Abstract
Summary Background Data
Quick intraoperative parathyroid hormone assays are widely used as a guide to the adequacy of resection during parathyroid surgery. However, some authors have reported a 15% error rate of these assays because of the presence of false-positive and false-negative results. Recently the authors have found that most commercial intact PTH (iPTH) assays cross-react with non-(1-84) PTH (likely 7-84 PTH) and that the proportional levels of non-(1-84) PTH in patients were variable in a much wider range, accounting mostly for 20% to 60% of the immunoreactivity in samples obtained from hyperparathyroid patients. A cyclase activating PTH (CAP) measured by a novel immunoradiometric assay was shown to measure specifically 1-84 PTH. Using a CAP assay, the authors studied the rate of decline of CAP after parathyroidectomy and compared it with iPTH as measured by the Nichols intact PTH immunoradiometric assay.
Methods
This study comprised 29 patients with primary hyperparathyroidism (pHPT) caused by a single adenoma and 7 patients with secondary hyperparathyroidism (secondary HPT) who underwent parathyroidectomy. Blood samples were drawn after anesthesia, before excision of one enlarged parathyroid gland in pHPT and of the last gland in secondary HPT, and at 5, 10, and 15 minutes after excision. The 7-84 PTH level was calculated by subtracting the CAP value from the iPTH value.
Results
The percentage of 7-84 PTH in iPTH in plasma samples was 27.5 ± 14.4% in pHPT and 39.6 ± 15.1% in secondary HPT. In pHPT patients the plasma CAP and iPTH value decreased to 23.4 ± 10.8 and 32.0 ± 11.3% of the preexcision level at 5 minutes, 10.6 ± 7.7 and 21.1 ± 8.8% at 10 minutes, and 8.5 ± 4.9 and 16.1 ± 6.8% at 15 minutes after removal of the enlarged gland, respectively. At 5 minutes, CAP levels of all 29 pHPT patients had decreased to less than 40% of the preparathyroidectomy level; however, 7 (24%) patients still had an iPTH level of more than 40%. In secondary HPT patients, CAP and iPTH values had dropped to 43.3 ± 20.2 and 66.1 ± 19.7% at 5 minutes, 28.6 ± 16.6 and 53.6 ± 18.1% at 10 minutes, and 14.2 ± 9.0 and 41.0 ± 12.9% at 15 minutes after removal of the last enlarged gland, respectively. At 10 minutes, CAP levels of all seven secondary HPT patients had decreased to less than 50% of the preexcision level; however, three (43%) patients still had an iPTH level of more than 50%. In pHPT and secondary HPT, the 7-84 PTH level had dropped to 57.4 ± 85.9 and 62.1 ± 84.9%, respectively, of the preexcision value 15 minutes after removal of the enlarged gland or glands.
Conclusions
The percentage of 7-84 PTH in iPTH in plasma samples varies substantially between patients with HPT. In both pHPT and secondary HPT, the plasma CAP value decreased more rapidly than iPTH after parathyroidectomy, depending on the amount of 7-84 PTH in circulation. These results suggest that the CAP assay may be a more useful adjunct to parathyroidectomy than the currently used iPTH assay.
Primary hyperparathyroidism (pHPT) is one of the most common causes of hypercalcemia. The goal of surgical treatment for pHPT is the restoration of calcium homeostasis by removing hyperfunctioning parathyroid glands. Surgery is successful in more than 95% of patients. 1–3 Failure of surgery results from the inability to find or identify hyperfunctioning glands. 4–7 During surgery, distinguishing between single-gland disease and multiglandular disease as the cause of pHPT is critical. In addition to visual assessment, several methods have been introduced to ensure a successful operation, including the use of a frozen section of the excised parathyroid glands with intracellular fat staining, 8 intraoperative density measurement, 9 urinary cyclic 3′5′ adenosine monophosphate, 10 and ionized calcium 11 as a marker of end-organ activity of parathyroid hormone (PTH). These are insufficient for a variety of reasons. Several groups, including ours, 7,12–19 have reported that the intraoperative quick intact PTH assay (QPTH) developed by Irvin et al. 20 in part as an adjunctive to successful parathyroidectomy can provide useful information for achieving a successful operation. However, the accurate predictive value of the postoperative outcome has been approximately 95%, ranging from 84% to 100%, because of the presence of false-positive and false-negative results. 17–24
Circulating PTH shows a high degree of immunoheterogeneity because of the occurrence of various fragments of PTH in circulation. The C-terminal and midregional PTH fragments are not biologically active and cannot bind to PTH-1 receptor; however, these small PTH fragments are accumulated in patients with chronic renal failure. Measurement of PTH has been challenged by the fact of the immunoheterogeneity of PTH molecules in the circulation.
