Abstract
Objective
To review the effectiveness of preoperative biliary drainage (PBD) in patients with obstructive jaundice resulting from tumors.
Summary Background Data
This was a systematic review, including a meta-analysis, of randomized controlled trials and comparative cohort studies conducted worldwide and published between 1966 and September 2001, classified on methodologic strength and subdivided into level 1 (randomized controlled trials) and level 2 (comparative cohort studies).
Methods
Comparison was made of PBD versus no PBD in jaundiced patients undergoing resection of a tumor. Outcome measures were in-hospital death rate, overall complications resulting from the treatment modality (drainage- and surgery-related complications), and hospital stay. Effect sizes were calculated and combined in meta-analyses. Relative differences (%) were calculated to compare effects on outcome measures.
Results
Five randomized controlled studies comprising 302 patients met the inclusion criteria for level 1 studies, and 18 cohort studies comprising 2,853 patients met the criteria for level 2 studies. Meta-analysis of level 1 studies showed no difference in the overall death rate between patients who had PBD and those who had surgery without PBD. The overall complication rate, however, was significantly adversely affected by PBD compared with surgery without PBD. At level 2, there was no difference in the death rate between the two treatment modalities. The overall complication rate, however, was significantly adversely affected by PBD compared with surgery without PBD. If PBD had been without complications, then complications would be in favor of drainage based on level 1 studies, and no difference based on level 2 studies. Further, PBD was not able to reduce the length of postoperative hospital stay compared with surgery without PBD; instead, it prolonged the stay.
Conclusions
This meta-analysis shows that PBD with current standards for patients with obstructive jaundice resulting from tumors carries no benefit and should not be performed routinely. The potential benefit of PBD in terms of postoperative rates of death and complications does not outweigh the disadvantage of the drainage procedure. Only if PBD-related complications could be reduced by 27% and consequently diminish hospital stay could PBD be beneficial. Further randomized controlled trials with improved PBD techniques are necessary.
Surgery in jaundiced patients with tumors carries an increased risk of postoperative complications. 1,2 Several risk factors have been identified; among these, preoperative hyperbilirubinemia has been identified as a potential risk factor for poor outcome. 3–5 To avoid death and complications, preoperative biliary drainage (PBD) has been proposed as a means of reversing the pathophysiologic disturbances seen in jaundiced patients. In 1935, Whipple already had performed a staged surgical approach with a preliminary bypass to reduce jaundice and improve hepatic function. 6 Interest in the staged approach was renewed with the advent of a nonoperative first stage, external and later internal biliary drainage. In the late 1970s, the first studies on PBD reported a reduced postoperative death rate in jaundiced patients. 7,8 Since then, numerous studies, randomized as well as retrospective, have compared the outcome of PBD with surgery without PBD.
Studies in experimental animals have shown benefit of PBD, especially after internal drainage when the enterohepatic circulation was restored. 9,10 Clinical studies have failed to show this benefit, and some studies even reported a deleterious effect. 11–17
Despite the lack of a beneficial effect in many centers, most jaundiced patients undergo surgery for tumors after preoperative drainage. PBD is mainly performed because of logistic problems, such as time needed for further staging and the expected waiting time for surgery. The objective of this meta-analysis was to examine the effectiveness of PBD in jaundiced patients with tumors, to guide clinicians in their management of these patients, and to identify areas of uncertainty for future research.
METHODS
Assessment of the Quality of Studies
Studies comparing surgery with PBD versus surgery without PBD for jaundiced patients with tumors were identified. Studies were classified as level 1 if: 1) the study groups were properly randomized for PBD and control; 2) the outcome measure of death and complications resulting from PBD was specified; 3) the outcome measure of postoperative death and complications was specified; and 4) the length of hospital stay resulting from the drainage procedure and after surgery was specified. Studies that used contemporary nonrandomized control patients or used a posthoc analysis for the outcome after PBD and after surgery in jaundiced patients but fulfilled criteria 2, 3, and 4 were classified as level 2 studies.
The quality of the studies was assessed by two authors independently with attention to the following methodologic standards for clinical trials:18 methods and efficacy of randomization; blinding in evaluation of results; estimation of sample size; handling of withdrawals; information about patient characteristics; evaluation of patient enrollment; and assessment of therapeutic intervention. Each study was given an overall quality score based on the above criteria, which was then used to rank the studies. Interrater agreement was measured by the intraclass correlation. 19
These criteria were applied independently by two researchers, and any disagreement was resolved by group discussion.
Search Strategy
A computer-assisted search in Medline, Embase, Current Contents, and the Cochrane Database of Systematic Reviews databases (OVID) covering the period January 1966 to September 2001 was performed in September 2001 to identify published human trials in the English literature of peer-reviewed medical journals. For this purpose, the terms “preoperative biliary drainage” and “postoperative complications” were entered in a free text search. This search was updated by electronic searches and hand searches of cross-references in relevant journals. Potentially eligible studies were evaluated for methodologic strength independently by two authors.
The main subject of this review was the difference in outcome of the treatment modality between patients having PBD and those having surgery without PBD. Because of the small numbers and the lack of uniformity in studies, we considered the different drainage procedures (ie, external and internal drainage) and different surgical procedures (eg, resection, bypass surgery for unresectable disease, and laparotomies) each as one entity. Drainage procedure-related complications, early postoperative complications, total length of hospital stay, and in-hospital death were considered primary outcome measures; if data on one of these parameters were not given in the article, then that study was not included in the analysis for that specific parameter.
