or head and neck involvement, the CDC recommends treatment with intravenous ciprofloxacin along with one or two additional agents with in vitro activity against anthrax, including rifampin, vancomycin, penicillin, ampicillin, chloramphenicol, imipenem, clindamycin, or clarithromycin. Penicillin and ampicillin should not be used alone to treat anthrax because of concerns about beta-lactamase activity. 23 When treated with antibiotics, the death rate of cutaneous anthrax is less than 1%, increasing to 10% to 20% without treatment. 32
Apart from antibiotics, treatment of localized cutaneous anthrax relies on routine local wound care with sterile moist dressings changed regularly, as well as elevation of the affected region to retard edema formation. Surgical debridement of the eschar is contraindicated until after the patient has been adequately treated with antibiotics because of the risk of systemic dissemination of the bacillus. 33 Vesicular fluid becomes sterile 5 to 24 hours after treatment with antibiotics. 34 After adequate medical treatment, excision of the eschar with skin grafting may be carried out, although smaller lesions typically heal without scarring. 35,36 Because of the massive tissue edema associated with anthrax, large and circumferential lesions on the extremities could lead to compartment syndrome and vascular compromise. 37 Any invasive measurement of compartment pressures or fasciotomy, if indicated, should avoid the primary lesion until adequate antibiotic treatment has been administered.
Gastrointestinal Anthrax
Gastrointestinal anthrax accounts for approximately 2.5% to 5% of naturally occurring cases worldwide and is associated with a death rate ranging from 25% to 60%. 32,38,39 The gastrointestinal tract can be affected directly by the consumption of food contaminated with anthrax bacilli or by hematogenous spread from systemic disease, usually originating in the respiratory tract, as confirmed by autopsies performed on patients after accidental exposure at Sverdlovsk in 1979. In this series, 39 of the 42 patients had multiple submucosal hemorrhagic lesions in the gastrointestinal tract, particularly the small bowel, stomach, and colon. Mesenteric lymphadenitis was found in 9 of the 42. 4 It is unknown how many other patients not included in the series had either primary or secondary gastrointestinal involvement, how many underwent surgery on the affected gastrointestinal tract, and what the overall death rate associated with gastrointestinal anthrax was in the Sverdlovsk incident.
In cases of direct exposure, spores can germinate in the oropharynx or the lower tract, with clinical manifestations occurring 2 to 5 days after ingestion. 40 In the oropharyngeal form, the characteristic necrotic, ulcerated lesion with overlying eschar may be evident in the posterior pharynx, with accompanying cervical edema and lymphadenopathy. Pharyngeal edema may lead to dysphagia and airway compromise. 41–43
Similarly, in the lower gastrointestinal tract, spores can deposit at sites of mucosal disruption and germinate, usually in the terminal ileum and cecum, although cases affecting the stomach and duodenum have been reported. 28,44,45 The spore thus appears to be unaffected by gastric acid. Once germination takes place in the wall of the gastrointestinal tract, macrophages engulf the vegetative form and are taken up by regional lymph nodes, leading to necrotizing, hemorrhagic mesenteric lymphadenitis as well as hematogenous and lymphatic dissemination. The primary point of entry becomes ulcerated, hemorrhagic, and necrotic. 46,47 Gastrointestinal manifestations caused by hematogenous dissemination from another site of primary infection are marked by multifocal submucosal hemorrhages extending into the muscularis propria and scattered throughout the intestinal tract. 4
Patients typically present with fever and diffuse abdominal pain and tenderness. Rebound tenderness and signs of an acute abdomen are often present. 48,49 Because of gastrointestinal ulceration and necrosis, hematemesis, coffee-ground emesis, and bloody diarrhea often develop, and intestinal perforation may occur. 40,44,49,50 Patients go on to accumulate massive ascites within 2 to 4 days after the onset of abdominal pain. The ascitic fluid may be clear, blood-tinged, or turbid, and Gram stain demonstrates gram-positive bacilli. 28,45,48,49 Systemic dissemination of the organism is accompanied by sepsis and shock. 48,49,51
Medical management of gastrointestinal anthrax consists of early institution of antibiotics, aggressive volume resuscitation with both crystalloid and colloid, and prompt correction of electrolyte imbalances. In the setting of bioterrorism, the CDC recommends intravenous ciprofloxacin or doxycycline and one or two additional antimicrobials, as listed above. Therapy can be further adjusted as antibiotic susceptibility data are available and the clinical situation permits. The recommended course is 60 days, but patients may be switched from intravenous to oral therapy once the clinical condition improves or if resources available do not allow for ongoing intravenous therapy. 2,23 Oral-pharyngeal manifestations require airway management, with possible intubation or establishment of a surgical airway if edema precludes the former. Steroids may play a role in lessening the extent of pharyngeal edema. 52,53
Management of direct and hematogenous anthrax infections in the lower gastrointestinal tract involves both primary medical treatment and potential surgical resection of affected areas. Most previously reported patients with lower gastrointestinal tract anthrax have died of systemic shock before surgery could be performed; however, there are cases in which prompt recognition of peritonitis led to exploration and resection of nonviable portions of the bowel. 44,45,48,49,51 In addition, autopsy findings in patients with gastrointestinal anthrax have shown bowel perforation, gastric ulceration, and segments of necrotic intestine. 4,28,45 Thus, gastrointestinal anthrax demands treatment of both the primary process and any resultant insults to the bowel.
