Abstract
Objective
To assess the feasibility, safety, and outcome of laparoscopic liver resection for malignant liver tumors.
Summary Background Data
The precise role of laparoscopy in resection of liver malignancies (hepatocellular carcinoma [HCC] and liver metastases) remains controversial despite an increasing number of publications reporting laparoscopic resection of benign liver tumors.
Methods
A retrospective study was performed in 11 surgical centers in Europe regarding their experience with laparoscopic resection of liver malignancies. Detailed questionnaires were sent to each surgeon focusing on patient characteristics, clinical data, type and characteristics of the tumor, technical details of the operation, and early and late clinical outcome. All patients had radiologic investigations at follow-up to exclude disease recurrence.
Results
From February 1994 to December 2000, 37 patients with malignant liver tumors were included in this study. Ten patients had HCC, including 9 with cirrhotic liver, and 27 patients had liver metastases. The mean tumor size was 3.3 cm, and 89% of the tumors were located in the left lobe or in the anterior segments of the right liver. Liver procedures included 12 wedge resections, 9 segmentectomies, 14 bisegmentectomies (including 13 left lateral segmentectomies), and 2 major hepatectomies. The transfusion rate, the use of pedicular clamping, the conversion rate (13.5% in the whole series), and the complication rate were significantly greater in patients with HCC. There were no deaths. Postoperative complications occurred in eight patients (22%). The surgical margin was less than 1 cm in 30% of the patients. During a mean follow-up of 14 months, the 2-year disease-free survival was 44% for patients with HCC and 53% for patients having hepatic metastases from colorectal cancer. No port-site metastases were observed during follow-up.
Conclusions
In patients with small malignant tumors, located in the left lateral segments or in the anterior segments of the right liver, laparoscopic resection is feasible and safe. The complication rate is low, except in patients with HCC on cirrhotic liver. By using laparoscopic ultrasound, a 1-cm free surgical margin should be routinely obtained. The late outcome needs to be evaluated in expert centers.
Since the event of laparoscopic cholecystectomy, 1 minimally invasive surgery has been applied to solid organs such as the spleen, 2 kidney, 3 adrenal glands, 4 and more recently the liver. 5–8 The first anatomic liver resection was reported by Azagra et al 9 in 1996; they performed a left lateral segmentectomy. An increasing number of publications have been reported concerning laparoscopic treatment of benign liver tumors by resection 10–14 or local ablation. 15,16 However, laparoscopic resection of liver malignancies remains controversial. 17 Indeed, the usual benefits of minimally invasive therapy (e.g., cosmetic aspect, rapid recovery, short postoperative hospital stay) are challenged by the paramount oncologic objective, which is the long-term disease-free survival. Similar to the situation in other gastrointestinal malignancies, 18 there are also concerns regarding potential tumor cell exfoliation and port-site metastases during laparoscopic procedures. The purpose of the present study was to analyze the feasibility, safety, and late outcome of patients undergoing laparoscopic resection for liver malignancies in a multicenter setting.
METHODS
From February 1994 to December 2000, 37 patients with malignant liver tumors were included in this study. The mean age of the patients was 62 years (median 64, range 38–79), and 57% of the patients were female. Five patients (13%) were classified according to the American Society of Anesthesiologists physical status score (ASA) as ASA III. 19 There were 10 patients with hepatocellular carcinoma (HCC); among them, 9 patients had liver cirrhosis (Child-Pugh A, n = 5; Child-Pugh B, n = 4) (Fig. 1). Twenty-seven patients had liver metastases, from colorectal origin in 12 patients, from a neuroendocrine primary tumor in 5 patients, from primary breast cancer in 5 patients, and from various primary malignancies in 5 other patients (pulmonary cancer, n = 2; ovarian cancer, n = 1; gastric cancer, n = 1; pancreatic cancer, n = 1). Thirty surgical procedures had previously been performed in these patients (open surgery, 23; laparoscopic, 7), including cholecystectomy (open, 1; laparoscopic, 1), colectomy (open, 9; laparoscopic, 5), partial gastrectomy (open, 2), adhesiolysis (open, 1), hysterectomy (open, 5), appendectomy (open, 4; laparoscopic, 1), and inguinal hernia (open, 1). Ten patients (HCC, 1; metas-tases, 9) had their tumor incidentally discovered during laparoscopic cholecystectomy.