Data Extraction
Studies that met the criteria for levels 1 or 2 were masked by an independent research assistant who was not involved with the abstraction of data by obscuring the author’s names and institutions, the location of the study, reference lists, and any other potential identifiers. Two investigators reviewed each study independently, using a standard form, and extracted data on methodology, outcomes, and quality criteria. 20 The unit of allocation and analysis, concealment of allocation, blinding, and statistical power were recorded. Agreement between reviewers for the selection of relevant articles was 100%.
Statistical Methods
Review Manager 4.1 software (Cochrane Collaboration, Oxford, UK; available from www.cochrane.dk) was used to analyze the data. Estimates of the effectiveness of the intervention were expressed as odds ratios by using a fixed effect model (Peto method). To perform a statistical overview of hospitalization, standard methods for combining information from 2 × 2 tables were used. 21 Probability values were calculated using the chi-square or Fisher exact test when appropriate. The corresponding 95% confidence intervals were calculated by the test-based method using the Maentel-Haenszel chi-square statistic. 21 Probability values for comparisons of means were calculated by applying the formula for the Student test on mean differences, the total number of patients in each treatment arm, and an estimate of the standard deviation, taking a weighted average of the standard deviation in the publications in which they were mentioned.
RESULTS
Study Characteristics
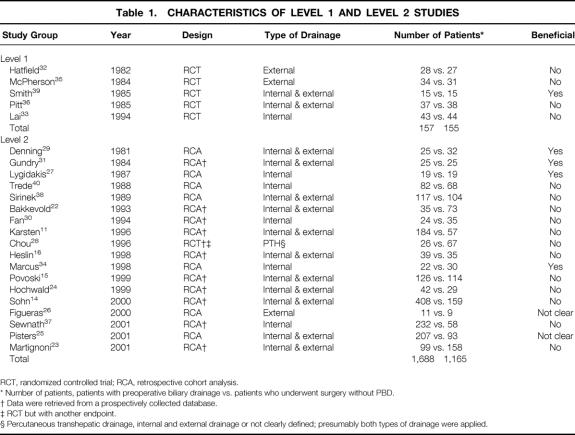
Twenty-three studies 11,14–16,22–40 were retrieved that were relevant and potentially eligible; the reference lists of these articles were also searched, but no further appropriate studies were found (Table 1). There were five randomized studies 32,33,35,36,39 that were analyzed as level 1 studies. The 18 nonrandomized studies were analyzed as level 2 studies and consisted of 11 prospective cohort studies 11,14–16,22–24,28,30,31,37 and 5 retrospective cohort studies. 25–27,29,34,38,40 The mean quality score of the trials was 2.75 (standard deviation 0.5), or 55% of the best quality for trial reporting. 41
Table 1. CHARACTERISTICS OF LEVEL 1 AND LEVEL 2 STUDIES
RCT, randomized controlled trial; RCA, retrospective cohort analysis.
* Number of patients, patients with preoperative biliary drainage vs. patients who underwent surgery without PBD.
† Data were retrieved from a prospectively collected database.
‡ RCT but with another endpoint.
§ Percutaneous transhepatic drainage, internal and external drainage or not clearly defined; presumably both types of drainage were applied.
Study Quality
Level 1 Studies
At level 1, four of the five studies reported enough information to determine that allocation had been adequately concealed (eg, consecutively numbered envelopes that contained the assigned treatment);33,35,36,39 in the fifth article, by Hatfield et al., the authors stated only that the study was randomized. 32 Four studies used masked assessors 33,35,36,39 and one had masked participants. 32 Only two studies (McPherson et al., 35 Lai et al. 33) reported a power calculation. All five randomized trials recorded that patient consent had been obtained, and four studies reported approval from an ethics committee. 32,33,35,36 Just one of the five randomized trials (Lai et al. 33) used solely internal PBD in their patients. Two studies used solely external PBD 32,35 and two studies used both types of drainage procedures. 36,39 Altogether, 312 patients were enrolled in the level 1 studies; 157 (50.3%) patients had PBD and 155 (49.7%) had surgery without PBD.
Only Smith et al. reported benefit on the use of PBD in terms of lowering postoperative rates of death and complications. 39 Two trials were terminated prematurely. The study by McPherson et al. was terminated because of high numbers of drainage procedure-related complications. 35 In the second study, Lai et al. stated that the estimated sample size was inadequate, but because the hospital death rates of the two treatment groups were close, inclusion of the remaining patients as planned would have added no further information. 33 Hatfield et al. stopped their trial after entering 55 patients, with eight deaths (four per group), although this was not fully explained in their report. 32 Pitt et al. also reported equal death rates in both trial arms. 36 Smith et al. did not make a power estimate, and so the reason why only 30 patients were enrolled cannot be explained. 39
Level 2 Studies
At level 2, one study was included where cohorts of patients were randomized for type of surgery (invaginated pancreaticojejunostomy vs. duct to mucosa anastomosis), 28 but the subgroup results were reported according to the use of PBD. In 11 studies, data were obtained from prospectively collected databases. 11,14–16,22–24,28,30,31,37 In seven studies data were collected retrospectively. 25–27,29,34,38,40
One trial reported on the use of external biliary drainage only, 26 5 trials reported on the use of only internal PBD, 27,30,34,37,40 in 11 studies both internal and external drainage were used, 11,14–16,22–25,29,31,38 and in 1 study it was not clear which type of drainage was used, 28 but presumably both types were used. Altogether, 2,853 patients were enrolled in the level 2 studies: 1,688 (59.2%) patients had PBD and 1,165 (40.8%) had surgery without PBD.