Because there are scant data to direct specific principles, knowledge of the effects of anthrax on the gastrointestinal tract and good surgical judgment must serve as the guides to management. Instances of ongoing sepsis or progressive abdominal pain and tenderness in patients treated promptly with antibiotics and restoration of intravascular volume would certainly be suspicious for gastrointestinal injury and should be managed as any other case of ischemic or perforated bowel. Also, progressive gastrointestinal bleeding refractory to medical treatment should be managed by localization of the bleeding source and surgical resection, because blood loss will exacerbate the severe hypovolemia characteristic of anthrax and will lead to an increased death rate. 44,48
Inhalation Anthrax
Inhalation anthrax accounts for 2.5% to 5% of the cases occurring sporadically; however, it is believed that inhalation anthrax will be the first manifestation to present after the release of aerosolized spores. 38,39 All 42 patients reported in the autopsy series after accidental aerosolized release at Sverdlovsk showed evidence of inhalation anthrax, and 64 of the 79 patients with presumed inhalation anthrax reported from the incident died, suggesting that it may be a primary cause of death in a bioterrorist attack. 3,4 In other reports, inhalation anthrax was found to be nearly uniformly fatal. 28,54 The pulmonary system is the mode of entry of the 1- to 5-μm anthrax spores, but it is not the site of primary pathology. Rather than causing a pneumonia, the bacilli are taken up by alveolar macrophages and transported to mediastinal and peribronchial lymph nodes, where germination continues, resulting in hemorrhagic mediastinitis. From there, the vegetative form of the bacillus spreads systemically. 28,46,54,55 A focus of hemorrhagic pneumonitis often develops, possibly related to the portal of systemic entry from the pulmonary system. 4
The incubation period from the time of exposure to the development of first symptoms, usually fever, nonproductive cough, myalgia, and malaise, is highly variable. Based on the Sverdlovsk cases, the modal incubation period was 10 days, with symptoms developing 2 days to 6 weeks after presumed exposure, suggesting prolonged viability of the spore form in the lungs. 3 It is estimated that the dose of inhaled anthrax spores sufficient to result in 50% deaths would be 2,500 to 55,000. 56 The pathognomonic sign on a chest radiograph in a previously healthy patient with symptoms of inhalation anthrax is a widened mediastinum (Fig. 2). Pleural effusions may also be seen. 57 With 1 to 3 days of the onset of initial symptoms, patients go on to develop symptoms of local progression and systemic dissemination with dyspnea, stridor, diaphoresis, fever, chills, and frank shock. 2,28,54 Death occurred a mean of 3 days after the onset of symptoms in the Sverdlovsk series. 3
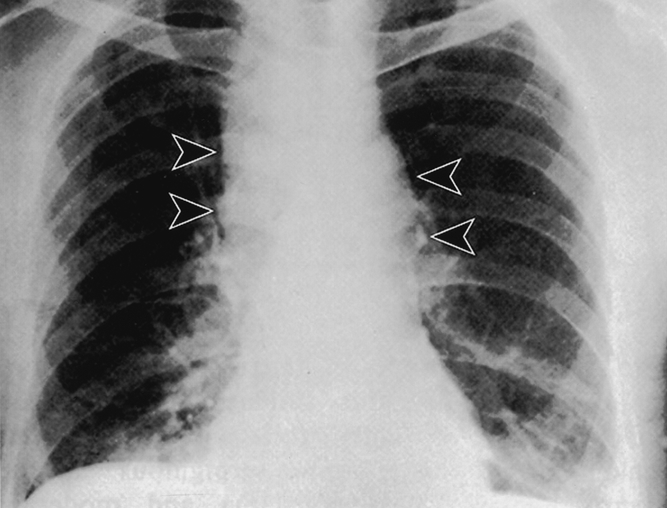
Figure 2. Chest radiograph of a patient with inhalation anthrax. Lobulated mediastinal widening (arrowheads) consistent with lymphadenopathy characteristic of inhalation anthrax. (Inglesby TV, Henderson DA, Bartlett JG, et al. Anthrax as a biological weapon: medical and public health management. JAMA 1999; 281:1735–1745. Used with permission.)
