Abstract
Objective
To evaluate the clinical usefulness of serum vascular endothelial growth factor (VEGF) levels in gastric cancer patients.
Summary Background Data
Vascular endothelial growth factor plays an important role in the formation of new blood vessels involved in the growth and metastatic spread of solid tumors, but there is limited information regarding the clinical significance of serum VEGF levels in cancer patients.
Methods
Serum VEGF concentrations were measured by an enzyme linked immunosorbent assay in 61 healthy controls and in 58 gastric cancer patients before surgery, and then again at 7 and 30 days after surgery. The association between preoperative serum VEGF levels, clinicopathological features and patient survival, and their changes following surgery were evaluated.
Results
Serum VEGF levels in gastric cancer patients were significantly higher than those in controls. There was a significant association between serum VEGF levels and disease stage, as well as invasion depth of the tumor and the presence of distant metastases. Serum VEGF levels decreased significantly after radical resection of the primary tumor and increased in patients with unresectable tumors. Multivariate regression analysis showed that serum VEGF level is an independent prognostic factor for survival.
Conclusions
Serum VEGF levels in gastric patients are significantly higher compared with normal controls and correlate with local tumor extent, disease stage, and the presence of distant metastases. Preoperative serum VEGF concentration decreases significantly after radical resection of the primary tumor and is an independent prognostic factor for patient survival suggesting that determination of serum VEGF levels may be clinically useful.
The fact that solid tumor growth and metastasis is angiogenesis-dependent 1 suggests a potential value of blood and tissue angiogenic markers as prognostic and survival determinants. Vascular endothelial growth factor (VEGF) is a dimeric, heparin-binding glycoprotein that functions as a potent mitogen of vascular endothelial cells, providing an opportunity for their migration and organization for the neovascularization of micrometastases. 2,3 Vascular endothelial growth factor exists in four isoforms resulting from alternative exon splicing of its ribonucleic acid (RNA) transcript. 4,5 Of these isoforms, only VEGF121 and VEGF165 are secreted in soluble form, with VEGF165 being the predominant soluble isoform. The larger transcripts, (VEGF189 and VEGF206), are bound to heparin-containing proteoglycans in the extracellular matrix. 6
Experimental studies using different approaches clearly demonstrate that VEGF promotes tumor growth, angiogenesis, and metastasis formation. 7–10 Vascular endothelial growth factor expression at both the mRNA and protein level has also been demonstrated in vivo in a variety of gastrointestinal tumors (including gastric cancer 11,12), with the intensity of its immunohistochemical expression reflecting intratumoral microvessel density, the presence of lymph node and hepatic metastases, and cancer-specific survival. 12 Soluble forms of VEGF are also detectable in the serum and other biologic fluids from cancer patients with the most elevated levels being found in disseminated cancer. 13–15 However, there are limited data regarding the clinical and prognostic significance of serum VEGF (sVEGF) levels in gastric cancer patients.
In this study, we evaluated sVEGF levels in healthy controls and in gastric cancer patients. We then correlated these levels with clinicopathologic features and patient survival. The effect of surgery on preoperative sVEGF levels was also evaluated.
PATIENTS AND METHODS
Study population
Fifty-eight consecutive patients with newly diagnosed and histologically confirmed gastric cancer were included in this study. There were 38 men and 20 women with a median age of 68 (range, 49–89) years. Patients who had received chemotherapy, radiotherapy, or blood transfusion before surgery were excluded from the study. Fifty cases were of primary gastric cancer, whereas in eight patients the tumor had developed in the gastric remnant many years after resectional surgery for benign gastroduodenal ulcer disease. Tumor staging was based on clinical information, radiologic reports (chest radiography, abdominal ultrasonography and computerized tomography), operative findings, and pathology reports, with reports of staging made in accordance with the TNM staging system for gastric cancer. 16 Tumors were histologically classified as intestinal or diffuse according to their Lauren type, 17 and graded as well, moderately, or poorly differentiated based on the predominant cell type. Patients were followed prospectively and the date and cause of death was recorded. The median follow-up of the patients was 11 (range, 2–46) months.
Control subjects consisted of 61 age- and sex-matched healthy volunteers (median age 71 [range, 44–86] years; 33 men and 28 women). The absence of disease was confirmed by clinical history, physical examination, and routine laboratory tests including liver and renal function tests. The regional ethics committee approved the project and written informed consent was obtained from all patients and controls before their inclusion.