The first-generation competitive PTH radioimmunoassays using antiserum against the C-terminal or midregional portion of the hormone had been used for about three decades. Because of poor clinical diagnostic sensitivity and specificity, as well as the uncertainty of which molecules were measured, the second-generation “intact” PTH (iPTH) assays were developed in the late 1980s. These assays are based on the two-site “sandwich” assay principle and greatly improved the assay performance characteristics. These iPTH assays using two distinct antibodies against the carboxyl- and amino-terminal portions of the hormone were reported to measure 1-84 PTH only. 25,26 However, Brossard 27 and Gao et al. 28 reported that most commercial iPTH assays cross-react with non-(1-84) PTH, a PTH fragment that shows similar hydrophobicity as synthetic 7-84 PTH. Further studies showed that the non-(1-84) PTH fragment is amino-terminally truncated and has a high potency to inactivate 1-84 PTH function. 27,29 Moreover, this 7-84 PTH fragment is produced mainly from parathyroid glands directly. 30 We reported that the proportional levels of 7-84 PTH in patients with either pHPT (165 patients) or secondary HPT (318 patients) were variable in a much wider range, accounting for approximately 20% to 60% of the measurable value of iPTH. 31
Recently, a cyclase activating PTH (CAP) measured by a novel immunoradiometric (IRMA) assay was shown to measure specifically 1-84 PTH exclusively. 27,30–33 The implications of these findings are as follows: 1) the decline of iPTH levels after parathyroidectomy varies depending on the amount of non-(1-84)PTH; 2) this might account for false-negative surgical outcome results in currently used QPTH assays; and 3) the CAP level could provide a more accurate predictive value for postoperative calcium levels than the iPTH level. We therefore studied the decline of CAP after parathyroidectomy and compared them with the iPTH assay using the Nichols assay in primary and secondary HPT.
METHODS
Patients
We studied 29 patients with pHPT caused by a single adenoma and 7 patients with secondary HPT receiving hemodialysis who underwent surgery between July 2000 and March 2001. All patients with secondary HPT received regularly hemodialysis. The clinical and biochemical data for the patients are summarized in Table 1. All patients with pHPT were diagnosed as having sporadic disease. Four had a history of thyroidectomy for benign thyroid nodules (n = 3) or papillary thyroid cancer (n = 1). One patient was referred to us for persistent pHPT. Preoperative localization studies were performed on all patients with ultrasonography, 99mTc sestamibi scanning, and magnetic resonance imaging.
Table 1. PREOPERATIVE CLINICAL AND BIOCHEMICAL DATA
HPT, hyperparathyroidism; iPTH, intact parathyroid hormone; BGP, bone GLA protein; NTx, N-telopeptides; ND, not determined.
* Values are given as mean ± standard deviation.
† Corrected calcium level was calculated by the following formula: [calcium concentration (mg/dL) plus (4-albumin (g/dL))].
Preoperative Laboratory Tests
Blood and urine samples were collected after an overnight fast. Urine samples were not available in patients with secondary HPT. Serum levels of alkaline phosphatase, total calcium, albumin, and inorganic phosphate were measured by routine automated procedures, and ionized calcium was determined by an electrolyte analyzer (NOVA Biochemical, Waltham, MA). The corrected calcium level was calculated with the formula [calcium concentration (mg/dL) plus (4-albumin (g/dL))]. Serum bone GLA protein (BGP) was measured by Mitsubishi Yuka BGP IRMA kit (Tokyo, Japan) using a mouse monoclonal antibody to human BGP. Preoperative serum iPTH was measured by a two-site immunochemiluminometric assay. 34 To assess bone resorption, we measured urinary excretions of the collagen type 1 cross-linked N-telopeptides by enzyme-linked immunosorbent assay (Osteomark, Ostex Intl., Seattle, WA), and the results were corrected using urinary creatinine concentration assessed using a standard calorimetric method. The protocol was approved by the staff at the Noguchi Thyroid Clinic. All subjects gave written informed consent for participation.