Diagnosis of inhalation anthrax in the setting of bioterrorism is based on a high index of suspicion as well as nonspecific symptoms that mimic a viral upper respiratory infection and widened mediastinum on chest radiograph. Although Gram stain and culture of sputum is unlikely to yield the diagnosis, initial confirmation of clinical suspicion can be obtained by Gram stain and culture of pleural fluid showing gram-positive bacilli. Gram stain and culture of the peripheral blood and/or cerebrospinal fluid may also yield a diagnosis after systemic dissemination has occurred. 28,54 The CDC recommends intravenous ciprofloxacin or doxycycline and one or two additional antimicrobials, as listed above, for the treatment of inhalation anthrax in the setting of bioterrorism. Therapy can be further adjusted as antibiotic susceptibility data are available and the clinical situation permits. The recommended course is 60 days, but patients may be switched from intravenous to oral therapy once the clinical condition improves or if resources available do not allow for ongoing intravenous therapy. Postexposure prophylaxis is based on the risk of exposure. Ciprofloxacin or doxycycline administered orally is recommended by the CDC until sensitivity can be determined. Prophylaxis should be continued for 60 days. 2,23,58
Anthrax Sepsis and Shock
Systemic anthrax infection is marked by hypotension, anoxia, and initial respiratory alkalosis followed by respiratory and metabolic acidosis, all consistent with the shock state. Also, electrolyte derangements include hypocalcemia, hypoglycemia, and hyperkalemia. 28,59,60 It is likely that shock is mediated by toxin-induced macrophage secretion of tumor necrosis factor-alpha and interleukin-1-beta. 15–17 Aggressive resuscitation with crystalloid and colloid, correction of electrolyte and acid–base abnormalities, and administration of intravenous antibiotics are the foundations for treating systemic anthrax sepsis. Invasive hemodynamic monitoring may be used to guide volume replacement and ensure its adequacy. Pressor support may also play a role in the management of anthrax sepsis as an adjunct to adequate resuscitation. Many of these patients develop oliguric or anuric renal failure and severe respiratory distress and should be supported appropriately. 2,28
In addition, patients should be monitored and treated for secondary gastrointestinal or meningeal involvement. Inhalation anthrax is the typical primary source in patients developing systemic dissemination that affects the gastrointestinal tract. 4 Anthrax meningitis more commonly occurs after hematogenous or lymphatic dissemination from skin infections, although it can also occur in the setting of primary gastrointestinal or inhalation anthrax. 4,61 In the autopsy series from Sverdlovsk, approximately half of the patients had meningeal involvement, presumably after inhalation anthrax. 4 Anthrax meningitis may present with fever, fatigue, myalgia, nausea, vomiting, seizures, agitation, and delirium. Rapid neurologic decline and death may follow 1 to 6 days after the onset of illness. 28,62 The cerebrospinal fluid is often bloody and usually stains for gram-positive bacilli, which can provide support for the clinical diagnosis. 63 Besides antibiotics, surviving patients have also been treated with steroids. 64,65
DIAGNOSIS
Microbiologic studies are essential not only for confirmatory diagnosis of anthrax but also for antibiotic susceptibility testing in the setting of bioterrorism. 2 Gram stain of fluid from vesicular lesions, ascitic fluid, pleural effusions, cerebrospinal fluid, and blood obtained after systemic dissemination will usually show gram-positive bacilli. 2,28,66 Culture with appropriate bacteriologic testing can further confirm the clinical diagnosis. 67 The microbiology laboratory should be notified that anthrax is clinically suspected, because identification of Bacillus species on Gram stain or in cultures is usually regarded as a contaminant. 68
