Abstract
Objective
To determine the effects of hypothermia and rewarming on changes in the villus microcirculation induced by intestinal ischemia–reperfusion (I/R).
Summary Background Data
The small intestine is extremely sensitive to I/R injury, and although hypothermia can reduce cellular injury, its capacity to influence the villous microcirculation after intestinal I/R is unclear, especially after the return to normothermic conditions.
Methods
Core body temperature of PVG rats was maintained at either 36° to 38°C (n = 12) or 30° to 32°C (n = 24) and then subjected to 30 minutes of intestinal ischemia. A subgroup of hypothermic animals (n = 12) were returned to normothermic conditions 120 minutes after clamp removal. The mucosal surface was visualized in an exteriorized ileal segment and macromolecular leak (MML) and leukocyte adhesion were monitored using in vivo microscopy (n = 6 in each group). MML from individual villi and numbers of adherent leukocytes within villi were determined for 2 to 4 hours after clamp removal. Heart rate and mean blood pressure were monitored in all animals. Control animals underwent sham surgery (n = 12).
Results
Ten of 12 normothermic animals failed to survive the reperfusion period, whereas all hypothermic animals and 11 of 12 of the hypothermic animals that were returned to normothermic conditions survived. MML was significantly increased in all animals subjected to I/R, although leakage was more marked in animals subjected to continuous normothermia. Enhanced leukocyte adhesion and decreased blood flow were observed only in normothermic animals.
Conclusions
Hypothermia might prove to be an effective strategy for preventing adverse side effects in clinical settings in which intestinal I/R can be predicted.
Ischemic injury to the small intestine can result from a number of clinical procedures, including thoracoabdominal aortic aneurysm repair and cardiopulmonary bypass, in addition to being an inevitable consequence of small bowel transplantation. 1–4 The intestinal mucosa is extremely sensitive to ischemia–reperfusion (I/R) injury, and even short periods of ischemia can induce substantial local tissue damage with the potential for other problems, including the development of remote organ damage and systemic shock. 5–7 A better understanding of the factors contributing to loss of tissue integrity after intestinal I/R and strategies for the preservation of the vasculature within the villi is crucial for maintaining effective absorptive and barrier functions.
The response to ischemia is biphasic, with the reperfusion phase being more detrimental and leading to an exacerbation of the original ischemic damage. 8,9 Key factors in the pathophysiology of I/R injury include endothelial activation and the upregulation of cell adhesion molecules during the ischemic phase, and an increase in leukocyte adhesion and vascular resistance in the tissue microcirculation during reperfusion. 10 Leukocyte adhesion in the reperfusion phase is accompanied by the release of reactive oxygen metabolites and the secretion of proteases such as collagenase and elastase. 11–13 The ensuing inflammatory process increases vascular permeability and promotes the extravasation of circulating leukocytes.
Strategies for ameliorating I/R damage have primarily focused on inhibiting leukocyte adhesion. However, it is well recognized that decreased temperatures reduce cellular metabolism, enzyme activity, and energy consumption. Systemic or local tissue cooling has therefore proved to be beneficial in both cerebrovascular and cardiovascular surgery. 14,15 Despite these demonstrated benefits, the effects of hypothermia on the microcirculatory disturbances associated with intestinal ischemia have not been investigated.
The effects of I/R injury on the mesenteric and submucosal microcirculation have been reported in a number of in vivo studies, with the suggestion that leukocyte adhesion within submucosal vessels is responsible for damage to the overlying layer. 16–18 However, tissue injury after intestinal ischemia is preferentially localized to the mucosal villi, with the underlying submucosal layers being relatively unaffected. 19,20 It is therefore more likely that leukocyte adhesion and loss of vascular integrity within the mucosal microcirculation itself contributes to villus damage. This study used fluorescent in vivo microscopy to evaluate the effects of hypothermia on leukocyte adhesion and vessel leakage in the villus microcirculation after intestinal I/R, and the influence on animal survival. We also determined whether a period of hypothermia could elicit beneficial responses in animals subsequently restored to normothermic conditions. This latter approach is important for evaluating the potential clinical applicability of this strategy for preventing the consequences of intestinal ischemia.