Blood samples and assays
Peripheral venous blood samples were drawn into sterile glass tubes (Vacutainer, Becton Dickinson, Plymouth, UK) in the morning between 8 and 9 hours after an overnight fast. Blood samples were allowed to coagulate at room temperature for 30 minutes and centrifuged at 2000 g for 10 minutes. Serum was separated, aliquoted, and stored at -70°C until assay. Serum samples from patients with gastric cancer were collected and stored on admission and 7 and 30 days following surgery.
Serum VEGF concentrations were determined using a commercially available enzyme-linked immunoassay (ELISA) designed to measure VEGF165 levels (Quantikine, R&D Systems Europe, Abingdon, UK). The assay employs the quantitative sandwich enzyme immunoassay technique using Sf21-expressed recombinant human VEGF165 and antibodies raised against the recombinant protein. The assay exhibits no significant cross-reactivity with other angiogenesis factors and has a sensitivity of 9.0 pg/mL. Optical density was measured at 450 nm using a microtitre plate reader (MR 5000, Dynatech Laboratories, Chantilly, VA, USA). All samples were assayed in duplicate.
Serum CEA and CA 19—9 determinations were also made by commercial ELISAs (Tosoh, Tokyo, Japan) as part of the routine preoperative patient evaluation.
Statistical Analysis
Statistical analysis was performed using the SPSS statistical software package. Data were tested for normality and were found to be nonnormally distributed. Accordingly, all data are presented as median value (interquartile range), with nonparametric analyses being employed to assess differences. The Kruskal-Wallis analysis of variance (ANOVA), the Mann-Whitney U test, and the Wilcoxon rank test were used to evaluate differences between multiple groups, unpaired and paired observations, respectively. Correlations were evaluated using the Spearman rank test. Survival curves were calculated using the Kaplan-Meier method and survival comparisons were made by the log-rank test. The Cox proportional hazards regression model was utilized for multivariate analyses after univariate analysis defined relevant prognostic variables. Significance was presumed at P < .05.
RESULTS
Serum VEGF Levels in Healthy Controls
Serum VEGF levels were detectable in all control subjects. Their median sVEGF level was 186 (101–266) pg/mL. There was no significant difference in sVEGF levels between males and females (198 [103–237] pg/mL versus 179 [100–288] pg/mL, respectively;P = .92). No correlation was found between sVEGF levels and age (r = .12;P = .32).
Serum VEGF Levels in Gastric Cancer Patients
Preoperative sVEGF levels (435 [282–730] pg/mL) in patients with gastric cancer were significantly higher than those in controls (P < .0001). There was no significant difference between males and females regarding sVEGF levels (409 [269–879] pg/mL versus 446 [361–682] pg/mL, respectively;P = .64), nor there was any correlation between sVEGF levels and age (r = .16;P = .22).
The relationships between sVEGF levels and clinicopathologic variables are shown in Table 1. There was a significant correlation between sVEGF levels and disease stage with higher sVEGF levels detected as the disease stage increased (P = .01). Although the relationship between sVEGF levels and the invasion depth of the tumor was not statistically significant by ANOVA (P = .09), further analysis using the Mann-Whitney U test showed that patients with tumors penetrating the serosa (T3–4) had significantly higher sVEGF levels when compared to those with tumors limited to the gastric wall (T1–2) (523 [300–900] pg/mL versus 311 [190–503] pg/mL, respectively;P = .04). Patients with distant metastases had significantly higher sVEGF levels when compared with those without metastatic disease (P = .004).