Only four studies reported a beneficial effect of the use of PBD in terms of lowering postoperative complication rates. 27,29,31,34 These studies did not consider complications resulting from to PBD and complications resulting from surgery as a separate issue.
Level 1 Studies
Clinical and Surgical Characteristics
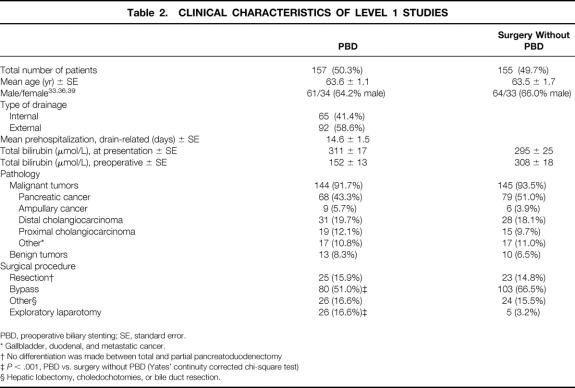
The number of patients treated with either PBD or surgery without PBD was nearly equal between both trial arms (Table 2). The mean age of all patients studied in the prospective randomized trials was 63.4 years and did not differ between the two study arms. Both patient groups had an equal male/female ratio. Internal biliary drainage was performed in 41% of the cases. The mean preoperative hospital stay for the drainage procedure was 14.6 ± 1.5 days. Mean bilirubin at presentation in the PBD group was slightly higher compared with the surgery without PBD group, but this difference was not significant. PBD led to a 50% reduction of plasma bilirubin levels. More than 90% of the tumors were malignant in both groups, adenocarcinoma of the pancreas being the most common malignancy. Only a few of the patients in both groups were treated with pancreatoduodenectomy. More than 50% of the patients in each group underwent bypass surgery (P = NS). Significantly more patients in the PBD group (17%) underwent exploratory laparotomy compared with the surgery without PBD group (3%).
Table 2. CLINICAL CHARACTERISTICS OF LEVEL 1 STUDIES
PBD, preoperative biliary stenting; SE, standard error.
* Gallbladder, duodenal, and metastatic cancer.
† No differentiation was made between total and partial pancreatoduodenectomy
‡P < .001, PBD vs. surgery without PBD (Yates’ continuity corrected chi-square test)
§ Hepatic lobectomy, choledochotomies, or bile duct resection.
Death Rate, Complications, and Hospital Stay Resulting From the Treatment Strategy
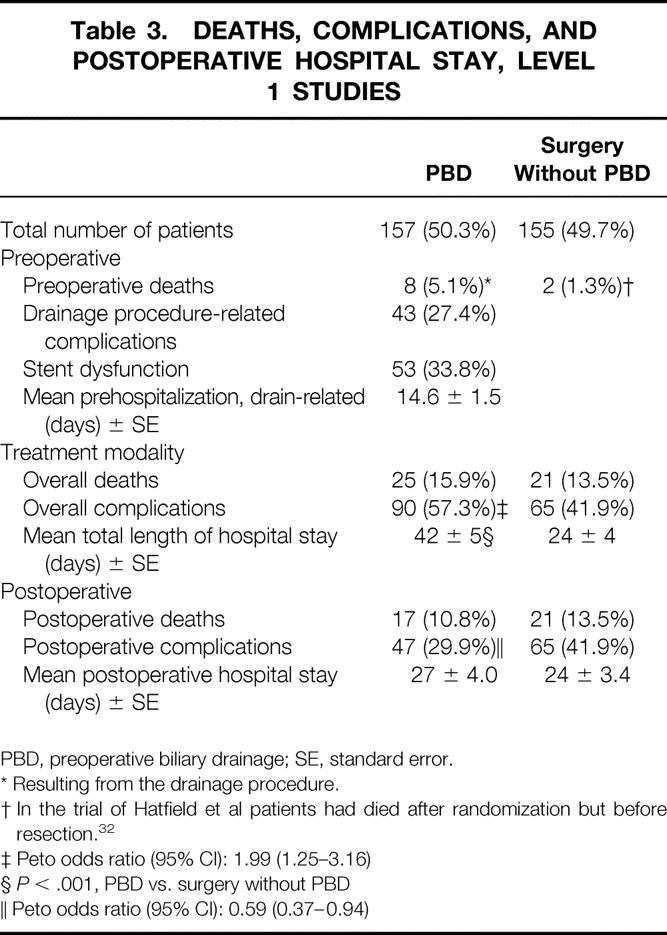
The preoperative death rate was higher in the PBD group compared with the surgery without PBD group: 5.1% versus 1.3% (P = NS;Table 3). In the PBD group eight patients died as a result of complications of the drainage proce-dure;32,33,35 in the surgery without PBD group two patients died after randomization but before resection as a result of biliary peritonitis and metastasis. 32 Twenty-seven percent of the patients in this trial arm had complications from the drainage procedure, including perforation of the duodenal wall (n = 10), bleeding (n = 15), and pancreatitis (n = 18). Early stent dysfunction occurred in one third of the patients, leading to a 9% rate of cholangitis in the PBD group.