METHODS
Animals
All studies were performed using adult male PVG rats (Harlan, UK) weighing 250 to 300 g. Animals were assigned to one of four groups, each of which comprised a total of 12 animals: group 1—normothermic I/R (36°–38°C throughout experiment, 150 minutes); group 2—hypothermic I/R (30°–32°C throughout experiment, 150 minutes); group 3—hypothermia I/R (30°–32°C, 150 minutes), followed by normothermia (36°–38°C, 120 minutes); and group 4—normothermic, non-I/R sham controls (36°–38°C throughout experiment, 150 minutes).
Animals were fasted overnight before experimentation and housed individually in wire-bottomed cages to prevent coprophagy. Experimental procedures were carried out with appropriate Home Office approval (PPL 40/1787) and in accordance with the Animals (Scientific Procedures) Act of 1986.
Animal Preparation
The preparation of animals for in vivo microscopy and the collection and analysis of data have been detailed previously. 21 Briefly, animals were anesthetized with a subcutaneous injection of diazepam (5 mg/mL, Dumex Ltd., UK) and Hypnorm (fentanyl citrate 0.315 mg/mL and fluanisone 10 mg/mL, Janssen Pharmaceutical Ltd., UK) in a ratio of 1:1 in a volume of 0.1 mL/100 g body weight. Additional anesthesia was administered as required. A cannula placed in the left carotid artery monitored mean arterial blood pressure and heart rate and also provided access for the administration of fluorochromes. Animals were allowed to stabilize for a period of 30 minutes after anesthesia and monitoring had been established.
Experimental Protocol
The core body temperature of I/R animals subjected to hypothermia was lowered to 30° to 32°C within 15 minutes before experimentation using a cooling fan. In group 2, hypothermia was maintained throughout the 30 minutes of ischemia and 120 minutes of reperfusion. In group 3 animals were rewarmed using a heating pad. Rewarming commenced after 90 minutes of cold reperfusion, and the body temperature returned to 36° to 38°C within 30 minutes. The core body temperature of sham control (group 4) and normothermic I/R (group 1) animals was maintained at 36° to 38°C throughout the experiment.
The superior mesenteric artery was identified and isolated at laparotomy in all three I/R experimental groups, and the vessel was occluded for 30 minutes with a nontraumatic vessel clamp. Sham control animals underwent laparotomy alone. In all animals, including the sham controls, a segment of ileum was exteriorized, and the mucosal surface was exposed by making a 20-mm incision along the antimesenteric border with an electric microcautery.
Macroscopic evidence of patchy necrosis and hemorrhage along the entire length of the intestine was apparent immediately after clamp removal in normothermic animals (group 1), and microscopically these areas were associated with blood flow stasis. This patchy appearance is consistent with previous findings that totally denuded villi can be adjacent to overtly normal villi. 20 Given the patchy response, the incision to allow observation of the mucosal surface for observation in animals subjected to intestinal ischemia was always made in a viable area to allow the effects of I/R injury in an area of reflow to be assessed.
A glass microscope slide was mounted on Perspex pegs of a specially designed animal board, onto which the small intestine was gently extended and held in place with stay sutures. The mucosal surface was visualized by opening the incision using microclamps, and the small bowel preparation was covered with an impermeable membrane (Saran wrap) to prevent dehydration.
Fluorescent In Vivo Microscopy
The animal, warming pad, and Perspex board were transferred to the stage of a Nikon Optiphot-2 microscope (Nikon, UK) equipped with a mercury arc lamp for epi-illumination fluorescent light microscopy. Images were acquired using a charged-coupled device camera (CCD, Hitachi, UK), displayed on a high-resolution monitor (Sony PVM-1443; Sony, UK), and recorded on video (Sony SLV-373-UB; Sony, UK) for off-line analysis. The final magnification of the images on the monitor was ×590.