Blood Samples During Surgery
EDTA plasma samples were drawn via a peripheral arterial catheter after anesthesia (basal), before excision of one enlarged parathyroid gland in HPT and of the last gland in secondary HPT, and at 5, 10, and 15 minutes after excision. The QPTH assay was performed by a two-site immunochemiluminometric assay at 10 minutes in all patients. 19,34 A decrease in the QPTH of 50% or more in the 10-minutes-after-excision sample compared with the higher value of either the basal or preexcision sample was used to predict successful parathyroidectomy in pHPT. Part of the EDTA plasma samples were kept at −70°C until later analysis of iPTH and CAP.
Parathyroid Hormone Assay for Stored Samples
Immunoradiometric CAP and iPTH assays of stored EDTA plasma samples were performed by Scantibodies Laboratory Inc. (Santee, CA). The method of CAP assay was reported previously. 30,31 In brief, two antibodies were produced in two different goats, one for the 39-84 region and the other for the 1-4 region of the human PTH molecule. Antihuman PTH (1-4) antibody is labeled with 125I, whereas the 39-84 region-specific antibody is captured onto 5/16-inch polystyrene beads. The plasma normal range of CAP was 7 to 36 pg/mL. The detection limit was approximately 1 pg/mL, and the interassay and intraassay coefficients of variation were found to be 2% and 7%, respectively. CAP assays currently take about 20 hours and were not used in the management of these patients. The iPTH assay was performed using Allegro Intact PTH (I-Nichols, San Juan Capistrano, CA). Normal values range from 10 to 65 pg/mL. The 7-84 PTH level was calculated by subtracting the CAP value from the iPTH value. The identification of 7-84 PTH has been confirmed previously. 27,30,33
Statistics
Data are expressed as mean ± standard deviation unless otherwise indicated. Continuous variables for pHPT and secondary HPT were compared by the Wilcoxon test. A generalized linear regression analysis was performed for the kinetics of CAP and iPTH with SAS-JMP version 4.0.2 software (SAS Institute Inc., Cary, NC). Correlation was determined by calculating the Pearson correlation coefficient.
RESULTS
The iPTH values versus the CAP are plotted in Figure 1.
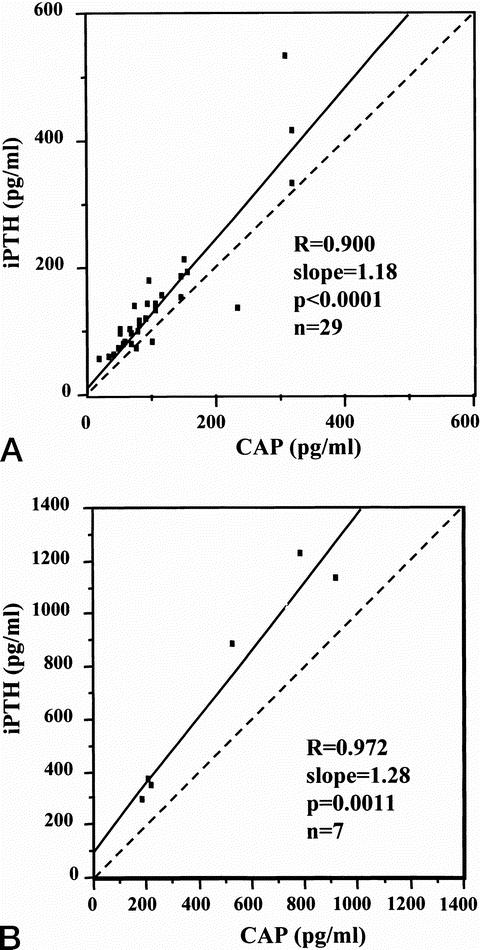
Figure 1. Regression parameters of preoperative plasma intact parathyroid hormone (iPTH) values measured by the Nichols and cyclase activating parathyroid hormone (CAP) in primary (A) and secondary (B) hyperparathyroidism.