Table 1. RELATIONSHIP BETWEEN SERUM VEGF LEVELS AND PATHOLOGICAL VARIABLES IN GASTRIC CANCER PATIENTS
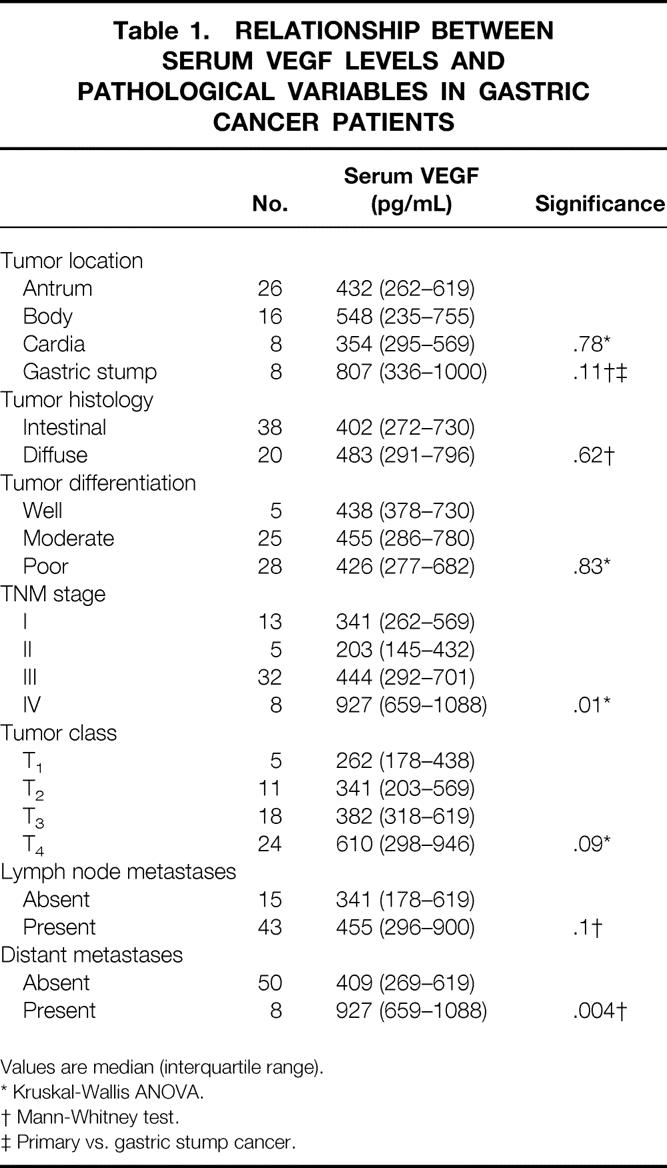
Values are median (interquartile range).
* Kruskal-Wallis ANOVA.
† Mann-Whitney test.
‡ Primary vs. gastric stump cancer.
There were no significant associations between sVEGF levels and tumor location (antrum, body or cardia), primary versus gastric stump cancer, intestinal versus diffuse histology, degree of differentiation or the presence of lymph node metastases. No correlation was detected between sVEGF levels and preoperative serum levels of either CEA (r = 0.16;P = .22) or CA 19—9 (r = 0.18;P = .16).
The Effect of Surgery on Serum VEGF Levels
The effect of surgical resection of the tumor was evaluated by sequential measurement of sVEGF levels before surgery and at 7 and 30 days following surgery. Radical resection including subtotal or total gastrectomy and removal of the regional perigastric lymph nodes (D1 dissection) was performed in 42 patients. Sixteen patients had unresectable tumors and underwent only by-pass surgery.
In the radical resection group, sVEGF levels at 7 days after surgery increased significantly compared with preoperative levels (556 [388–863] pg/mL versus 372 [262–608] pg/mL, respectively;P = .0015). There was a subsequent decrease with 30-day postoperative levels (242 [138–387] pg/mL) being significantly lower than preoperative levels (P = .0005). In those patients who underwent palliative by-pass only, there was a significant increase in sVEGF at 7 days compared with preoperative levels (934 [704–1282] pg/mL versus 701 [402–946] pg/mL, respectively;P = .0006) with their levels at 30 days (1006 [763–1260] pg/mL) remaining significantly higher (P = .0004) when compared with preoperative values (Fig. 1).
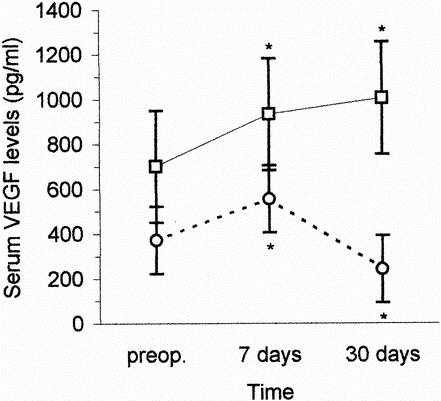
Figure 1. Changes in serum VEGF concentrations after radical resection of the tumor (○ and following unresectable disease (□). Values are median (interquartile range). *Statistically significant differences versus preoperative values (Wilcoxon rank test).