After release of the clamp in the I/R groups, or at the equivalent time point in the control group, animals received either fluorescein isothiocyanate conjugated to bovine serum albumin (FITC-BSA; 0.2 mL/100 g body weight; n = 6) or acridine orange (0.1 mL/100 g body weight, n = 6) as a bolus injection via the carotid cannula. FITC-BSA allows visualization of blood flow, and microvascular permeability to macromolecules (macromolecular leakage [MML]) appears as a fluorescent flare in the interstitium. Acridine orange labels leukocytes and allows their adhesion/retention in the microvasculature to be monitored. MML, blood flow, and leukocyte adherence were assessed in two separate areas of the exposed mucosa. Recordings were taken every 15 minutes after the administration of fluorochromes for a period of 2 hours in groups 1, 3, and 4 and for 4 hours in group 2, where hypothermic animals were subsequently rewarmed.
Quantification of Macromolecular Leakage
The leakage of FITC-BSA from the vessels was measured by mapping out an area of interest adjacent to up to four individual villi on the video screen. Interstitial fluorescence at each time point during the experiment was measured using computerized analysis (Capiscope, KK Research Technology Ltd., UK), and the recorded image was stored as a palette of 255 gray levels. The mean of the gray levels alongside the four individual villi was determined and arbitrary values for leak were derived. An increase in fluorescence represents a proportional increase in leak. 22 Blood flow in the mucosal vessels was assessed qualitatively.
Quantification of Leukocyte Adhesion
The number of leukocytes adherent to the endothelium of up to four individual villi (∼400–500 μm in length) was determined from the recorded videos. A leukocyte was considered to be adherent if it remained stationary for at least 30 seconds. Data are expressed as the number of adherent leukocytes per villus at each time point. Leukocytes that had emigrated to the interstitium but remained in close proximity to the capillaries were often difficult to distinguish from those that were adherent within the blood vessel. Therefore, both subpopulations of stationary leukocytes were regarded as adherent in this study. The difficulty in distinguishing these adherent leukocyte populations resulted primarily from images being obtained from thick tissue, the small diameter of the capillaries, and the presence of the mucosal surface in different focal planes.
Statistical Analysis
In the experimental and control groups, MML was compared using the Kruskal-Wallis and then the Mann-Whitney test for unpaired, nonparametric data. Heart rate, blood pressure, and leukocyte adherence before and at given time points during the experiments were compared using analysis of variance followed by a two-tailed Student t test. All data are expressed as mean ± standard error of the mean. Results were considered to be of statistical significance when P < .05.
RESULTS
Survival
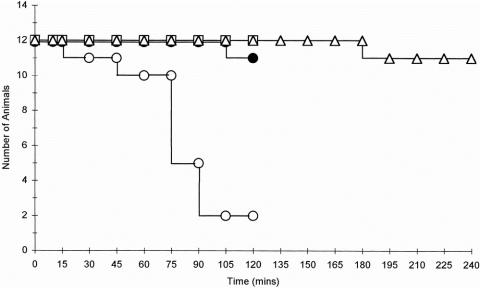
Eleven of the 12 control animals (group 4) survived the experimental period, whereas 10 of the 12 animals that were subjected to intestinal ischemia during normothermic conditions (group 1) died (Fig. 1). The majority of animals in this group died after 90 minutes, with respiratory distress being a common feature. In contrast, all 12 animals subjected to ischemia under hypothermic conditions (group 2) survived the 2-hour reperfusion period with no evidence of respiratory distress. Eleven of the 12 hypothermic animals that were subsequently rewarmed (group 3) also survived the 4-hour reperfusion period, and there was no evidence of respiratory distress in these animals at any time point.
Figure 1. Survival of control animals and ischemia–reperfusion (I/R) animals subjected to normothermic and hypothermic conditions. Eleven of the 12 control animals (•-•) and 2 of the 12 normothermic animals subjected to intestinal I/R (○-○) survived the 2-hour reperfusion period. However, all 12 animals subjected to hypothermia (□-□) survived and 11 of 12 hypothermic animals that were subsequently rewarmed (▵-▵) survived. Data presented are for the reperfusion phase.