Serologic and immunologic testing using enzyme-linked immunosorbent assay or indirect microhemagglutination for bacterial exotoxins or titers of antibody to protective antigen and capsular components may be used, but antibodies are not present until late in the course of the infection. 2,28,68–71 A skin test using an extract from an attenuated strain of B. anthracis is available and is diagnostic in 82% of patients 1 to 3 days and in 99% of patients 4 weeks after the onset of symptoms. 72,73 Polymerase chain reaction techniques used to amplify markers specific to B. anthracis exist but are not widely available outside research and government laboratories. 2,28,74–81
VACCINATION
There are currently two vaccines available for immunization against anthrax: an inactivated cell-free extract produced in the United States and a human live attenuated vaccine produced and used in the former Soviet Union. Because of the residual virulence of a live attenuated strain of B. anthracis, this form has been deemed unsuitable for use in the United States. 2,82–84 Anthrax Vaccine Adsorbed (AVA) is a noninfectious filtrate from the culture of an attenuated strain of B. anthracis that uses the protective antigen exotoxin to confer immunologic protection. The vaccine was licensed by the U.S. Food & Drug Administration in 1970 and has been used to vaccinate individuals with high-risk occupational exposure to anthrax as well as U.S. military forces. 84,85 Six doses are required at 0, 2, and 4 weeks and 6, 12, and 18 months, with annual boosters thereafter. 84,85 Few serious adverse events have been associated with the vaccine, with mild local reactions occurring in approximately 30% of those receiving the vaccine and systemic reactions consisting of malaise, lassitude, fever, and chills in less than 0.2%. 84 The efficacy of AVA has been established largely from animal studies because the high death rate associated with anthrax would preclude any human challenge studies, although there is anecdotal data from vaccinated and nonvaccinated individuals in high-risk occupations. The cumulative data suggest that immunization with AVA affords most subjects immunity against a lethal challenge with aerosolized anthrax. 86–99 There is currently no recommendation for mass immunization against anthrax. 23,58,84 However, in the event of a bioterrorist event, vaccination is recommended concurrently with antibiotics for both prophylaxis and treatment. 2,28
INFECTION CONTROL
Human-to-human contagion has not been reported, suggesting that the primary infectious form of anthrax is the spore. 21,28 From case reports of patients with anthrax undergoing surgery, there is no evidence that special precautions were taken. 35,44,48 In addition, there are no distinct guidelines for protection of the surgical team while operating on patients with anthrax. Standard universal precautions appear at this time adequate for protection. Instruments and materials contaminated during an operation on a patient with anthrax should be autoclaved or incinerated, as is the usual practice. There is no specific recommendation of antibiotic prophylaxis or vaccination for healthcare workers exposed to patients with anthrax. 2,23,28,30
CONCLUSIONS
Although bioterrorism-associated anthrax infections reported in the United States have to date been limited, preparation for further isolated infections as well as possible large-scale exposure seems essential. As such, surgeons should be familiar with both the medical and more specific surgical aspects of diagnosing and treating anthrax in its localized and disseminated forms. Because there is no way to detect an intentional, covert release of anthrax, only heightened clinical suspicion based on understanding the pathogenesis of B. anthracis will be effective in combating the disease.
Footnotes
Correspondence: Lisa Colletti, MD, 2922H Taubman, Box 0331
Ann Arbor, MI 48109-0331.
E-mail: colletti@umich.edu
Accepted for publication February 28, 2002.
References
- 1.Office of Technology Assessment, US Congress. Proliferation of Weapons of Mass Destruction. Washington, DC: US Government Printing Office; 1993;53–55. Publication OTA-ISC-559.