In pHPT, the mean weight of a single adenoma was 801 ± 1058 mg (range 134–5,382). All the patients with pHPT underwent successful parathyroidectomies (>50% reduction in QPTH at 10 minutes confirmed in the operating room) and were normocalcemic or hypocalcemic after surgery and in follow-up studies ranging from 3 to 10 months. There were no intraoperative or postoperative complications.
Six of the seven patients with secondary HPT underwent removal of four hyperplastic parathyroid glands (total mean weight, 3,485 ± 1,640 mg) with forearm autotransplantation, and the level of iPTH of these patients was below the reference range (10–65 pg/mL) on the first postoperative day. For the seventh patient, we could not find other parathyroid glands except for one enlarged gland (1,874 mg), which was excised without autotransplantation. In this patient the level of iPTH on the first postoperative day was 5 pg/mL; it was 19.5 pg/mL 6 months after surgery under the control of relative hypocalcemia (8.0–8.5 mg/dL) with active vitamin D. All patients underwent successful parathyroidectomies and were normocalcemic or hypocalcemic after surgery and in follow-up studies ranging from 3 to 6 months. One patient had transient recurrent nerve palsy.
The percentage of 7-84 PTH in iPTH in plasma samples varied from 0.7% to 66.0% (27.5 ± 14.4%) in pHPT and from 18.6% to 69.0% (39.6 ± 15.1%) in secondary HPT. Three patients with pHPT having a higher value of CAP than iPTH were excluded. A significant difference in the 7-84 PTH percentage was found between the two groups (P = .0001). In HPT patients the plasma CAP and iPTH values had decreased to 23.4 ± 10.8 and 32.0 ± 11.3% of the preexcision level at 5 minutes, 10.6 ± 7.7 and 21.1 ± 8.8% at 10 minutes, and 8.5 ± 4.9 and 16.1 ± 6.8% 15 minutes after removal of an enlarged gland, respectively (P = .0001) (Fig. 2). At 5 minutes, the CAP levels of all 29 pHPT patients had decreased to less than 40% of the preparathyroidectomy higher level; however, 7 (24%) patients still had an iPTH level of more than 40%. In secondary HPT patients, the CAP and iPTH values had dropped to 43.3 ± 20.2 and 66.1 ± 19.7% at 5 minutes, 28.6 ± 16.6 and 53.6 ± 18.1% at 10 minutes, and 14.2 ± 9.0 and 41.0 ± 12.9% at 15 minutes, respectively (P = .0001) (Fig. 3). At 10 minutes, CAP levels of all seven secondary HPT patients had decreased to less than 50% of the preexcision level of the last enlarged parathyroid gland; however, three (43%) patients still had an iPTH level of more than 50%. The 7-84 PTH value in pHPT and secondary HPT patients had decreased to 57.4 ± 85.9 and 62.1 ± 84.9%, respectively, at 15 minutes after removal of the enlarged gland or glands compared with the preexcision values.
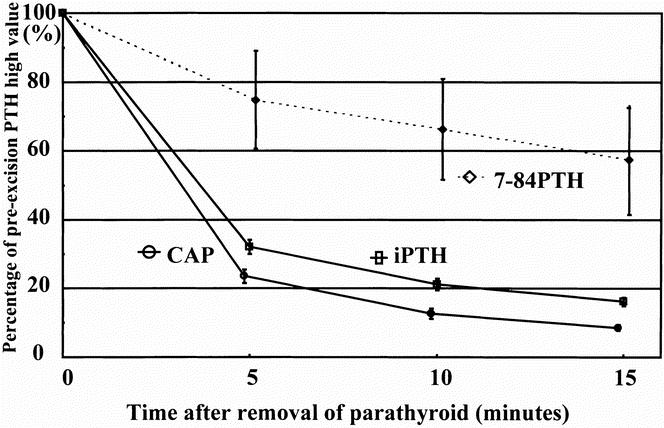
Figure 2. Decline of cyclase activating parathyroid hormone (CAP), intact parathyroid hormone (iPTH), and 7-84 parathyroid hormone (PTH) after excision of one enlarged parathyroid gland in the 29 patients with primary hyperparathyroidism. Results are shown as the percentage of the higher value of either basal or preexcision and means (standard error). There were significant differences in the percentage decrease among them (P = .0001) by a generalized linear regression analysis. CAP was significantly lower than iPTH at all times (P = .0001) by analysis of variance. The 7-84 PTH level was calculated by subtracting the CAP value from the iPTH value.