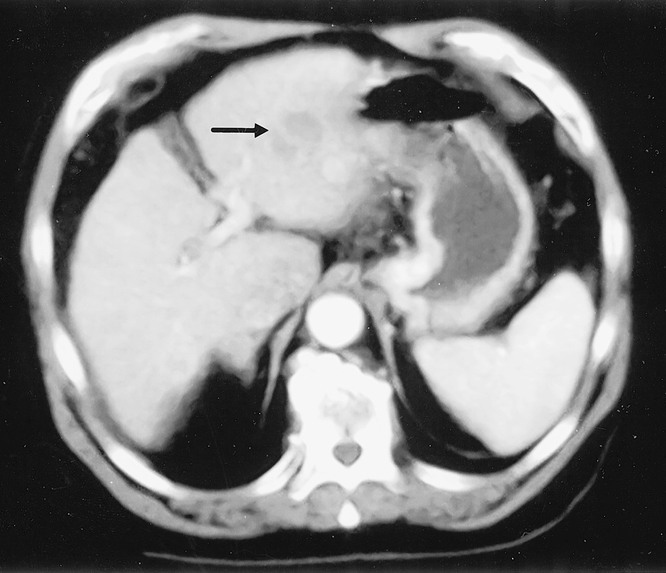
Figure 1. Patient with Child-Pugh B liver cirrhosis suffering from a 4 cm deep-sited hepatocellular carcinoma (arrow), located in the left lateral segment of the liver and treated by laparoscopic left lateral segmentectomy.
The tumor was unique in 27 patients and multiple in 5 (two tumors in 3 patients and four tumors in 2 patients). Five other patients had multiple (≥10) bilobar liver metastases. The mean size of the resected tumor was 3.3 cm (median 3; range 1–6). The distribution of segmental location of the tumor according to the Couinaud classification 20 is shown in Table 1. Most of these tumors were superficial (<1 cm from the liver surface). Fifteen of the 16 patients with deep tumors had tumors in the left lateral segments of the liver.
Table 1. SEGMENTAL INTRAHEPATIC LOCATION OF RESECTED LIVER TUMOR
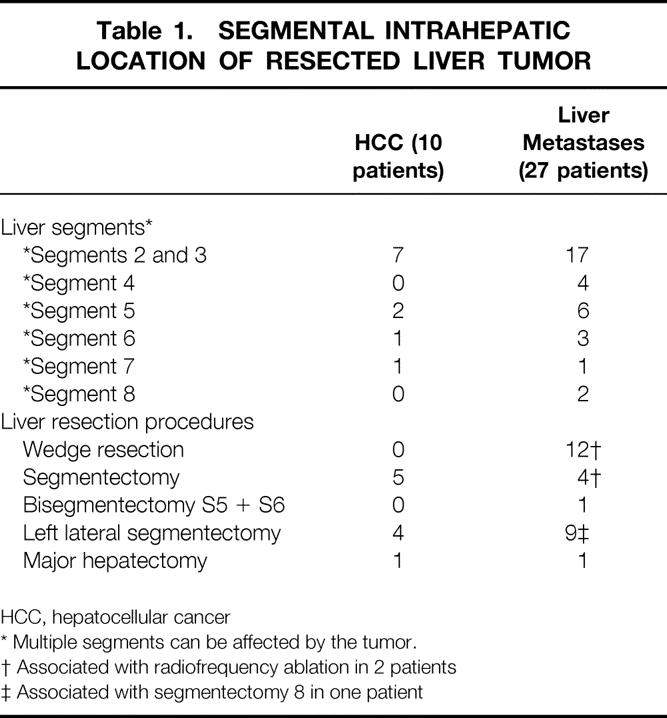
HCC, hepatocellular cancer
* Multiple segments can be affected by the tumor.
† Associated with radiofrequency ablation in 2 patients
‡ Associated with segmentectomy 8 in one patient
Laparoscopic liver resection was performed with the patient in the supine position. Pneumoperitoneum with carbon dioxide was used. Abdominal pressure was monitored and maintained at less than 15 mm Hg. Pneumoperitoneum was performed in 34 patients (92%) with the Veress needle. Abdominal lift technique was not used. The mean number of trocars was five (median 5; range 3–6). Tumor location was explored visually in all patients and by laparoscopic ultrasound in 24 patients (65%). Liver parenchymal transection was performed using crushing forceps in 15 patients (40%), hook coagulator in 9 patients (24%), harmonic shears in 13 patients (35%), stapling device in 10 patients (27%), and ultrasonic dissector in 8 patients (22%). Intraparenchymal vascular control was obtained by monopolar cautery in 5 patients (13%), intraperitoneal ligation in 1 patient (3%), harmonic shears in 2 patients (6%), clips in 29 patients (78%), and an Endostapler in 16 patients (43%), including 12 patients (75%) undergoing left lateral segmentectomy or major hepatectomy. An atraumatic Lucane liver clamp was used in two patients (6%). 13 Hemostasis of the transection line was obtained in 22 patients by monopolar cautery (30%), by harmonic shears in 1 patient (3%), by argon beam coagulator in 8 patients (22%), by hemostatic swabs in 2 patients (6%), and by fibrin glue in 6 patients (16%). Control of biliary leak at the liver surface was assessed by inspection in all cases or by intraoperative cholangiography in four patients (11%). Extraction of the surgical specimen was always performed using an Endo-bag, through an enlarged trocar site in 27 patients (73%), through a minilaparotomy in 5 patients (13.5%), or by conversion to an open approach in 5 patients (13.5%). Peritoneal drainage was used in 34 patients (92%).