- 2.Inglesby TV, Henderson DA, Bartlett JG, et al. Anthrax as a biological weapon: medical and public health management. JAMA 1999; 281: 1735–1745. [DOI] [PubMed] [Google Scholar]
- 3.Meselson M, Guillemin J, Hughes-Jones M, et al. The Sverdlovsk anthrax outbreak of 1979. Science 1994; 266: 1202–1208. [DOI] [PubMed] [Google Scholar]
- 4.Abramova FA, Grinberg LM, Yampolskaya OV, et al. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci USA 1993; 90: 2291–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titball RW, Turnbull PC, Hutson RA. The monitoring and detection of Bacillus anthracis in the environment. J Appl Bacteriol 1991; 70 (suppl): 9S–8S. [PubMed] [Google Scholar]
- 6.Manchee RJ, Stewart WD. The decontamination of Gruinard Island. Chem Br. July 1988;690–691.
- 7.Watson A, Keir D. Information on which to base assessments of risk from environments contaminated with anthrax spores. Epidemiol Infect 1994; 113: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino S, Sasakawa C, Uchida I, et al. Cloning and CO2-dependent expression of the genetic region for encapsulation from Bacillus anthracis. Mol Microbiol 1988; 2: 371–376. [DOI] [PubMed] [Google Scholar]
- 9.Dai Z, Sirard J-C, Mock M, et al. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol Microbiol 1995; 16: 1171–1181. [DOI] [PubMed] [Google Scholar]
- 10.Dragon DC, Rennie RP. The ecology of anthrax spores. Can Vet J 1995; 36: 295–301. [PMC free article] [PubMed] [Google Scholar]
- 11.Titball RW, Turnbull PC, Hutson RA. The monitoring and detection of Bacillus anthracis in the environment. J Appl Bacteriol 1991; 70 (suppl): 9S-18S. [PubMed] [Google Scholar]
- 12.Leppla SH. The anthrax toxin complex. In: Alouf J, Freer JH, eds. Sourcebook of bacterial protein toxins. London: Academic Press, 1991: 277–302.
- 13.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA 1982; 79: 3162–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppla SH. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res 1984; 17: 189–198. [PubMed] [Google Scholar]
- 15.Hanna PC, Acosta D, Collier RJ. On the role of macrophages in anthrax. Proc Natl Acad Sci USA 1993; 90: 10198–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna PC, Kruskal BA, Ezekowitz RA, et al. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol Med 1994; 1: 7–18. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna PC, Kochi S, Collier RJ. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol Biol Cell 1992; 3: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman JW, Nitecki DE. Studies on the relation of a prior immune response to immunogenicity. Immunology 1967; 13: 577–583. [PMC free article] [PubMed] [Google Scholar]
- 19.Makino SI, Uchida I, Terakado N, et al. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol 1989; 171: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwartouw HT, Smith H. Polyglutamic acid from Bacillus anthracis grown in vivo: structure and aggressin activity. Biochem J 1956; 63: 437–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mock M, Fouet A. Anthrax. Annu Rev Microbiol 2000; 55: 647–671. [DOI] [PubMed] [Google Scholar]
- 22.Albrink WS. Pathogenesis of inhalation anthrax. Bacteriol Rev 1961; 25: 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR 2001; 50: 909–919. [PubMed] [Google Scholar]
- 24.Taylor JP, Dimmitt DC, Ezzell JW, et al. Indigenous human cutaneous anthrax in Texas. South Med J 1993; 86: 1–4. [DOI] [PubMed] [Google Scholar]
- 25.Human cutaneous anthrax—North Carolina. Arch Dermatol 1988; 124:1324. [DOI] [PubMed]
- 26.Leads from the MMWR. Human cutaneous anthrax—North Carolina, 1987. JAMA 1988; 260:616. [PubMed]
- 27.Human cutaneous anthrax—North Carolina. MMWR 1988; 37:413–414. [PubMed]
- 28.Dixon TC, Meselson M, Guillemin J, et al. Anthrax. N Engl J Med 1999; 341: 815–826. [DOI] [PubMed] [Google Scholar]
- 29.Edwards MS. Anthrax. In: Feigin RD, Cherry JD, eds. Textbook of pediatric infectious diseases, 3rd ed, Vol. 1. Philadelphia: WB Saunders, 1992: 1053–1056.