Correlations Between Circulating VEGF Levels and Patient Survival
Elevated serum VEGF levels were defined as being greater than the 95th percentile value in the healthy control group in accordance with the recommendations of Werther et al. 18 This resulted in a cut-off value of 533 pg/mL for sVEGF concentrations. Using this cut-off value, elevated sVEGF levels were found in 24 patients (41%).
During the study, 39 patients died from gastric cancer progression and 19 patients remained alive, three of them with documented tumor recurrence. Prognostic variables evaluated in a univariate analysis included age (entered as continuous variable), gender, histologic tumor type, differentiation, tumor location (antrum, body, cardia or gastric stump), TNM stage, depth of gastric wall invasion (T class), lymph node involvement, the presence or absence of distant metastases, and sVEGF level (normal vs. elevated). Univariate analysis showed tumor location, TNM stage, depth of gastric wall invasion, lymph node metastasis, distant metastasis, and preoperative sVEGF to be significant factors affecting overall survival (Table 2). Log-rank analysis showed elevated preoperative sVEGF levels to correlate with poor overall survival (P < .0001;Fig. 2). Multivariate regression analysis showed the presence of distant metastasis (hazard ratio, 9.77; 95% CI, 1.23–77.34;P = .03), depth of gastric wall invasion (hazard ratio, 3.4; 95% CI, 1.44–8.01;P = .005), and preoperative sVEGF levels (hazard ratio, 2.91; 95% CI, 1.32–6.38;P = .007), to be significant independent factors for cancer-specific survival in decreasing order of potency.
Table 2. UNIVARIATE ANALYSIS FOR PREDICTORS OF SURVIVAL IN 58 PATIENTS WITH GASTRIC CANCER
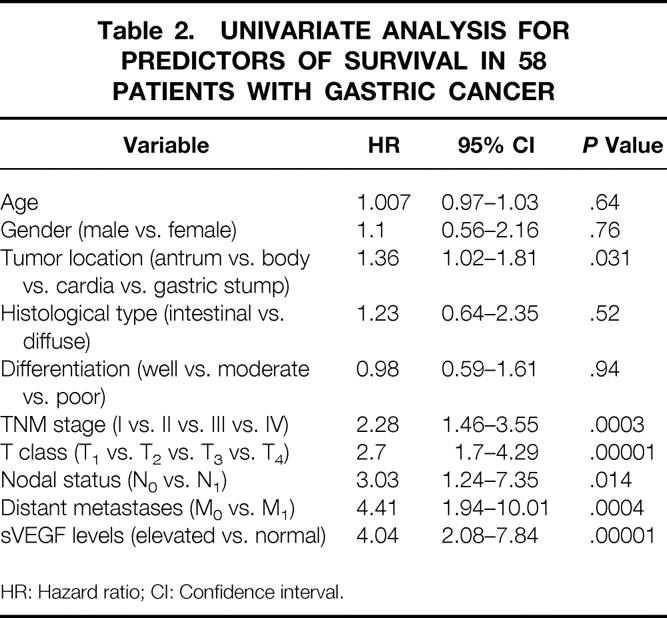
HR: Hazard ratio; CI: Confidence interval.
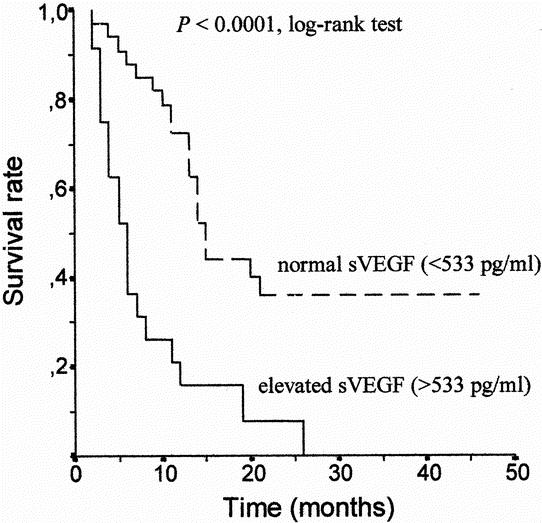
Figure 2. Kaplan-Meier survival curve in relation to serum VEGF levels in 58 patients with gastric cancer. The survival rate of patients with elevated serum levels of VEGF (>533 pg/mL) was significantly lower than that of patients with non-elevated levels (log-rank test P < 0.0001).