Evaluation criteria included type and details of the operative procedure; early postoperative course, including complications and reoperation rate; postoperative hospital stay; and late outcome, including disease recurrence. Postoperative death and complications were assessed at a postoperative delay of 2 months. All patients had radiologic investigations at follow-up to exclude disease recurrence (by ultrasound in 7 patients, computed tomography in 29 patients, and magnetic resonance imaging in 4 patients).
Statistical analysis included the chi-square test or Student t test when indicated. Survival curves were calculated according to the Kaplan-Meier method. Actuarial survival was calculated only in the group of patients undergoing a resection with curative intent.
RESULTS
According to the Goldsmith and Woodburne classification, 21 the type of liver resection is shown in Table 1. A case of wedge resection for colorectal liver metastase is illustrated in figure 2. One patient underwent a double resection, including left lateral segmentectomy and removal of segment 8. Hand-assisted hepatectomy was used in three patients (8%), including one case of segmentectomy 7, one case of left lateral segmentectomy, and one case of right hepatic lobectomy. Excluding the 10 patients with incidental detection during laparoscopic cholecystectomy, another associated laparoscopic procedure was performed in seven patients (19%), including cholecystectomy (n = 2), adrenalectomy (n = 1), colectomy (n = 1) (Fig. 3), pancreatic biopsy (n = 1), and radiofrequency ablation of a second liver metastasis (n = 2). Portal triad clamping was used in six patients (16%); it was unilateral in one patient and total in five patients. The mean duration of portal triad clamping was 31 minutes (median 31; range 15–50). This maneuver was used for right hepatic lobectomy (one patient), left lateral segmentectomy (one patient), and segmentectomy in four patients (segmentectomy 2, segmentectomy 5, segmentectomy 6, and segmentectomy 7 in one patient each). Portal triad clamping was used in 4 of the 10 patients with HCC (40%) and in 2 of 27 patients with liver metastases (7%) (P = .035). Conversion to open surgery (5 patients [13.5%]) was significantly more frequent in patients with HCC (4/10 [40%]) than in those with liver metastases (1/27 [4%]) (P = .014).
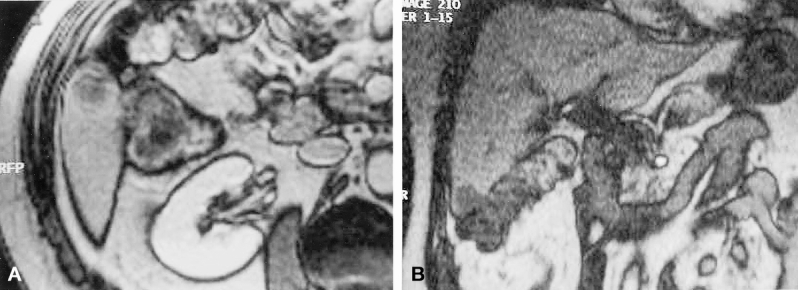
Figure 2. Detection on routine carcinologic screening of 1 cm liver metastase located in segment VI of the right lobe of the liver in a patient previously operated for colorectal cancer. Despite the presence of dense peritoneal adhesions, the patient was considered as an excellent candidate for laparoscopic wedge resection with a 1-cm free surgical margin. (A) Transverse view of magnetic resonance imaging. (B) Frontal view of magnetic resonance imaging.