Heart Rate and Blood Pressure
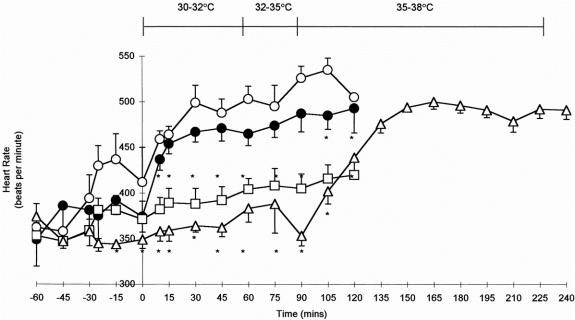
Heart rates in the control and normothermic ischemia groups were similar, but progressively increased during the course of the experiment (Fig. 2). Heart rate in the hypothermic animals in the reperfusion period was significantly lower than that in both the control and normothermic ischemia animals (P < .05). Rewarming of hypothermic animals increased the heart rate to that observed in controls.
Figure 2. Heart rates in control animals (•-•); normothermic ischemia–reperfusion (I/R) animals (○-○); hypothermic I/R animals (□-□); and rewarmed hypothermic I/R animals (▵-▵). *P < .05 versus control (two-tailed Student t test). Data are means ± standard error of the mean. The body temperature profile of rewarmed hypothermic animals is indicated.
Clamping of the mesenteric artery induced a transient increase in the mean arterial blood pressure (mBP; 103.0 ± 3.4 mm Hg to 124.0 ± 2.6 mm Hg;P < .001) in the warm ischemia group. mBP returned to baseline levels after 15 minutes of warm ischemia, at which level it remained until 60 minutes after reperfusion. Thereafter, mBP decreased significantly, and 10 of the 12 animals in this group died within 2 hours of reperfusion. Because the vascular disturbances in animals subjected to warm ischemia occurred immediately after reperfusion, it is likely that this was induced by, rather than resulted from, the decreased blood pressure observed after 75 minutes of reperfusion. mBP was maintained at baseline levels throughout the experiment in control animals and in both groups of animals subjected to hypothermia.
Blood Flow
Mucosal blood flow was rapid and stable throughout the experimental period in control animals, with homogeneous blood flow observed in all areas of interest and also within the individual villi themselves. Blood flow in normothermic I/R animals was comparable to controls immediately after reperfusion but declined toward the end of the experiment, and complete cessation of flow often occurred before the animal died. When observed, complete cessation of flow was present in all villi within that area, indicating that the effect of I/R on capillary perfusion was homogenous within the region and individual villi under observation. There was no apparent reduction in blood flow in the animals subjected to hypothermia.
Macromolecular Leakage
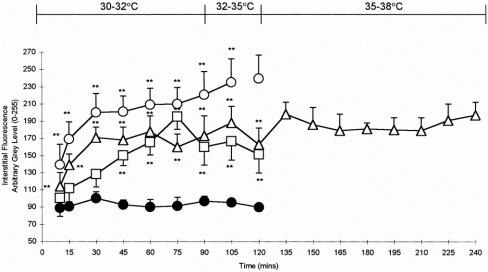
Vascular integrity was maintained in control animals, with no increases in interstitial fluorescence surrounding the villi observed during the experimental period (Figs. 3, 4). Interstitial fluorescence increased from a gray level of 138.2 ± 10.0 at 10 minutes to 220.57 ± 27 at 90 minutes in animals subjected to warm ischemia (P < .01). MML was both rapid and sustained at all time points, and hemoconcentration and congested capillaries were observed in a number of areas. Although MML also increased in hypothermic animals and peaked at 75 minutes (195.2 ± 14.8;P < .01), this was not as rapid, nor of the same magnitude, as that observed in the warm ischemia group. MML remained elevated but stable in hypothermic animals that were subsequently rewarmed, but did not approach levels observed at earlier time points in normothermic animals.