Table 3. DEATHS, COMPLICATIONS, AND POSTOPERATIVE HOSPITAL STAY, LEVEL 1 STUDIES
PBD, preoperative biliary drainage; SE, standard error.
* Resulting from the drainage procedure.
† In the trial of Hatfield et al patients had died after randomization but before resection. 32
‡ Peto odds ratio (95% CI): 1.99 (1.25–3.16)
§P < .001, PBD vs. surgery without PBD
∥ Peto odds ratio (95% CI): 0.59 (0.37–0.94)
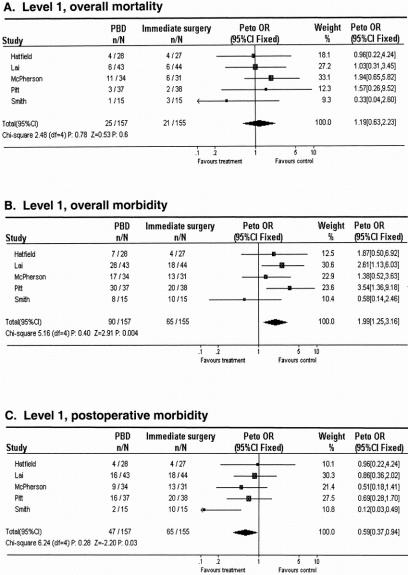
The overall death rate resulting from the treatment strategy was slightly higher in the PBD group compared with the surgery without PBD group: 15.9% versus 13.5% (P = NS;Fig. 1A). The overall complication rate resulting from the treatment strategy was significantly higher in the PBD group than in the surgery without PBD group: 57.3% versus 41.9% (Peto odds ratio [95% confidence interval]: 1.99 [1.25–3.16]; see Fig. 1B). Mainly as a result of the period before hospital admission of approximately 15 days, the mean total length of hospital stay was significantly increased in the PBD group (42 ± 5 days) compared with 24 ± 4 days in the surgery without PBD group (P < .01).
Figure 1. Meta-analysis for aggregated data in level 1 studies: effect of preoperative biliary drainage (PBD; treatment arm) and surgery without PBD (control arm) on overall death rate (A), overall complication rate (B), and postoperative complication rate (C) in patients undergoing pancreaticobiliary surgery for suspected periampullary tumors. Values less than 1 indicate that the treatment is better than control.
If drainage-related complications were excluded from analysis, then the postoperative death rate was still only slightly lower in the PBD group compared with the surgery without PBD group: 10.8% versus 13.5% (P = NS). However, postoperative complications were significantly lower after PBD than after surgery without PBD: 29.9% versus 41.9% (Peto odds ratio [95% confidence interval]: 0.59 [0.37–0.94]; see Fig. 1C). The mean postoperative hospital stay was 27 ± 4 days for stented patients and 24 ± 3 days for patients who underwent surgery without PBD (P = NS).
Level 2 Studies
Clinical and Surgical Characteristics
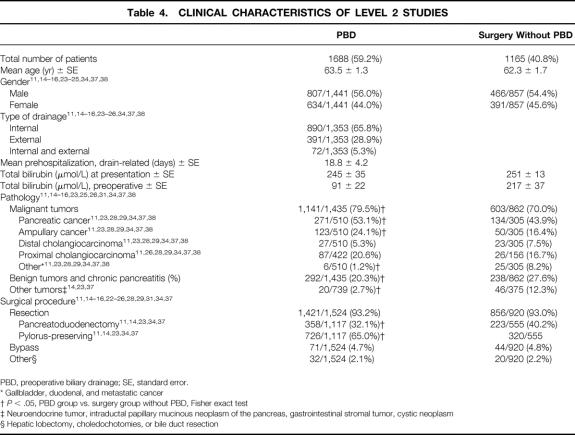
The number of patients treated with PBD was 59% (Table 4). The mean age of all patients studied in the collected trials was 62.9 years and did not differ between both groups, nor was there a difference in the male/female ratio. Sixty-six percent of the patients were drained internally, 29% externally, and 5% underwent both types of drainage procedures. Mean preoperative hospital stay for the drainage procedure was 18.8 ± 4.2 days. Mean bilirubin at presentation was 248 ± 23 and did not differ in the groups. In the PBD group, mean plasma bilirubin levels decreased by 63% from 245 μmol/L to 91 μmol/L toward surgery. Eighty percent of the patients undergoing PBD and 70% of the patients undergoing surgery without PBD had an underlying malignancy (P < .05). Adenocarcinoma of the pancreas was the most common malignancy in both groups. The majority of patients in both groups underwent surgical resection. Only five trials reported on the type of resection, 11,14,23,34,37 and significantly more patients in the surgery without PBD group (40%) underwent classical pancreatoduodenectomy compared with the PBD group (32%;P < .05).
Table 4. CLINICAL CHARACTERISTICS OF LEVEL 2 STUDIES
PBD, preoperative biliary drainage; SE, standard error.