DISCUSSION
In the present study, we evaluated serum VEGF levels in healthy controls and in gastric cancer patients using a validated ELISA, which has been shown to be highly sensitive and specific for the detection of the main VEGF isoform, namely the VEGF165 isoform, although VEGF121 is also detectable to a lesser degree. 19
Our study shows a marked difference between preoperative sVEGF concentrations in gastric cancer patients when compared with age and sex-matched controls, with a significant association between these levels and tumor stage, local tumor extent, and the presence of distant metastases. Previous studies have shown that patients with advanced stage and metastatic cancer of different histologic types have higher sVEGF levels when compared with those with localized tumors. 13–15 In gastric cancer, Kitamura et al. 20 have also shown significantly higher sVEGF levels in patients with stage IV disease when compared with those that have stage I tumors, and a significant association between sVEGF levels and serosal involvement, T class and venous infiltration by the tumor. Similarly, the immunohistochemical expression of VEGF in gastric cancer has been associated with increased microvessel density, lymphatic and venous invasion and lymph node and liver metastases. 12 Our findings are in agreement with these observations showing an association with TNM stage, depth of gastric wall invasion and distant metastasis, which is maintained in multivariate analysis and which correlates preoperative sVEGF levels with cancer-specific survival.
A weak point in this study is that we have not examined the association between tissue VEGF expression and serum levels in the same patients. Several studies have correlated tissue VEGF expression with clinicopathologic features in gastric carcinoma, 12,21 but there is limited data at present. This suggests that in some tumors there is no clear correlation between tissue expression as detected by immunohistochemistry or immunoassay, and circulating VEGF levels. 22,23 The factors that regulate VEGF expression in gastric carcinoma are poorly understood, and will influence the detection of circulating VEGF. Mutations of the ras and p53 genes, which are important in gastric carcinogenesis, both up-regulate VEGF expression. 24,25 The levels of VEGF expression will also be affected by the local cytokine milieu, 26 the extent of intratumoral hypoxia, 27 and by variations in VEGF RNA splicing, which alters the ratio of detectable VEGF isoforms involved in the activation of endothelial cell growth within the tumor neovasculature. 28
Despite evidence of elevated sVEGF concentrations in cancer patients there has been considerable recent debate concerning its origin and the validity of serum over plasma VEGF measurement in cancer patients. Soluble VEGF levels were detectable in the serum of normal healthy volunteers, in accordance with previous reports. 13–15 Although in vitro 10 and in vivo 13,29 studies provided evidence that VEGF is released into the circulation by the primary tumor, its kinetics and degradation (as well as the effect on serum estimation of soluble VEGF-binding receptors and carrier proteins) are poorly characterized. Hematopoietic cell lineages including platelets, megakaryocytes, monocytes, and lymphocytes, 30,31 as well as release from normal tissues, 32 are also contributing to detectable circulating levels of VEGF. The latter implies a role for VEGF in the maintenance of physiologic endothelial integrity.
The presence of VEGF on platelets and its release during clotting may affect serum VEGF levels; their values reflect platelet-derived VEGF at least to some extent and they are consistently higher when compared with plasma samples. 33,34 However, cancer-derived serum VEGF is biologically active and appears to correlate with tumor growth kinetics, 35 implying a valid biologic effect for sVEGF in tumor biology. 36 Moreover, there are advantages of preoperative serum measurement as well as in the follow-up of patients after resection for cure over subjective and poorly standardized immunohistochemistry or complex extraction procedures. 37 As a result, we measured serum VEGF levels only in this study with a validated ELISA, which has shown high precision and parallelism in serial sample dilution. 19
In our study, radical resection of the tumor resulted in a significant decrease in preoperative sVEGF over a 30-day period, although a transient increase was observed one week after surgery, which could be associated with increased angiogenesis related to wound healing. 38 In contrast, all cases undergoing palliative by-pass only failed to show this sustained postoperative reduction in sVEGF levels. This finding supports further the hypothesis that soluble VEGF fragments are produced and released by the primary tumor. A decrease in sVEGF concentrations after partial response to chemotherapy and an increase in patients who had progressive disease after chemotherapy have both been shown previously in patients with recurrent gastric cancer. 20 These findings, along with our results, suggest that sVEGF might be able to be used as a measure of surgical completeness. Whether sVEGF can be used for the detection of tumor recurrence requires further study with longitudinal follow-up data.