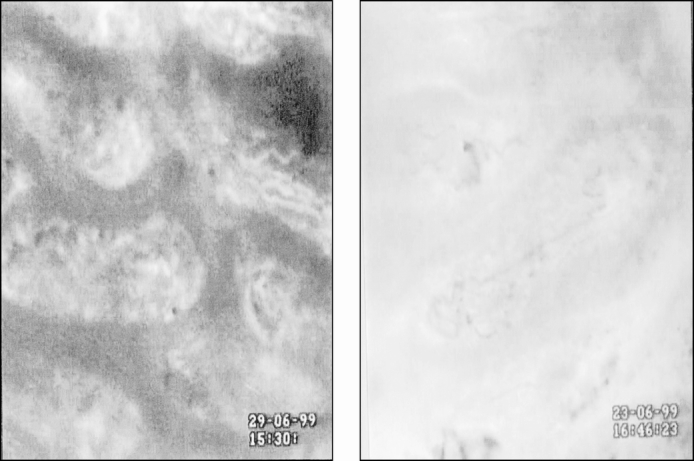
Figure 3. Interstitial fluorescence after administration of fluorescein isothiocyanate conjugated to bovine serum albumin (FITC-BSA) to (A) control and (B) normothermic animals subjected to intestinal ischemia. FITC-BSA was contained within the vessels in control animals, which appear white against a relatively black background. The capillaries within the villus are easily identified. Ischemia–reperfusion induced leakage of FITC-BSA (MML) from the capillaries into the interstitium, and a brighter flare surrounded the villi. Congestion of red blood cells was often observed within the villus capillaries. Magnification ×590.
Figure 4. Leakage of fluorescein isothiocyanate conjugated to bovine serum albumin (FITC-BSA) from the intestinal villus microcirculation in control animals and ischemia–reperfusion (I/R) animals subjected to normothermic and hypothermic conditions. Control animals (•-•); normothermic I/R animals (○-○); hypothermic I/R animals (□-□); rewarmed hypothermic I/R animals (▵-▵). *P < .05, **P < .01 versus control (Mann-Whitney test). No comparisons were performed after 105 minutes in the normothermic group because only two animals survived beyond this point. Data are means ± standard error of the mean. The body temperature profile of rewarmed hypothermic animals is indicated. Data presented are for the reperfusion phase.
Leukocyte Adhesion
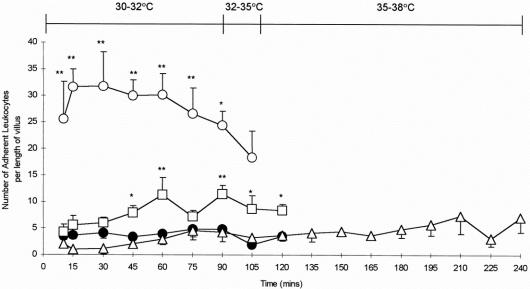
Only occasional adherent leukocytes were observed within the villi of control animals throughout the experimental period (Figs. 5, 6). I/R during normothermic conditions induced a rapid, sustained, and significant increase in leukocyte adherence within both the mucosal capillaries and the supplying arteriole (P < .01). Although leukocyte adhesion was also observed in the hypothermia groups, this was not only delayed but also of significantly lower magnitude than that observed after warm ischemia. Leukocyte adhesion did not increase on rewarming.
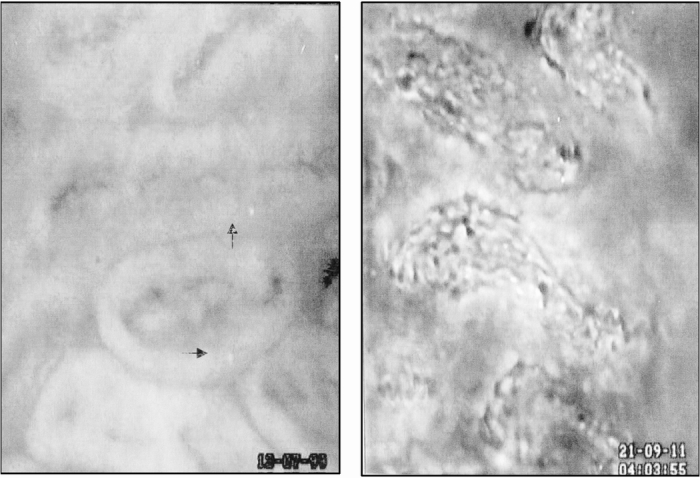
Figure 5. Intestinal villus microcirculation in (A) control animals and (B) normothermic animals subjected to intestinal ischemia after the administration of acridine orange. Few adherent leukocytes were observed within the villus microcirculation of control animals (arrows). Reperfusion of the small intestine induced the adherence of numerous leukocytes within the supplying arteriole and the capillaries. Control villi were associated with a thick epithelial layer of cells, whereas this healthy architecture was lost within 15 to 30 minutes of reperfusion in normothermic ischemia–reperfusion animals. Magnification ×590.