* Gallbladder, duodenal, and metastatic cancer
†P < .05, PBD group vs. surgery group without PBD, Fisher exact test
‡ Neuroendocrine tumor, intraductal papillary mucinous neoplasm of the pancreas, gastrointestinal stromal tumor, cystic neoplasm
§ Hepatic lobectomy, choledochotomies, or bile duct resection
Death Rate, Complications, and Hospital Stay Resulting From the Treatment Strategy
Only six trials reported on the death rate (0.3%) resulting from the drainage procedure (Table 5). 11,27,29,31,37,38 Ten percent of the patients treated with PBD had drainage procedure-related complications, mostly perforation of the duodenal wall. Stent dysfunction occurred in 26% of this population, mostly leading to cholangitis. The time before hospital admission was considerable, on average 19 days, as a result of drainage procedures and related complications. It is not clear whether this also included the time needed for staging.
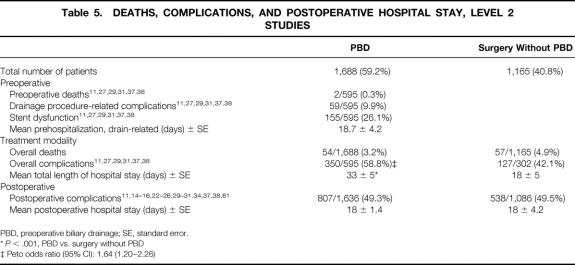
Table 5. DEATHS, COMPLICATIONS, AND POSTOPERATIVE HOSPITAL STAY, LEVEL 2 STUDIES
PBD, preoperative biliary drainage; SE, standard error.
*P < .001, PBD vs. surgery without PBD
‡ Peto odds ratio (95% CI): 1.64 (1.20–2.26)
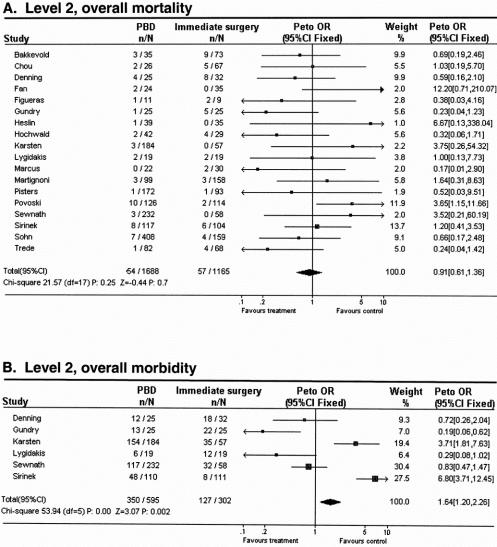
The overall death rate as a result of the treatment strategy was slightly lower in the PBD group compared with the surgery without PBD group: 3.2% versus 4.9% (P = NS;Fig. 2A). Six studies reported enough information on overall complications, 11,27,29,31,37,38 which was only significantly higher in the PBD group than in the surgery without PBD group: 58.8% versus 42.1% (Peto odds ratio [95% confidence interval]: 1.64 [1.20–2.26]; see Fig. 2B).
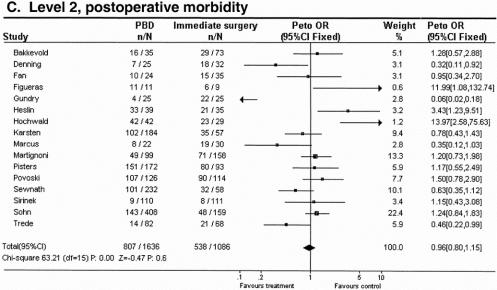
Figure 2. Meta-analysis for aggregated data in level 2 studies: effect of preoperative biliary drainage (PBD; treatment arm) and surgery without PBD (control arm) on overall death rate (A), overall complication rate (B), and postoperative complication rate (C) in patients undergoing pancreaticobiliary surgery for suspected tumors. Values less than 1 indicate that the treatment is better than control.
Figure. Continued
The postoperative complication rate was equal between PBD and surgery without PBD: 49.3% versus 49.5% (see Fig. 2C). The mean postoperative hospital stay was 14 ± 1 days in stented patients and 18 ± 4 days in patients with surgery without PBD; although 4 days shorter for the PBD group, there was no significant difference compared with the surgery without PBD group.
DISCUSSION
This systematic review does not provide evidence for a clinical benefit of using PBD in jaundiced patients with tumors planned for surgery. Indeed, it even shows that this treatment strategy increases the overall complication rate. Moreover, the increase in total hospital stay resulting from these concurrent problems adds more inconvenience for the patient, despite the considerable improvement in success rates and decreased complication rates that have occurred in the past decade. Decreasing drainage-related complications could be a way of improving the beneficial effects of PBD that may be expected based on experimental data.
Although in recent years the postoperative complication rate has declined dramatically, surgery in patients with obstructive jaundice remains associated with a substantial rate of complications, even in high-volume centers. Major perioperative strategies to improve postoperative outcome have emerged. Although at first it was propagated to perform PBD, randomized trials showed that there is no evidence that surgery with PBD is better than surgery without PBD. In several centers, PBD was rejected because of reports of adverse events of the procedure (eg, increased incidence of wound infection and anastomotic leakage). 14,15,37 But although there is no scientific evidence of the beneficial effects of PBD, as many as 6 of 10 patients are still being treated that way as a temporary measure to prevent cholangitis after diagnostic endoscopic retrograde cholangiopancreatography (ERCP) or to reduce obstructive jaundice because of an expected delay in surgery resulting from the need for further preoperative assessment or a long waiting time before surgery. 42
The most important drawback of most trials is the lack of uniformity. First, three studies used external biliary drainage only, 26,32,35 six studies reported on the use of only internal biliary drainage, 27,30,33,34,37,40 and most studies used both. 11,14–16,22–25,28,29,31,36,38,39 Although both decompress the biliary tract, the pathophysiologic consequence of internal drainage is entirely different from that of external drainage in terms of restoration of the enterohepatic cycle, 43,44 colonization of the biliary tract, 12,45 and inflammatory reaction of the biliary tract. 13 Second, different levels of biliary obstruction are compared as one. Biliary drainage of a proximal tumor with intrahepatic stenosis of the bile duct is different from a distal obstruction. Third, different types of operations are compared, although it is evident that the complication and death rates of a pancreatoduodenectomy are greater than a bypass procedure.