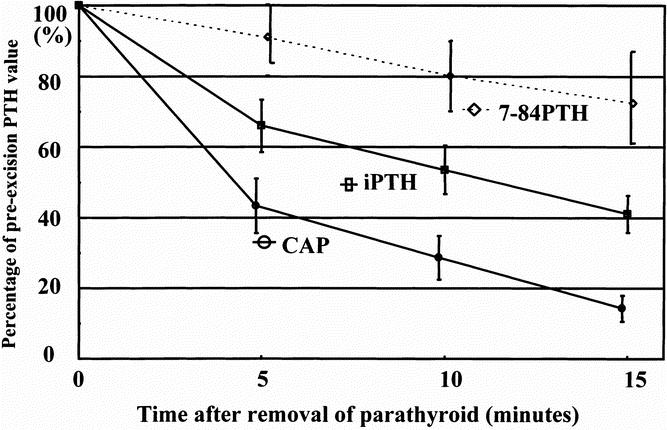
Figure 3. Decline of cyclase activating parathyroid hormone (CAP), intact parathyroid hormone (iPTH), and 7-84 parathyroid hormone (PTH) after excision of the last parathyroid gland in the seven patients with secondary hyperparathyroidism. Results are shown as the percentage of the preexcision and means (standard error). There were significant differences in the percentage decrease among them (P = .0001) by a generalized linear regression analysis. CAP was significantly lower than iPTH at all times (P = .001) by analysis of variance. The 7-84 PTH level was calculated by subtracting the CAP value from the iPTH value.
DISCUSSION
In the past 10 years, the management of HPT has changed significantly because of improved preoperative localization techniques 35–37 and QPTH. 7,12–16,18,19,38 The traditional operative approach of identifying all four parathyroid glands and resecting the abnormal gland, with biopsy of a normal-appearing gland, has changed to more limited neck exploration in patients with sporadic HPT and one abnormal parathyroid gland identified before surgery. 39–42
Since QPTH was introduced in our institution, we have performed parathyroidectomy through a small incision or endoscopic surgery in selected pHPT patients with a localized single adenoma when concomitant thyroid disease was not present. 19,42 Some authors have considered the use of QPTH during surgical treatment of pHPT; this is of value by providing the surgeon evidence that no remaining hyperfunctioning parathyroid tissue is present. 7,12–16,38 Others have found such monitoring less important. 43,44 When reviewing the large series using QPTH, however, the accurate predictive value of the postoperative outcome has been approximately 95% because of the presence of false-positive and false-negative results. 17–24 Sokoll et al. 22 have recently reported that the overall accuracy of the QPTH in predicting surgical success was 88% using the criterion of a 50% decrease at 5 to 10 minutes, and 97% including the subset of patients with delayed decreases of PTH, from the study of 195 patients with pHPT. We also have had several patients (false-negative results) whose iPTH did not decrease 50% after 10 minutes with postoperative eucalcemia. 19 If the half-life of PTH is on the order of 1 to 5 minutes, 45 at least 50% reduction should be achieved 10 minutes after removal of the hyperfunctioning gland. Although the utility of QPTH is based on the critical assumption that the half-life of PTH is constant among patients, the apparent half-life has been shown to vary over a wide range. 21 The unknown mechanisms of the delayed apparent PTH metabolism could be solved from the present study.