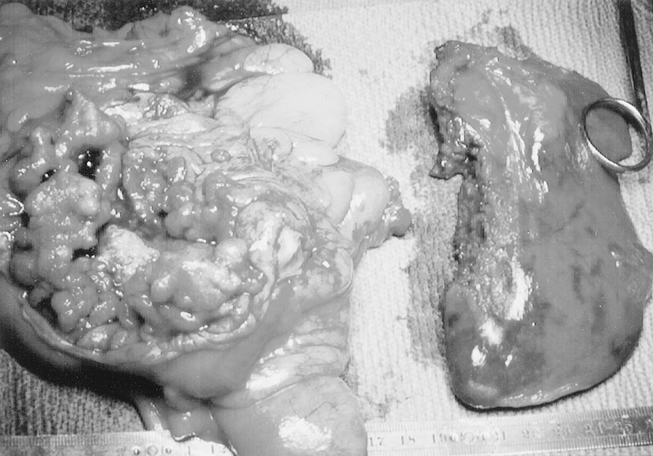
Figure 3. Detection during laparoscopic right colectomy for cancer of a synchronous 3 cm liver metastases, located in the left lateral segment of the liver, detected by intraoperative ultrasound and treated by associated laparoscopic left lateral segmentectomy during the same operation: operative view of the resected specimens.
Perioperative complications included bleeding in five patients (13.5%), from the liver parenchyma (n = 3), left portal vein (n = 1), and splenic laceration (n = 1). Perioperative bleeding required conversion in four of these patients (80%). In another patient, already reported by Descottes et al, 13 the procedure was converted to an open approach because of presumed invasion of the surgical margin during the resection of an HCC; this feature was not confirmed at laparotomy. Perioperative and postoperative blood transfusion was required in six patients (16%), and the incidence of transfusion was greater in HCC patients (4/10 [40%]) compared with patients with liver metastases (2/27 [7%]) (P = .035). Five patients (13.5%) had a blood transfusion volume greater than 500 mL. Among these patients, the hepatic procedures included segmentectomy 5 (two patients), left lateral segmentectomy (one patient), bisegmentectomy 5 and 6 (one patient), and right hepatic lobectomy (one patient).
There were no deaths in this series. Postoperative complications and reoperation rates are shown in Table 2. The occurrence of left bile duct stricture in a patient undergoing laparoscopic right hepatic lobectomy is illustrated in figure 4. The mean postoperative hospital stay was 7 days (median 6; range 2–16). The postoperative hospital stay was significantly affected by the type of liver tumor and by the extent of liver resection. The mean stay in HCC patients was 10 days (median 10, range 6–16); in patients with liver metastases it was 6 days (median 6, range 2–16) (P = .02). The mean stay was 8 days (median 7, range 3–16) when more than one liver segment was resected and 6 days (median 5.7, range 2–10) when less than one liver segment was resected (P = .04).
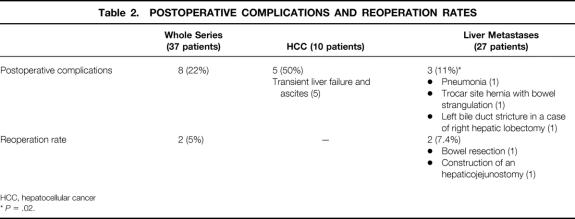
Table 2. POSTOPERATIVE COMPLICATIONS AND REOPERATION RATES
HCC, hepatocellular cancer
*P = .02.

Figure 4. Postoperative endoscopic retrograde cholangiography in a patient undergoing a right hepatic lobectomy for a 6-cm neuroendocrine liver metastase located deeply in segment VI-VII of the right lobe of the liver: presence of an iatrogenic stricture of the left hepatic duct, requiring reoperation for construction of an hepatico-jejunostomy.
A liver resection with “an intent to cure” was performed in 25 patients (10 patients with HCC, 15 with liver metastases). A diagnostic “noncurative” resection was performed in 12 patients with liver metastases (colorectal, 2; neuroendocrine, 4; others, 6) as a result of bilobar and multiple distribution in 8 patients, presence of extrahepatic disease in 1 patient, and incidental laparoscopic discovery of unknown visible metastases in 3 patients with incomplete liver workup.
At pathologic examination, the transection line was invaded in 1 (6.7%) of the 15 patients with liver metastases operated on with an intent to cure. The surgical margin was less than 1 cm in three patients with HCC (30%) and in three patients with liver metastases (20%); among them two patients presented with local recurrence at the transection line during oncology follow-up. The incidence of invaded or less-than-1-cm surgical margin was more frequent when laparoscopic ultrasound was omitted (3/5 patients [60%]) than when laparoscopic ultrasound was used (4/20 patients [20%]), but the difference was not statistically significant.
In the 12 patients with colorectal liver metastases, adjuvant chemotherapy was used in 8 (67%). In the five patients with neuroendocrine liver metastases, two underwent surgical resection of the primary endocrine tumor (pancreatoduodenectomy and left pancreatectomy in one patient each), one patient underwent metabolic radiotherapy, one patient received chemotherapy based on streptozocin, and two patients were treated with long-acting subcutaneous somatostatin analogs. Three of the five patients with breast liver metastases and two of the five patients with liver metastases from various origins were treated with adjuvant chemotherapy.