Another point of variation in the studies was the duration of PBD: the range was 12 to 26 days in level 1 studies and 10 to 32 days in level 2 studies. The wide range can be explained by patients requiring early surgery for drainage procedure-related complications and other patients having a slow decrease in the plasma bilirubin level, but also a waiting time for surgery. To date the optimal duration of PBD remains undetermined. The duration of biliary drainage should probably be at least 4 weeks. Even if the bilirubin level has decreased to normal levels, hepatic function will be fully restored only after at least 4 to 6 weeks. Koyama et al. showed that it takes more than 6 weeks of decompression before hepatic mitochondrial functions return to normal. 46 Aronson et al. demonstrated that at the histologic level liver damage was completely reversible within 8 weeks of internal bile flow restoration in cholestatic rats. 47 On the other hand, McPherson et al. demonstrated by using the antipyrine clearance method that hepatic function does not improve consistently during drainage. 48 Also, depressed cell-mediated immunity, impaired hepatic reticuloendothelial function, 49 and altered lymphocyte transformation have been documented, 30,50 and these functions are unlikely to improve within 4 weeks. A too-short period of PBD is probably not able to reverse the various metabolic abnormalities associated with obstructive jaundice, whereas in patients with an underlying malignancy some of these parameters may not be reversible at all. Increasing drainage time increases the risks of stent clogging and secondary inflammatory changes to the bile duct wall. If these complications require readmission to the hospital, this might lead to postponing surgery.
Stenting is routinely applied after a diagnostic ERCP in patients with obstructive jaundice to prevent cholangitis. However, at present other noninvasive imaging techniques as spiral computed tomography and magnetic resonance imaging/magnetic resonance cholangiopancreatography, have taken over from the diagnostic ERCP. Subsequently, the ideal strategy should probably be a diagnostic workup without invasive visualization of the bile duct and accurate selection of patients for endoscopic palliative stenting or immediate surgery. In patients with severe jaundice (bilirubin > 150 μmol/L) and/or cholangitis, PBD might still be indicated, although evidence is lacking.
It is conceivable that there are specific subgroups of jaundiced patients with tumors undergoing surgery without PBD and having a greater risk of developing postoperative complications who would substantially benefit from a complication-free PBD. At present, such a subgroup has not been convincingly identified, probably because of the lack of uniformity in the identified studies.
Although PBD seemed to reduce the incidence of postoperative complications, the absolute benefit was relatively small, even detrimental. Given this modest benefit of PBD for unselected jaundiced patients with tumors, PBD cannot be recommended routinely.
Methodologic Issues
One of the reasons to exclude historical controls from this analysis was that many factors, such as an improved awareness of the importance of nutrition, may have changed over time and may have resulted in a reduction in the number of deaths. 8,51,52 Moreover, the technique itself has improved, with reduced rates of death and complications, and has shifted from external to internal drainage.
The quality of the studies at level 1 was moderate, and sample sizes were often small. Another point of concern is the preoperative mean bilirubin level of 152 μmol/L in patients despite PBD. Because hyperbilirubinemia has been proven to be a potential risk factor in hepatopancreatobiliary surgery, 3,5,53 this must be decreased much further.
Unfortunately only one study, by Sohn et al., analyzed whether internal drainage was better than external drainage; it concluded that there was no difference in terms of death or complications. 14 However, experimental studies have proven the benefit of internal biliary drainage, among others by improving nutritional status, 54 reducing endotoxemia, 55 normalizing abnormal lipid status, 56 improving immune functions, 57 and even reducing the death rate. 9 For the same reason the question of whether PBD is helpful in patients with hilar obstruction cannot be answered. Only the study by Lai et al. 33 at level 1 and the studies by Hochwald et al. 24 and Figueras et al. 26 at level 2 reported data on the benefit of PBD in patients with proximal cholangiocarcinoma. None of the studies showed a benefit for PBD. Therefore, there were not enough data available to perform a decent analysis of this matter. It is, however, known from other studies that biliary drainage of proximal cholangiocarcinomas is associated with high rates of complications. 58 Moreover, (extended) hepatic resection in patients with severe obstructive jaundice leads to more liver insufficiency and subsequent higher rates of death and complications. 59–61
A cost–benefit analysis should be an integral part of any future study because the excess cost of the PBD procedure should be taken into account. In only two studies was this issue discussed. Marcus et al. suggested that PBD for tumor resection in jaundiced patients would be cost-effective;34 Pitt et al. demonstrated in their randomized trial that patients randomized to receive a stent had increased hospital costs. 36
Finally, trials should be consistent in inclusion and exclusion criteria. Most trials enrolled patients with infectious and coagulation abnormalities in addition to obstructive jaundice with lesions at varying levels of the biliary tree, and various curative and palliative surgical procedures. In addition, patients with cholangitis should be excluded.