We have found a significant association between the preoperative sVEGF level and overall survival, with sVEGF acting as an independent prognostic factor in multivariate analysis. This finding has been confirmed in the only other study of this type of which we are aware where plasma VEGF levels were measured in patients with gastric carcinoma. 39 These findings reflect inherent VEGF expression in gastric tumors and its role in prognosis, 40–42 as well as the importance of a tissue expression VEGF phenotype (particularly in unresectable gastric cancer). 43 Moreover, this VEGF-rich tissue phenotype appears to exert a greater impact on survival and chemo-responsiveness in inoperable cases than other conventional markers, such as histologic type and tumor extent (locally advanced or visceral metastatic disease). The association of VEGF expression in gastric carcinoma with other important angiogenic markers, such as platelet-derived endothelial growth factor, suggests that such tumors may be prognostically divisible based on their angiogenic profile, perhaps permitting their categorization in phase III trials with angiogenesis inhibitors. 44,45 An improved understanding of circulating VEGF dynamics in gastric cancer will assist in the development of new antiangiogenesis therapies and perhaps best define those patients with potentially chemo-sensitive tumors.
In conclusion, this study has demonstrated higher sVEGF levels in gastric cancer patients when compared with normal controls. These levels correlate with local tumor extent, disease stage, and the presence of distant metastases. Preoperative sVEGF concentration decreases significantly following radical resection of the primary tumor and is an independent prognostic factor for patient survival.
Acknowledgments
The authors thank their colleagues from the First Surgical Department, First IKA Hospital, Athens, Greece, for providing sera on gastric cancer patients.
Footnotes
This study was supported in part by a grant from the Medical School of the University of Athens, Greece.
Correspondence: Anastasios J. Karayiannakis, MSc, MD, Second Department of Surgery, Democritus University of Thrace, Medical School, 6 I. Kaviri Street, 68 100 Alexandroupolis, Greece.
E-mail: akarayan@usa.net
Accepted for publication November 29, 2001.
References
- 1.Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267: 10931–10934. [PubMed] [Google Scholar]
- 2.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: 1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Houck KA, Jakeman LB, et al. The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991; 47: 211–218. [DOI] [PubMed] [Google Scholar]
- 4.Houck KA, Ferrara N, Winer J, et al. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol 1991; 5: 1806–1814. [DOI] [PubMed] [Google Scholar]
- 5.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266: 11947–11954. [PubMed] [Google Scholar]
- 6.Houck KA, Leung DW, Rowland AM, et al. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 1992; 267: 26031–26037. [PubMed] [Google Scholar]
- 7.Claffey KP, Brown LF, del Aguila LF, et al. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 1996; 56: 172–181. [PubMed] [Google Scholar]
- 8.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993; 362: 841–844. [DOI] [PubMed] [Google Scholar]
- 9.Melnyk O, Shuman MA, Kim KJ. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res 1996; 56: 921–924. [PubMed] [Google Scholar]
- 10.Warren RS, Yuan H, Matli MR, et al. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995; 95: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993; 53: 4727–4735. [PubMed] [Google Scholar]
- 12.Maeda K, Chung YS, Ogawa Y, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996; 77: 858–863. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Toi M, Kondo S, et al. Concentrations of vascular endothelial growth factor in the sera of normal controls and cancer patients. Clin Cancer Res 1996; 2: 821–826. [PubMed] [Google Scholar]
- 14.Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999; 85: 178–187. [PubMed] [Google Scholar]
- 15.Salven P, Manpaa H, Orpana A, et al. Serum vascular endothelial growth factor is often elevated in disseminated cancer. Clin Cancer Res 1997; 3: 647–651. [PubMed] [Google Scholar]
- 16.Kennedy BJ. The unified international gastric cancer staging classification system. Scand J Gastroenterol 1987; 22 (Suppl 133): 11–13. [Google Scholar]
- 17.Lauren P. The two histological main types of gastric carcinoma. Difffuse and so-called intestinal type carcinoma. An attempt at a histoclinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 18.Werther K, Christensen IJ, Brunner N, et al. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. Eur J Surg Oncol 2000; 26: 657–662. [DOI] [PubMed] [Google Scholar]