The mean follow-up in the whole series was 14 months (median 12; range 2–72). Details of patient status at follow-up are shown in Table 3. In the patients with HCC, the mean follow-up was 15 months (median 12; range 4–26); four patients are alive without recurrence, one patient died without recurrence as a result of rupture of esophageal varices, two patients died of liver recurrence, and two patients are alive with liver recurrence, treated by arterial chemoembolization in one patient. In the patients with HCC, the overall and disease-free survival rates were 83.3% and 58.3% at 1 year and 62.5% and 43.7% at 2 years, respectively.
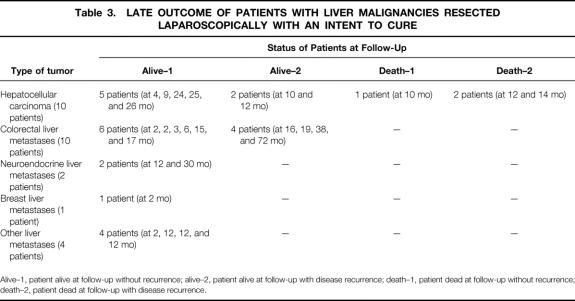
Table 3. LATE OUTCOME OF PATIENTS WITH LIVER MALIGNANCIES RESECTED LAPAROSCOPICALLY WITH AN INTENT TO CURE
Alive–1, patient alive at follow-up without recurrence; alive–2, patient alive at follow-up with disease recurrence; death–1, patient dead at follow-up without recurrence; death–2, patient dead at follow-up with disease recurrence.
In the 10 patients with liver metastases undergoing a curative resection, the mean follow-up was 19 months (median 16; range 2–72); six patients are alive without recurrence and four patients are alive with recurrent disease, treated by chemotherapy in three patients and additional laparoscopic radiofrequency ablation in one patient. In patients with colorectal liver metastases resected with an intent to cure, the overall and disease-free survival rates were 100% and 100% at 1 year and 100% and 53.3% at 2 years, respectively. For the five patients with neuroendocrine liver metastases, three patients undergoing diagnostic resection died with disease persisting at a mean delay of 17 months (median 18; range 12–20). The two remaining patients are alive without recurrence at 12 and 30 months respectively after surgery. Of four patients undergoing diagnostic resection (out of five patients with breast liver metastases), two died of disease persistence at 6 and 18 months; two patients are alive with recurrence at 5 and 12 months after surgery. The last patient with curative resection is alive without recurrence 2 months after surgery.
Of the patients with liver metastases of various origin, four patients are alive without recurrence at 2, 12, 12, and 12 months; the last patient resected with a diagnostic intent died of disease persistence 4 months after surgery.
DISCUSSION
The application of minimally invasive surgery to liver malignancies remains controversial. Indeed, the functional postoperative advantages of minimally invasive surgery, as reported in benign gastrointestinal conditions, 22,23 differ from those involving the treatment of malignant diseases. In these circumstances, the paramount criteria of evaluation are 5- and 10-year disease-free survival rates. Up to now, none of the rare articles reporting limited experience with laparoscopic resection of HCC 6,13,14,24–34 or liver metastases 5,7,8,10,11,13,14,27,31,33–37 provided information concerning the late outcome of such patients. The problems of tumor cell seeding and port-site metastases have been addressed by several reports involving laparoscopic treatment of other types of gastrointestinal malignancies. 18 Information regarding these concerns about laparoscopic resection of liver malignancies remains inconclusive because of the limited number of reported patients.
In laparoscopic resection for liver malignancies, the same oncologic rules should be applied as in open surgery, including “no-touch” technique, R0 radical resection, and achievement of a 1-cm free surgical margin. 38,39 Indeed, late survival of those patients has been correlated with the surgical margin, either in patients with HCC 38 or in those with colorectal liver metastases. 39 Although the 1-cm tumor-free resection margin has been recently challenged, 40 the achievement of a tumor-free surgical margin needs to be the key purpose of any laparoscopic procedure for resection of liver malignancy. Our multicentric series showed that during laparoscopic resection, one third of our patients did not meet this requirement. This fact is clearly related to the lack of digital palpation during the laparoscopic approach. This limitation was encountered in a series of limited liver resections for small and superficial tumors, and this insufficiency should be solved by the routine use of laparoscopic contact ultrasonography, which will allow determination of the precise location of liver transection in relation to the tumor margin. In fact, a lower incidence of insufficient surgical margin was observed in our series in the subgroup of patients in whom laparoscopic ultrasound was used. Although the difference was not statistically significant, we believe, as do others, 41,42 that laparoscopic ultrasound provides the surgeon with a strategic addition in the treatment of patients with liver malignancy.