Figure 6. Leukocyte adhesion in the villi of controls and animals subjected to intestinal ischemia under normothermic and hypothermic conditions. Control animals (•-•); normothermic ischemia–reperfusion (I/R) animals (○-○); hypothermic I/R animals (□-□); rewarmed hypothermic I/R animals (▵-▵). *P < .05, **P < .01 versus control (two-tailed Student t test). Results are mean ± standard error of the mean. The body temperature profile of rewarmed hypothermic animals is indicated. Data presented are for the reperfusion phase.
Villus Morphology
Acridine orange also allowed villus morphology to be observed. There was a thick epithelial layer of cells surrounding the supplying arteriole and capillaries in the villi of control animals (see Fig. 5), and a similar morphologic appearance was observed throughout the experimental period in both groups of animals subjected to hypothermia. Healthy villus architecture was lost within 15 to 30 minutes of reperfusion in warm ischemia animals, and the epithelial layer became very thin or absent. The villi within hemorrhagic areas of this group were often denuded.
DISCUSSION
This is the first in vivo study to show that whole-body hypothermia inhibits mucosal villus microcirculatory disturbances and prevents the deaths associated with warm intestinal I/R injury. Further, protection is maintained after the restoration of normothermic conditions, suggesting that responses elicited during the ischemic and early reperfusion phases are responsible for the observed microcirculatory and systemic effects.
The mechanism by which hypothermia reduces I/R-induced tissue injury and death is unclear, but several possibilities exist. The function of adhesion molecules is temperature-dependent, and protection may therefore be related to the reduction of leukocyte adhesion. 23 Hypothermia has also been shown to suppress lymphocyte function 24 and to reduce vasoconstriction of supplying arterioles during ischemia of the rat cremaster muscle. 25
The postischemic intestine releases proinflammatory molecules such as hydrogen peroxide, superoxide radicals, and cytokines (tumor necrosis factor-α) into the portal and systemic circulation. As a result, circulating neutrophils are primed, and their sequestration in organs susceptible to neutrophil-mediated injury, such as the lungs, induces remote organ damage and the potential for multiple organ failure. 7,26,27 Hypothermia reduces the production of reactive oxygen metabolites by leukocytes and has been shown to decrease the production of proinflammatory thromboxanes and increase the synthesis of prostaglandins in burn injuries. 28,29
Although hypothermia significantly reduced the adhesion of leukocytes to the endothelium, it had little effect on MML, suggesting that the protective effects of hypothermia were less dependent on the maintenance of tissue integrity. This is in contrast to a previous study in which hypothermia was observed to reduce thermal injury-induced leakage in a guinea pig model of dorsal scald burns. 30 The differences have yet to be explained but may be related to the severity of injury induced in the two experimental systems.
Animals subjected to warm I/R appeared to suffer from respiratory distress during the latter phases of the reperfusion stage, and it is likely that pulmonary injury was responsible for the high death rate in this animal group. This proposition has been supported by a previous study in the same experimental model that showed the presence of collapsed alveoli having thick interstitial walls resulting from a dense neutrophilic infiltrate in the lungs of animals subjected to intestinal ischemia (Kalia et al., manuscript submitted). The decreased surface area available for gaseous exchange most likely accounts for the respiratory distress observed in these animals.