- 19.Span PN, Grebenchtchikov N, Geurts-Moespot J, et al. EORTC receptor and biomarker study group report: a sandwich enzyme-linked immunosorbent assay for vascular endothelial growth factor in blood and tumor tissue extracts. Int J Biol Markers 2000; 15: 184–191. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura M, Toi M, Arai K, et al. Concentrations of vascular endothelial growth factor in the sera of gastric cancer patients. Oncol Rep 1998; 5: 1419–1424. [DOI] [PubMed] [Google Scholar]
- 21.Saito H, Tsujitani S, Kondo A, et al. Expression of vascular endothelial growth factor correlates with hematogenous recurrence in gastric carcinoma. Surgery 1999; 125: 195–201. [PubMed] [Google Scholar]
- 22.Takano S, Yoshii Y, Kondo S, et al. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 1996; 56: 2185–2190. [PubMed] [Google Scholar]
- 23.Balsari A, Maier JA, Colnaghi MI, et al. Correlation between tumor vascularity, vascular endothelial growth factor production by tumor cells, serum vascular endothelial growth factor levels, and serum angiogenic activity in patients with breast carcinoma. Lab Invest 1999; 79: 897–902. [PubMed] [Google Scholar]
- 24.Rak J, Mitsuhashi Y, Bayko L, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995; 55: 4575–4580. [PubMed] [Google Scholar]
- 25.Saito H, Tujitani S, Ikeguchi M, et al. Neoangiogenesis and relationship to nuclear p53 accumulation and vascular endothelial growth factor expression in advanced gastric carcinoma. Oncology 1999; 57: 164–172. [DOI] [PubMed] [Google Scholar]
- 26.Dolecki GJ, Connolly DT. Effects of a variety of cytokines and inducing agents on vascular permeability factor mRNA levels in U937 cells. Biochem Biophys Res Commun 1991; 180: 572–578. [DOI] [PubMed] [Google Scholar]
- 27.Minchenko A, Bauer T, Salceda S, et al. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest 1994; 71: 374–379. [PubMed] [Google Scholar]
- 28.Houck KA, Leung DW, Rowland AM, et al. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 1992; 267: 26031–26037. [PubMed] [Google Scholar]
- 29.Landriscina M, Cassano A, Ratto C, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer 1998; 78: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 1995; 55: 4140–4145. [PubMed] [Google Scholar]
- 31.Möhle R, Green D, Moore MAS, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 1997; 94: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berse B, Brown LF, Van de Water L, et al. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992; 3: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks RE, Forbes MA, Kinsey SE, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 1998; 77: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermeulen PB, Salven P, Benoy I, et al. Blood platelets and serum VEGF in cancer patients. Br J Cancer 1999; 79: 370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirix LY, Vermeulen PB, Hubens G, et al. Serum basic fibroblast growth factor and vascular endothelial growth factor and tumour growth kinetics in advanced colorectal cancer. Ann Oncol 1996; 7: 843–848. [DOI] [PubMed] [Google Scholar]
- 36.Lee JK, Hong YJ, Han CJ, et al. Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol 2000; 17: 149–152. [PubMed] [Google Scholar]
- 37.Adams J, Carder PJ, Downey S, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 2000; 60: 2898–2905. [PubMed] [Google Scholar]
- 38.Nissen NN, Polverini PJ, Koch AE, et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998; 152: 1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshikawa T, Tsuburaya A, Kobayashi O, et al. Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma. Cancer Lett 2000; 153: 7–12. [DOI] [PubMed] [Google Scholar]
- 40.Tanigawa N, Amaya H, Matsumura M, et al. Correlation between expression of vascular endothelial growth factor and tumor vascularity and patient outcome in human gastric carcinoma. J Clin Oncol 1997; 15: 826–832. [DOI] [PubMed] [Google Scholar]
- 41.Maeda K, Kang SM, Onoda N, et al. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer 1999; 86: 566–571. [DOI] [PubMed] [Google Scholar]
- 42.Maehara Y, Kabashima A, Koga T, et al. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery 2000; 128: 408–416. [DOI] [PubMed] [Google Scholar]
- 43.Boku N, Chin K, Hosokawa K, et al. Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5-fluorouracil and cis-platinum. Clin Cancer Res 1998; 4: 1469–1474. [PubMed] [Google Scholar]
- 44.Maeda K, Kang SM, Ogawa M, et al. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial growth factor expression in gastric carcinoma. Int J Cancer 1997; 74: 545–550. [DOI] [PubMed] [Google Scholar]
- 45.Ikeguchi M, Oka S, Saito H, et al. The expression of thymidine phosphorylase and its correlation with angiogenesis in gastric adenocarcinoma. Anticancer Res 1999; 19: 4001–4005. [PubMed] [Google Scholar]