The laparoscopic approach probably is indicated in only a few patients with malignant liver diseases. Indeed, large tumors (deeply sited or posteriorly located in the right hepatic lobe) and tumors close to the portal bifurcation or the suprahepatic junction should not be considered for laparoscopic resection. Tumor selection in this series concerned small, superficial, or peripheral lesions, essentially located in the left lateral segment of the liver (segments 2 and 3) or in the anterior segments of the right liver, including the anterior part of the segment 4, 5, and 6.
Fong et al 33 have reported other limitations of laparoscopic management of liver tumors, such as difficulty of identifying tumor margins, difficulty in liver mobilization, and the presence of dense adhesions related to previous open resectional procedures for primary cancer. As shown in this and other series, 7,13,14,27,33,34,37 only limited liver resections or “réglée” left lateral segmentectomies can be safely performed through a laparoscopic approach. Despite hand-assisted performance, 31,33,34 and as illustrated by the occurrence of a major biliary complication in this series, the legitimacy of laparoscopic major hepatectomies 31,37 remains questionable. Indeed, when performed laparoscopically, tumor transgression and vascular injury during parenchymal transection are major concerns in these procedures. 33 Additionally, techniques of total vascular isolation of the liver in such procedures seem also questionably applicable during a totally laparoscopic approach.
However, in this selected group of patients, when the technique is appropriately performed by a surgical team expert in liver and laparoscopic surgery, the technique appears to be safe, with an acceptable complication rate. The most common perioperative complication was bleeding, as in the open approach, but obviously this complication was more difficult to control during laparoscopy, because 80% of procedures had to be converted to open surgery in this situation. Despite deleterious effects of pneumoperitoneum associated with the Pringle maneuver in animal models, 43,44 the use of this combination in this series was well tolerated in five patients with an occlusion time varying from 15 to 50 minutes. However, portal triad clamping was used in this series for liver resections, namely segmentectomies and left lateral segmentectomy, for which it should not have been used during an open approach. Finally, the laparoscopic approach carries an increased risk of gas embolism compared with the open approach. 16,25,45,46 Despite the absence of such potentially life-threatening complications in the present series, the use of gasless laparoscopy and cautious use of the argon beam coagulator to achieve hemostasis of the transection line is recommended by some authors. 6,25,26,28,44
Our data seem in contrast to the suggested benefits in other reports 32 regarding a better tolerance of selected patients with HCC when resection is performed laparoscopically or regarding widening of the surgical indications for resection. Indeed, despite a limited number of patients, resection of HCC was associated in this series with a higher incidence of perioperative bleeding, transfusions, postoperative complications, need for portal triad clamping, and conversion to an open approach. Until definitive information becomes available from experimental and centralized clinical studies, the role of laparoscopic resection of patients with HCC should be evaluated in expert centers with multidisciplinary hospital oncologic teams.
For patients with liver metastases, this series emphasizes the diagnostic and staging role of laparoscopic exploration. Indeed, in 33% of the patients, liver metastases were detected during another laparoscopic procedure. Again, the combined use of laparoscopic inspection, biopsy, and contact ultrasonography should be stressed. Despite improvements in imaging modalities, we believe that staging laparoscopy remains the best option to detect peritoneal implants or small superficial liver metastases. For this reason, explorative diagnostic laparoscopy should be suggested as the first step of any resection procedure for liver malignancies. 41,42 Again, the role of complete perioperative staging during primary laparoscopic colonic resection should emphasize the use of laparoscopic contact liver ultrasound, allowing in one patient of this series the association of radical colonic and liver resections.
Despite a limited number of patients undergoing surgery with a curative intent, this is the first time that some results concerning disease-free survival are reported in patients undergoing surgery for HCC or colorectal liver metastases. Because the number of patients and the duration of follow-up are still limited, our results cannot be compared with the results obtained by an open approach. However, the overall 1- and 2-year survival rates range between 68% and 80% and 55% and 68%, respectively, in large series of resected HCC on cirrhotic liver. 47–49 For patients with resected colorectal liver metastases, 39,50–52 overall 1- and 2-year survival rates ranging between 89% and 93% and 62% and 73%, respectively, have been reported. In the series by Scheele et al, 50 the 2-year disease-free survival rate was around 52%. Thus, further evaluation on a greater number of patients should be undertaken in large clinical series before adopting laparoscopic management of liver malignancy as an acceptable tool.