Hypothermia can have adverse effects. It may reduce blood flow within the microcirculation and increase blood viscosity and hematocrit. These effects may, therefore, to some extent at least, counteract the benefits of hypothermia at a cellular level. Clinically, moderate hypothermia (20°–25°C) may increase the risk of perioperative arrhythmias and other cardiac events, as well as suppressing the immune system and increasing the risk of infection. 31,32 Increased coagulopathy is a concern;31,32 however, mild hypothermia at the temperatures used in the current study (30°–32°C) appeared to induce no adverse effects either systemically or on local tissue perfusion.
This study has shown that intestinal I/R induces rapid microcirculatory breakdown in the mucosal layer with severe physiologic consequences. Whole-body hypothermia can maintain a functioning mucosal microcirculation and markedly improve animal survival after return to normothermic conditions. Intestinal I/R undoubtedly occurs in situations such as small bowel transplantation and aortic aneurysm repair. Therefore, the evaluation of hypothermia treatment in the clinical setting deserves further investigation.
Footnotes
Correspondence: Neena Kalia, PhD, Section of Surgical and Anaesthetic Sciences, K-Floor, Royal Hallamshire Hospital, Glossop Road, Sheffield, S10 2JF, UK.
E-mail: n.kalia@sheffield.ac.uk
Supported by the Wellcome Trust (grant 056087).
Accepted for publication November 20, 2001.
References
- 1.Soong CV, Blair PH, Halliday MI, et al. Bowel ischaemia and organ impairment in elective abdominal aortic aneurysm repair. Br J Surg 1994; 81: 965–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulds S, Cheshire NJ, Schachter M, et al. Endotoxin related early neutrophil activation is associated with outcome after thoracoabdominal aortic aneurysm repair. Br J Surg 1997; 84: 172–177. [PubMed] [Google Scholar]
- 3.Foulds S, Mireskandari M, Kalu P, et al. Visceral ischemia and neutrophil activation in sepsis and organ dysfunction. J Surg Res 1998; 75: 170–176. [DOI] [PubMed] [Google Scholar]
- 4.Gennaro M, Ascer E, Matano R, et al. Acute mesenteric ischaemia after cardiopulmonary bypass. Am J Surg 1993; 166: 231–236. [DOI] [PubMed] [Google Scholar]
- 5.Horie Y, Wolf R, Miyasaka M, et al. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology 1996; 111: 666–673. [DOI] [PubMed] [Google Scholar]
- 6.Ikai M, Iyoh M, Joh T, et al. Complement plays an essential role in shock following intestinal ischaemia in rats. Clin Exp Immunol 1996; 106: 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao F, Eppihimer MJ, Young JA, et al. Lung neutrophil retention and injury after intestinal ischemia/reperfusion. Microcirculation 1997; 4: 359–367. [DOI] [PubMed] [Google Scholar]
- 8.Grace PA. Ischaemia-reperfusion injury: a review. Br J Surg 1995; 81: 637–647. [DOI] [PubMed] [Google Scholar]
- 9.Granger DN. Ischemia-reperfusion: mechanism of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 1999; 6: 167–178. [PubMed] [Google Scholar]
- 10.Cavanagh SP, Gough MJ, Homer-Vanniasinkam S. The role of the neutrophil in ischaemia-reperfusion injury: potential therapeutic interventions. Cardiovasc Surg 1998; 6: 112–118. [DOI] [PubMed] [Google Scholar]
- 11.Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 1998; 114: 1066–1090. [DOI] [PubMed] [Google Scholar]
- 12.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischaemia. Am J Physiol 1986; 251: G567–G574. [DOI] [PubMed] [Google Scholar]
- 13.Kurtel H, Fujimoto K, Zimmerman BJ, et al. Ischemia-reperfusion-induced mucosal dysfunction: role of neutrophils. Am J Physiol 1991; 261: G490–G496. [DOI] [PubMed] [Google Scholar]