CONCLUSIONS
This meta-analysis shows that PBD for jaundiced patients with resectable tumors is not beneficial because of drainage-related procedures. Because of the lack of uniformity of the studies, it might be that a well-selected high-risk subgroup of patients might benefit from PBD, but this cannot be identified by use of a meta-analysis. Therefore, new, properly designed randomized controlled trials are necessary to identify patients who might benefit from PBD.
Footnotes
Correspondence: Dirk J. Gouma, MD, Department of Surgery, Suite G4-116, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
E-mail: d.j.gouma@amc.uva.nl
Accepted for publication January 25, 2002.
References
- 1.Armstrong CP, Dixon JM, Taylor TV, et al. Surgical experience of deeply jaundiced patients with bile duct obstruction. Br J Surg 1984; 71: 234–238. [DOI] [PubMed] [Google Scholar]
- 2.Greig JD, Krukowski ZH, Matheson NA. Surgical morbidity and mortality in one hundred and twenty-nine patients with obstructive jaundice. Br J Surg 1988; 75: 216–219. [DOI] [PubMed] [Google Scholar]
- 3.Blamey SL, Fearon KC, Gilmour WH, et al. Prediction of risk in biliary surgery. Br J Surg 1983; 70: 535–538. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JM, Armstrong CP, Duffy SW, et al. Factors affecting mortality and morbidity after surgery for obstructive jaundice. Gut 1984; 25: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawarada Y, Higashiguchi T, Yokoi H, et al. Preoperative biliary drainage in obstructive jaundice. Hepato-Gastroenterology 1995; 42: 300–307. [PubMed] [Google Scholar]
- 6.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg 1935; 102: 763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takada T, Hanyu F, Kobayashi S, et al. Percutaneous transhepatic cholangial drainage: direct approach under fluoroscopic control. J Surg Oncol 1976; 8: 83–97. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama T, Ikeda A, Okuda K. Percutaneous transhepatic drainage of the biliary tract: technique and results in 104 cases. Gastroenterology 1978; 74: 554–559. [PubMed] [Google Scholar]
- 9.Gouma DJ, Coelho JC, Schlegel JF, et al. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch Surg 1987; 122: 731–734. [DOI] [PubMed] [Google Scholar]
- 10.Greve JW, Maessen JG, Tiebosch T, et al. Prevention of postoperative complications in jaundiced rats. Internal biliary drainage versus oral lactulose. Ann Surg 1990; 212: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsten TM, Allema JH, Reinders M, et al. Preoperative biliary drainage, colonisation of bile and postoperative complications in patients with tumours of the pancreatic head: a retrospective analysis of 241 consecutive patients. Eur J Surg 1996; 162: 881–888. [PubMed] [Google Scholar]
- 12.Karsten TM, Davids PH, van Gulik TM, et al. Effects of biliary endoprostheses on the extrahepatic bile ducts in relation to subsequent operation of the biliary tract. J Am Coll Surg 1994; 178: 343–352. [PubMed] [Google Scholar]
- 13.Karsten TM, Coene PP, van Gulik TM, et al. Morphologic changes of extrahepatic bile ducts during obstruction and subsequent decompression by endoprosthesis. Surgery 1992; 111: 562–568. [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg 2000; 4: 258–267. [DOI] [PubMed] [Google Scholar]
- 15.Povoski SP, Karpeh MSJ, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg 1999; 230: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heslin MJ, Brooks AD, Hochwald SN, et al. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg 1998; 133: 149–154. [DOI] [PubMed] [Google Scholar]
- 17.Povoski SP, Karpeh MS, Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg 1999; 3: 496–505. [DOI] [PubMed] [Google Scholar]
- 18.Nicolucci A, Grilli R, Alexanian AA, et al. Quality, evolution, and clinical implications of randomized, controlled trials on the treatment of lung cancer. A lost opportunity for meta-analysis. JAMA 1989; 262: 2101–2107. [PubMed] [Google Scholar]
- 19.Bartko JJ. Measures of agreement: a single procedure. Stat Med 1994; 13: 737–745. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane Collaboration on Effective Professional Practice. The Cochrane Collaboration on Effective Professional Practice data collection checklist. York: Department of Health Sciences and Clinical Evaluation. University of York, 1996.
- 21.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research. Principles and quantitative methods. Belmont: Lifetime Learning Publications, 1982.