In conclusion, despite a limited number of patients, this series shows that laparoscopic resection of liver malignancies is feasible, with an acceptable complications rate in selected patients and liver tumors. The procedure should be performed by surgical teams expert in hepatobiliary and laparoscopic surgery, with the same oncologic rules of open resectional therapy, including “no-touch” tumor technique, radical R0 resection, and a free surgical margin. Laparoscopic ultrasound should be routinely used to achieve complete staging and an adequate margin. Because late disease-free survival is the paramount criterion of evaluation in these patients, further evaluation is mandatory with a greater number of patients prospectively enrolled in strict evaluation studies conducted by centers expert in the management of patients with cancer.
Footnotes
Correspondence: Professor J. F. Gigot, Hepato-Bilio-Pancreatic Unit, Saint-Luc University Hospital, Hippocrate Avenue, 10 B-1200, Brussels, Belgium.
E-mail: gigot@chir.ucl.ac.be
Accepted for publication December 4, 2001.
References
- 1.Dubois F, Berthelot G, Levard H. Cholecystectomie par coelioscopie. Presse Med 1989; 18: 980–982. [PubMed] [Google Scholar]
- 2.Gigot GF, de Ville de Goyet J, van Beers BE, et al. Laparoscopic splenectomy in adults and children: experience with 31 patients. Surgery 1996; 119: 384–389. [DOI] [PubMed] [Google Scholar]
- 3.Clayman RV, Kavoussi LR, Soper NJ. Laparoscopic nephrectomy. N Engl J Med 1991; 324: 1370–1371. [DOI] [PubMed] [Google Scholar]
- 4.Gagner M, Pomp A, Heniford BT, et al. Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 1997; 226: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor [abstract]. Surg Endosc 1992; 6: 99. [Google Scholar]
- 6.Croce E, Azzola M, Russo R, et al. Laparoscopic liver tumour resection with the Argon Beam. Endosc Surg 1994; 2: 186–188. [PubMed] [Google Scholar]
- 7.Rau HG, Meyer G, Cohnert TU, et al. Laparoscopic liver resection with the water-jet dissector. Surg Endosc 1995; 9: 1009–1012. [DOI] [PubMed] [Google Scholar]
- 8.Gugenheim J, Mazza D, Katkhouda N, et al. Laparoscopic resection of solid liver tumours. Br J Surg 1996; 83: 334–335. [DOI] [PubMed] [Google Scholar]
- 9.Azagra JS, Goergen M, Gilbart E, et al. Laparoscopic anatomical (hepatic) left lateral segmentectomy: technical aspects. Surg Endosc 1996; 10: 758–761. [DOI] [PubMed] [Google Scholar]
- 10.Samama G, Chiche L, Brefort JL, et al. Laparoscopic anatomical hepatic resection. Surg Endosc 1998; 12: 76–78. [DOI] [PubMed] [Google Scholar]
- 11.Marks J, Mouiel J, Katkhouda N, et al. Laparoscopic liver surgery. A report on 28 patients. Surg Endosc 1998; 12: 331–334. [DOI] [PubMed] [Google Scholar]
- 12.Katkhouda N, Hurtwitz M, Gugenheim J, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg 1999; 229: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Descottes B, Lachachi F, Sodji M, et al. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg 2000; 232: 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000; 232: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannitti DA, Heniford BT, Hale J, et al. Laparoscopic cryoablation of hepatic metastases. Arch Surg 1998; 133: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 16.Curley SA, Izzo F, Ellis FM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000; 32: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuschieri CN. Laparoscopic management of cancer patients. J R Coll Surg Edinb 1995; 40: 1–9. [PubMed] [Google Scholar]
- 18.Johnstone PAS, Rohde DC, Swartz SE, et al. Port-site recurrences after laparoscopic and thoracoscopic procedures in malignancy. J Clin Oncol 1996; 14: 1950–1956. [DOI] [PubMed] [Google Scholar]
- 19.American Society of Anesthesiologists. New classification of physical status. Anesthesiology 1963; 24: 111. [Google Scholar]
- 20.Couinaud D. Le foie: études anatomiques et chirurgicales. Paris: Masson, 1957.