- 14.Nishio S, Chen ZF, Yunoki M, et al. Hypothermia-induced ischaemic tolerance. Ann NY Acad Sci 1999; 890: 26–41. [DOI] [PubMed] [Google Scholar]
- 15.Jahania MS, Sanchez JA, Narayan P, et al. Heart preservation for transplantation: principles and strategies. Ann Thoracic Surg 1999; 68: 1983–1987. [DOI] [PubMed] [Google Scholar]
- 16.Beuk RJ, oude Egbrink MGA, Kurvers HAJM, et al. Ischemia/reperfusion injury in rat mesenteric venules: Red blood cell velocity and leukocyte rolling. J Pediatr Surg 1996; 31: 512–515. [DOI] [PubMed] [Google Scholar]
- 17.Bonke H, Kurvers HAJM, oude Egbrink S, et al. Leukocyte rolling and blood flow in the in vivo assessment of ischaemia-reperfusion damage in rat mesenteric venules. Transplant Proc 1994; 26: 1478–1480. [PubMed] [Google Scholar]
- 18.Beuk RJ, Heineman E, Tangelder G-J, et al. Total warm ischemia and reperfusion impairs flow in all rat gut layers but increases leukocyte-vessel wall interactions in the submucosa only. Ann Surg 2000; 231: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illyés G, Hamar J. Sequence of morphological alterations in a small intestinal ischaemia/reperfusion model of the anaesthetised rat. A light microscopy study. Int J Exp Path 1992; 73: 161–172. [PMC free article] [PubMed] [Google Scholar]
- 20.Kong SE, Blennerhassett CR, Heel KA, et al. Ischaemia-reperfusion injury to the intestine. Aust NZ J Surg 1998; 68: 554–561. [DOI] [PubMed] [Google Scholar]
- 21.Kalia N, Pockley AG, Wood RFM, et al. Effects of FK409 on intestinal ischaemia-reperfusion injury and ischaemia-induced changes in the rat mucosal villus microcirculation. Transplantation 2001; 72: 1875–1880. [DOI] [PubMed] [Google Scholar]
- 22.Miller FN, Joshua IG, Anderson GL. Quantitation of vasodilator induced macromolecular leakage by in vivo fluorescent microscopy. Microvasc Res 1982; 24: 56–67. [DOI] [PubMed] [Google Scholar]
- 23.Verrier E. The microvascular cell and ischaemia-reperfusion injury. J Cardiovasc Pharmacol 1996; 27: S26–S30. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Eliyahu S, Shankar G, Rosenne E, et al. Hypothermia in barbiturate-anaesthetised rats suppresses natural killer cell activity and compromises resistance to tumour metastasis. Anesthesiology 1999; 91: 732–740. [DOI] [PubMed] [Google Scholar]
- 25.Beris AE, Soucacos PN, Seaber AV, et al. Effects of cold ischaemia on reflow patterns in the rat cremaster muscle microcirculation. Int Angiol 1995; 14: 248–252. [PubMed] [Google Scholar]
- 26.Koike K, Moore EE, Moore FA, et al. Gut phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia-reperfusion. Am J Physiol 1995; 268: G397–G403. [DOI] [PubMed] [Google Scholar]
- 27.Baue AE. The horror autotoxicus and multiple-organ failure. Arch Surg 1992; 127: 1451–1462. [DOI] [PubMed] [Google Scholar]
- 28.Wenisch C, Narzt E, Sessler DI, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anaesth Analgesia 1996; 82: 810–816. [DOI] [PubMed] [Google Scholar]
- 29.Heggers JP, Robson MC. Burns: prostaglandins and thromboxane. Crit Care Clin 1985; 1: 59–77. [PubMed] [Google Scholar]
- 30.De Camara DL, Raine T, Robson MC. Ultrastructural aspects of cooled thermal injury. J Trauma 1981; 21: 911–919. [DOI] [PubMed] [Google Scholar]
- 31.Buggy DJ, Crossley AWA. Thermoregulation, mild perioperative hypothermia and post-anaesthetic shivering. Br J Anaesthesia 2000; 84: 615–628. [DOI] [PubMed] [Google Scholar]
- 32.Sessler DI. Mild perioperative hypothermia. N Engl J Med 1997; 336: 1730–1737. [DOI] [PubMed] [Google Scholar]