- 30.Lew D. Bacillus anthracis (anthrax). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practices of infectious disease. New York: Churchill Livingstone, 2000: 2215–2220.
- 31.Doganay M, Bakir M, Dokmetas I. A case of cutaneous anthrax with toxaemic shock. Br J Dermatol 1987; 117: 659–662. [DOI] [PubMed] [Google Scholar]
- 32.Brachman PS, Kaufmann A. Anthrax. In: Evans AS, Brachman PS, eds. Bacterial infections of humans. New York: Plenum, 1998.
- 33.Gold H. Treatment of anthrax. Fed Proc 1967; 26: 1563–1568. [PubMed] [Google Scholar]
- 34.Ronaghy HA, Azadeh B, Kohout E, et al. Penicillin therapy of human cutaneous anthrax. Curr Ther Res Clin Exp 1972; 14: 721–725. [PubMed] [Google Scholar]
- 35.Aslan G, Terzioglu A. Surgical management of cutaneous anthrax. Ann Plast Surg 1998; 41: 468–470. [PubMed] [Google Scholar]
- 36.Caksen H, Arabaci F, Abuhandan M, et al. Cutaneous anthrax in eastern Turkey. Cutis 2001; 67: 488–492. [PubMed] [Google Scholar]
- 37.Terziohlu A, Aslan G. Ulnar nerve lesion due to cutaneous anthrax. Ann Plast Surg 1999; 43: 644–645. [DOI] [PubMed] [Google Scholar]
- 38.Pile JC, Malone JD, Eitzen EM, et al. Anthrax as a potential biological warfare agent. Arch Intern Med 1998; 158: 429–434. [DOI] [PubMed] [Google Scholar]
- 39.Gold H. Anthrax: a report of one hundred seventeen cases. Arch Intern Med 1955; 96: 387–396. [DOI] [PubMed] [Google Scholar]
- 40.LaForce FM. Anthrax. Clin Infect Dis 1994; 19: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 41.Sirisanthana T, Nelson KE, Ezzell JW, et al. Serological studies of patients with cutaneous and oral-pharyngeal anthrax from northern Thailand. Am J Trop Med Hyg 1988; 39: 575–581. [DOI] [PubMed] [Google Scholar]
- 42.Kunanusont C, Limpakarnjanarat K, Foy HM. Outbreak of anthrax in Thailand. Ann Trop Med Parasitol 1989; 84: 507–512. [DOI] [PubMed] [Google Scholar]
- 43.Sirisanthana T, Navachareon N, Tharavichitkul P, et al. Outbreak of oral-pharyngeal anthrax. Am J Trop Med Hyg 1984; 33: 144–150. [DOI] [PubMed] [Google Scholar]
- 44.Nalin DR, Sultana B, Sahunja R, et al. Survival of a patient with intestinal anthrax. Am J Med 1977; 62: 130–132. [DOI] [PubMed] [Google Scholar]
- 45.Dutz W, Saidi F, Kohout E. Gastric anthrax with massive ascites. Gut 1970; 11: 352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutz W, Kohout E. Anthrax. Pathol Annu 1971; 6: 209–248. [PubMed] [Google Scholar]
- 47.Dutz W, Kohout-Dutz E. Anthrax. Int J Dermatol 1981; 20: 203–206. [DOI] [PubMed] [Google Scholar]
- 48.Tekin A, Bulut N, Unal T. Acute abdomen due to anthrax. Br J Surg 1977; 84: 813. [PubMed] [Google Scholar]
- 49.Alizad A, Ayoub EM, Makki N. Intestinal anthrax in a two-year-old child. Pediatr Infect Dis J 1995; 14: 394–395. [DOI] [PubMed] [Google Scholar]
- 50.Jena GP. Intestinal anthrax in man: a case report. Central Afr J Med 1980; 26: 253–4. [PubMed] [Google Scholar]
- 51.Bhat P, Mohan DN, Srinivasa H. Intestinal anthrax with bacteriological investigations. J Infect Dis 1985; 152: 1357–1358. [DOI] [PubMed] [Google Scholar]
- 52.Doust JY, Sarkarzadeh A, Kavoossi K. Corticosteroid in treatment of malignant edema of chest wall and neck (anthrax). Dis Chest 1968; 53: 773–774. [DOI] [PubMed] [Google Scholar]
- 53.Cataldi A, Labruyere E, Mock M. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol Microbiol 1990; 4: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 54.Shafazand S, Doyle R, Ruoss S, et al. Inhalation anthrax: epidemiology, diagnosis and management. Chest 1999; 116: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 55.Albrink WS. Pathogenesis of inhalation anthrax. Bacteriol Rev 1961; 25: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Defense Intelligence Agency. Soviet Biological Warefare Threat. Washington, DC: US Dept of Defense, 1986. Publication DST-161OF-057–86.