- 21.Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet 1957; 105: 310–318. [PubMed] [Google Scholar]
- 22.Barkun JS, Barkun AN, Sampalis JS, et al. Randomised controlled trial of laparoscopic versus mini cholecystectomy. Lancet 1992; 340: 1116–1119. [DOI] [PubMed] [Google Scholar]
- 23.Brunt LM, Doherty GM, Norton JA, et al. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg 1996; 183: 1–10. [PubMed] [Google Scholar]
- 24.Friedman RL, Hiatt JR, Korman JL, et al. Laparoscopic or open splenectomy for hematologic disease: which approach is superior? J Am Coll Surg 1997; 185: 49–54. [PubMed] [Google Scholar]
- 25.Hashizume M, Takenaka K, Yanaga K, et al. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc 1995; 9: 1289–1291. [DOI] [PubMed] [Google Scholar]
- 26.Intra M, Viania MP, Ballarini C, et al. Gasless laparoscopic resection of hepatocellular carcinoma (HCC) in cirrhosis. J Laparoendosc Surg 1996; 6: 263–270. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery 1996; 120: 468–475. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe Y, Sato M, Ueda S, et al. Laparoscopic hepatic resection: a new and safe procedure by abdominal wall lifting method. Hepato-Gastroenterology 1997; 44: 143–147. [PubMed] [Google Scholar]
- 29.Sato M, Watanabe Y, Ueda S, et al. Minimally invasive hepatic resection using laparoscopic and minithoracotomy. Arch Surg 1997; 132: 206–208. [DOI] [PubMed] [Google Scholar]
- 30.Mizoe A, Tomioka T, Inoue K, et al. Systematic laparoscopic left lateral segmentectomy of the liver for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 1998; 5: 173–178. [DOI] [PubMed] [Google Scholar]
- 31.Huscher CGS, Lirici MM, Chiodini S. Laparoscopic liver resections. Semin Laparosc Surg 1998; 5: 204–210. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Atty MY, Farges O, Jagot P, et al. Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg 1999; 86: 1397–1400. [DOI] [PubMed] [Google Scholar]
- 33.Fong Y, Jarnagin W, Conlon KC, et al. Hand-assisted laparoscopic liver resection. Arch Surg 2000; 135: 854–859. [DOI] [PubMed] [Google Scholar]
- 34.Cuschieri A. Laparoscopic hand-assisted surgery for hepatic and pancreatic disease. Surg Endosc 2000; 14: 991–996. [DOI] [PubMed] [Google Scholar]
- 35.Wayand W, Woisetshlager R. Laparoskopische resektion einer lebermetastase. Chirurg 1993; 64: 195–197. [PubMed] [Google Scholar]
- 36.Rau HG, Buttler E, Meyer G, et al. Laparoscopic liver resection compared with conventional partial hepatectomy. A prospective analysis. Hepato-Gastroenterology 1998; 45: 2333–2338. [PubMed] [Google Scholar]
- 37.Mouiel J, Katkhouda N, Gugenheim J, et al. Possibilities of laparoscopic liver resection. J. Hepatobiliary Pancreat Surg 2000; 7: 1–8. [DOI] [PubMed] [Google Scholar]
- 38.Masutani S, Sasaki Y, Imaoka S, et al. The prognostic significance of surgical margin in liver resection of patients with hepatocellular carcinoma. Arch Surg 1994; 129: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 39.Shirabe K, Tanekata K, Gion T, et al. Analysis of prognostic factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997; 84: 1077–1080. [PubMed] [Google Scholar]
- 40.Elias D, Cavalcanti A, Sabourin JC, et al. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol 1998; 69: 88–93. [DOI] [PubMed] [Google Scholar]
- 41.John TG, Greig JD, Crosbie JL, et al. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg 1994; 220: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahusen FD, Cuesta MA, Borgstein PJ, et al. Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg 1999; 230: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haberstroh J, Ahrens M, Munzar T, et al. Effects of the Pringle maneuver on hemodynamics during laparoscopic liver resection in the pig. Eur Surg Res 1996; 28: 8–13. [DOI] [PubMed] [Google Scholar]
- 44.Takagi S. Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc 1998; 12: 427–431. [DOI] [PubMed] [Google Scholar]
- 45.Yacoub OF, Cardona I, Coveler LA, et al. Carbon dioxide embolism during laparoscopy. Anesthesiology 1982; 57: 533–535. [DOI] [PubMed] [Google Scholar]
- 46.Moskop RJ, Lubarsky DA. Carbon dioxide embolism during laparoscopic cholecystectomy. South Med J 1994; 84: 414–415. [DOI] [PubMed] [Google Scholar]
- 47.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993; 218: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagasue N, Kohno H, Chang YC, et al. Liver resection for hepatocellular carcinoma: results of 229 consecutive patients during 11 years. Ann Surg 1993; 217: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990; 98: 733–738. [PubMed] [Google Scholar]
- 50.Scheele J, Stang RS, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 51.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997; 132: 505–511. [DOI] [PubMed] [Google Scholar]
- 52.Fong Y, Fornter J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]