- 22.Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Ann Surg 1993; 217: 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martignoni ME, Wagner M, Krahenbuhl L, et al. Effect of preoperative biliary drainage on surgical outcome after pancreatoduodenectomy. Am J Surg 2001; 181: 52–59. [DOI] [PubMed] [Google Scholar]
- 24.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg 1999; 134: 261–266. [DOI] [PubMed] [Google Scholar]
- 25.Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001; 234: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueras J, Llado L, Valls C, et al. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transplant 2000; 6: 786–794. [DOI] [PubMed] [Google Scholar]
- 27.Lygidakis NJ, van der Heyde MN, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand 1987; 153: 665–668. [PubMed] [Google Scholar]
- 28.Chou FF, Sheen-Chen SM, Chen YS, et al. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur J Surg 1996; 162: 477–481. [PubMed] [Google Scholar]
- 29.Denning DA, Ellison EC, Carey LC. Preoperative percutaneous transhepatic biliary decompression lowers operative morbidity in patients with obstructive jaundice. Am J Surg 1981; 141: 61–65. [DOI] [PubMed] [Google Scholar]
- 30.Fan ST, Lo CM, Lai EC, et al. T-lymphocyte function in patients with malignant biliary obstruction. J Gastroenterol Hepatol 1994; 9: 391–395. [DOI] [PubMed] [Google Scholar]
- 31.Gundry SR, Strodel WE, Knol JA, et al. Efficacy of preoperative biliary tract decompression in patients with obstructive jaundice. Arch Surg 1984; 119: 703–708. [DOI] [PubMed] [Google Scholar]
- 32.Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet 1982; 2: 896–899. [DOI] [PubMed] [Google Scholar]
- 33.Lai EC, Mok FP, Fan ST, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg 1994; 81: 1195–1198. [DOI] [PubMed] [Google Scholar]
- 34.Marcus SG, Dobryansky M, Shamamian P, et al. Endoscopic biliary drainage before pancreaticoduodenectomy for periampullary malignancies. J Clin Gastroenterol 1998; 26: 125–129. [DOI] [PubMed] [Google Scholar]
- 35.McPherson GA, Benjamin IS, Hodgson HJ, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg 1984; 71: 371–375. [DOI] [PubMed] [Google Scholar]
- 36.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg 1985; 201: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sewnath ME, Birjmohun RS, Rauws EAJ, et al. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg 2001; 192: 726–34. [DOI] [PubMed] [Google Scholar]
- 38.Sirinek KR, Levine BA. Percutaneous transhepatic cholangiography and biliary decompression. Invasive, diagnostic, and therapeutic procedures with too high a price? Arch Surg 1989; 124: 885–888. [DOI] [PubMed] [Google Scholar]
- 39.Smith RC, Pooley M, George CR, et al. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery 1985; 97: 641–648. [PubMed] [Google Scholar]
- 40.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg 1988; 207: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a meta-analysis. Br Med J 1998; 317: 1477–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillemoe KD. Preoperative biliary drainage and surgical outcome. Ann Surg 1999; 230: 143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey ME. Endotoxin, bile salts and renal function in obstructive jaundice. Br J Surg 1976; 63: 774–778. [DOI] [PubMed] [Google Scholar]
- 44.Cahill CJ. Prevention of postoperative renal failure in patients with obstructive jaundice—the role of bile salts. Br J Surg 1983; 70: 590–595. [DOI] [PubMed] [Google Scholar]
- 45.Blenkharn JI, McPherson GA, Blumgart LH. An improved system for external biliary drainage. Lancet 1981; 2: 781–782. [DOI] [PubMed] [Google Scholar]
- 46.Koyama K, Takagi Y, Ito K, et al. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg 1981; 142: 293–299. [DOI] [PubMed] [Google Scholar]
- 47.Aronson DC, Chamuleau RA, Frederiks WM, et al. Reversibility of cholestatic changes following experimental common bile duct obstruction: fact or fantasy? J Hepatol 1993; 18: 85–95. [DOI] [PubMed] [Google Scholar]
- 48.McPherson GA, Benjamin IS, Boobis AR, et al. Antipyrine elimination as a dynamic test of hepatic functional integrity in obstructive jaundice. Gut 1982; 23: 734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt DR, Allison ME, Prentice CR, et al. Endotoxemia, disturbance of coagulation, and obstructive jaundice. Am J Surg 1982; 144: 325–329. [DOI] [PubMed] [Google Scholar]
- 50.Hunt CE, Diani AR, Brown PK, et al. Diet-induced atherogenic hyperlipoproteinaemia and liver injury in cynomolgus macaques. Br J Exp Pathol 1986; 67: 235–249. [PMC free article] [PubMed] [Google Scholar]
- 51.Norlander A, Kalin B, Sundblad R. Effect of percutaneous transhepatic drainage upon liver function and postoperative mortality. Surg Gynecol Obstet 1982; 155: 161–166. [PubMed] [Google Scholar]
- 52.Gobien RP, Stanley JH, Soucek CD, et al. Routine preoperative biliary drainage: effect on management of obstructive jaundice. Radiology 1984; 152: 353–356. [DOI] [PubMed] [Google Scholar]
- 53.Dixon JM, Armstrong CP, Duffy SW, et al. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut 1983; 24: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gouma DJ, Roughneen PT, Kumar S, et al. Changes in nutritional status associated with obstructive jaundice and biliary drainage in rats. Am J Clin Nutr 1986; 44: 362–369. [DOI] [PubMed] [Google Scholar]
- 55.Saitoh N, Hiraoka T, Uchino R, et al. Endotoxemia and intestinal mucosal dysfunction after the relief of obstructive jaundice by internal and external drainage in rats. Eur Surg Res 1995; 27: 11–18. [DOI] [PubMed] [Google Scholar]
- 56.Sewnath ME, Levels JHM, Oude Elferink RP, et al. Endotoxin-induced mortality in bile duct-ligated rats after administration of reconstituted High-Density lipoprotein. Hepatology 2000; 32: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 57.Clements WD, McCaigue M, Erwin P, et al. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut 1996; 38: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987; 2: 57–62. [DOI] [PubMed] [Google Scholar]
- 59.Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma—a single-center experience. Surgery 2000; 127: 395–404. [DOI] [PubMed] [Google Scholar]
- 60.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000; 191: 38–46. [DOI] [PubMed] [Google Scholar]
- 61.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]