- 57.Vessal K, Yeganehdoust J, Dutz W, et al. Radiologic changes in inhalation anthrax. Clin Radiol 1975; 26: 471–474. [DOI] [PubMed] [Google Scholar]
- 58.CDC. Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR 2001; 50: 889–897. [PubMed] [Google Scholar]
- 59.Dahlgren CM, Buchanan LM, Decker HM, et al. Bacillus anthracis aerosols in goat hair processing mills. Am J Hyg 1960; 72: 24–31. [DOI] [PubMed] [Google Scholar]
- 60.Walker JS, Lincoln RE, Klein F. Pathophysiological and biochemical changes in anthrax. Fed Proc 1967; 26: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 61.Tabatabaie P, Syadati A. Bacillus anthracis as a cause of bacterial meningitis. Pediatr Infect Dis J 1993; 12: 1035–1037. [DOI] [PubMed] [Google Scholar]
- 62.Rangel RA, Gonzalez DA. Bacillus anthracic meningitis. Neurology 1975; 25: 525–530. [DOI] [PubMed] [Google Scholar]
- 63.Pluot M, Vital C, Aubertin J, et al. Anthrax meningitis: report of two cases with autopsies. Acta Neuropathol (Berl) 1976; 36: 339–345. [DOI] [PubMed] [Google Scholar]
- 64.Tahernia AC, Hashemi G. Survival in anthrax meningitis. Pediatrics 1972; 50: 329–333. [PubMed] [Google Scholar]
- 65.Tahernia AC. Treatment of anthrax in children. Arch Dis Child 1967; 42: 181–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penn CC, Klotz SA. Anthrax. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious diseases, 2nd ed. Philadelphia: WB Saunders, 1998: 1575–1578.
- 67.Parry JM, Turnbull PCB, Gibson JR. A color atlas of Bacillus species. London: Wolfe Medical, 1983: 272.
- 68.Turnbull PCB, Kramer JM. Bacillus. In Balows A, ed. Manual of clinical microbiology, 5th ed. Washington, DC: American Society for Microbiology, 1991: 296–303.
- 69.Harrison LH, Ezzell JW, Abshire TG, et al. Evaluation of serologic tests for diagnosis of anthrax after an outbreak of cutaneous anthrax in Paraguay. J Infect Dis 1989; 160: 706–710. [DOI] [PubMed] [Google Scholar]
- 70.Turnbull PC, Doganay M, Lindeque PM, et al. Serology and anthrax in humans, livestock and Etosha National Park wildlife. Epidemiol Infect 1992; 108: 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson-Winegar A. Comparison of enzyme-linked immunosorbent and indirect hemagglutination assays for determining anthrax antibodies. J Clin Microbiol 1984; 20: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shlyakhov E, Rubinstein E. Evaluation of the anthraxin skin test for diagnosis of acute and past human anthrax. Eur J Clin Microbiol Infect Dis 1996; 15: 242–245. [DOI] [PubMed] [Google Scholar]
- 73.Shlyakhov E, Rubinstein E, Novikov I. Anthrax post-vaccinal cell-mediated immunity in humans: kinetics pattern. Vaccine 1997; 15: 631–636. [DOI] [PubMed] [Google Scholar]
- 74.Andersen GL, Simchock JM, Wilson KH. Identification of a region of genetic variability among Bacillus anthracis strains and related species. J Bacteriol 1996; 178: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patra G, Vaissaire J, Weber-Levy M, et al. Molecular characterization of Bacillus strains involved in outbreaks of anthrax in France in 1997. J Clin Microbiol 1998; 36: 3412–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patra G, Sylvestre P, Ramisse V, et al. Isolation of a specific chromosomic DNA sequence of Bacillus anthracis and its possible use in diagnosis. FEMS Immunol Med Microbiol 1996; 15: 223–231. [DOI] [PubMed] [Google Scholar]
- 77.Ramisse V, Patra G, Garrigue H, et al. Identification and characterization of Bacillus anthracis by multiplex PCR analysis of sequences on plasmids pXO1 and pXO2 and chromosomal DNA. FEMS Microbiol Lett 1996; 145: 9–16. [DOI] [PubMed] [Google Scholar]
- 78.Keim P, Kalif A, Schupp J, et al. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol 1997; 179: 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klevytskya A, Schupp J, Price LB, et al. A multiplexed fluorescent PCR genotyping system based on ten highly informative single-locus markers for B. anthracis strain identification. Presented at the Third International Conference on Anthrax. Plymouth, England, 1998.
- 80.Price LB, Hugh-Jones M, Jackson PJ, Keim P. Genetic diversity in the protective antigen gene of Bacillus anthracis. J Bacteriol 1999; 181: 2358–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson PJ, Walthers EA, Kalif AS, et al. Characterization of the variable-number tandem repeats in vrrA from different Bacillus anthracis isolates. Appl Environ Microbiol 1997; 63: 1400–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turnbull PC. Anthrax vaccines: past, present and future. Vaccine 1991; 9: 533–539. [DOI] [PubMed] [Google Scholar]
- 83.Shlyakhov EN, Rubinstein E. Human live anthrax vaccine in the former USSR. Vaccine 1994; 12: 727–730. [DOI] [PubMed] [Google Scholar]
- 84.Friedlander AM, Pittman PR, Parker GW. Anthrax vaccine: evidence for safety and efficacy against inhalation anthrax. JAMA 1999; 282: 2104–2106. [DOI] [PubMed] [Google Scholar]
- 85.Anthrax vaccine, military use in Persian Gulf region [press release]. Washington, DC: US Dept of Defense; Sept. 8, 1998.
- 86.Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 1997; 278: 399–411. [DOI] [PubMed] [Google Scholar]
- 87.Brachman PS, Gold H, Plotkin SA, et al. Field evaluation of a human anthrax vaccine. Am J Public Health 1962; 52: 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.50 Federal Register 51058. 1985.
- 89.Turnbull PCB, Broster MG, Carman JA, et al. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun 1986; 52: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Little SF, Knudson GB. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun 1986; 52: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ivins BE, Welkos SL, Little SF, et al. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect Immun 1992; 60: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ivins BE, Fellows PF, Nelson GO. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea pigs. Vaccine 1994; 12: 872–874. [DOI] [PubMed] [Google Scholar]
- 93.Fellows PF, Linscott MK, Ivins BE. Anthrax vaccine efficacy against Bacillus anthracis strains of diverse geographical origin. Presented at 3rd International Conference on Anthrax, September 1998. Plymouth, England.
- 94.Ivins BE, Fellows P, Pitt L, et al. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine 1995; 13: 1779–1784. [DOI] [PubMed] [Google Scholar]
- 95.Fritz DL, Jaax NK, Lawrence WB, et al. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab Invest 1995; 73: 691–702. [PubMed] [Google Scholar]
- 96.Ivins BE, Fellows PF, Pitt MLM, et al. Efficacy of a standard human anthrax vaccine against Bacillus anthracis aerosol spore challenge in rhesus monkeys. Salisbury Med Bull 1996; 87: 125–126. [Google Scholar]
- 97.Pitt MLM, Ivins BE, Estep JE, et al. Comparison of the efficacy of purified protective antigen and MDPH to protect non-human primates from inhalation anthrax. Salisbury Med Bull 1996; 87: 130. [Google Scholar]
- 98.Ivins BE, Pitt MLM, Fellow PF, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 1998; 16: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 99.Pitt MLM, Little S, Ivins BE, et al. In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol 1999; 87: 304. [DOI] [PubMed] [Google Scholar]