We showed that the percentage of 7-84 PTH in iPTH in plasma samples varies and that the plasma CAP value decreases more rapidly than iPTH after parathyroidectomy, depending on the amount of 7-84 PTH in circulation, in both pHPT and secondary HPT. These results have a significant clinical implication for intraoperative QPTH assay design, development, and selection during the surgical treatment of HPT. Decline of true 1-84 PTH does not depend on 7-84 PTH, and the 50% decrease in plasma PTH is achieved earlier when CAP is measured. The replacement of iPTH assay with the CAP assay could shorten the operative time and also avoid further exploration on patients with false-negative results. The fact that CAP levels at 5 minutes had decreased to less than 40% of the preparathyroidectomy level in all patients with pHPT, but seven (24%) patients still had iPTH levels of more than 40%, shows the promising potential of the operative use of CAP instead of iPTH. However, both CAP and iPTH levels had decreased to less than 50%, which most investigators use as an indication that all abnormal parathyroid tissue has been removed.
The QPTH has been considered of significance to the parathyroid surgeon because it measures function; therefore, CAP measurement has both theoretical and practical advantages over iPTH, which shows cross-reactivity to 7-84 PTH. 27,30–33 These advantages are especially relevant for uremic patients because they usually have associated cardiovascular disease and a large amount of 7-84 PTH in circulation; moreover, anesthesiologists and surgeons are particularly keen to avoid unnecessary prolonged exploration, compared with pHPT patients. 7-84 PTH was reported to have antagonistic effects in vivo in rats at the levels of bone and kidney. 30 In our data and others monitoring iPTH during parathyroidectomy in patients with renal failure, 46 the rate of decline of iPTH levels was slightly slowed; others have found a slope similar to that of patients with pHPT. 43 Lokey et al. 46 have recently reported that the intraoperative decay of iPTH during surgery for secondary HPT is slower than for patients with normal renal function, and that 20 minutes after resection, a decline to less than 50% of the preoperative level predicts cure. In our series, CAP levels 10 minutes after excision of the last pathologic gland decreased to less than 50% of the preexcision level in all cases, although the number of patients studied was small. The kidney is a major site of PTH catabolism and of excretion of its C-terminal fragment. 47 Our results showing that a significant difference exists in the decline of 7-84 PTH after parathyroidectomy between pHPT and secondary HPT may have a bearing on the role of renal function for 7-84 PTH clearance.
The theoretical and practical superiority of the CAP measurements over iPTH measurements was discussed above, although the CAP assay is not currently available for intraoperative use. Technical modification to shorten assay time may be possible. In the past a standard 24-hour iPTH immunoradiometric or chemoluminometric assay was modified to achieve a total turnaround of 10 minutes by using the Quick Intraoperative iPTH reagent kit with the Quick-Pack portable chemiluminometer workstation (Nichols Institute Diagnostics, San Juan Capistrano, CA).
Three patients had preoperative CAP levels higher than iPTH levels. We did not count these three for the 7-84 PTH calculation. The reversed CAP and iPTH levels disappeared after parathyroidectomy, which partially indicates that this phenomenon was caused by a factor related to parathyroid glands. These samples were checked and found negative for specific assay interference, such as HAMA or heterophilic antibodies. We believe that the reversed CAP versus iPTH levels could show the existence of an N-terminal PTH fragment produced by the adenoma, and this PTH fragment could be detected by the CAP assay but not the iPTH assay. Gao et al. 48 reported that there is an N-terminal PTH fragment in some rare patients with primary hyperparathyroidism. There is no direct evidence for the existence of N-terminal PTH fragment in samples from these three patients, but it could be clinically significant because the CAP assay could measure not only the true intact PTH (1-84) but also a bioactive PTH fragment and could provide higher clinical diagnostic sensitivity for primary HPT. 49 Further study would be needed to confirm this hypothesis.
Our study shows that the percentage of 7-84 PTH in iPTH in plasma samples varies over a wide range between patients with pHPT and secondary HPT and that plasma CAP values decreased more rapidly than iPTH after parathyroidectomy, depending on the amount of 7-84 PTH. These results suggest that the CAP assay can be a more useful adjunct to parathyroidectomy than the currently used iPTH assay.
Footnotes
Correspondence: Hiroyuki Yamashita, MD, Noguchi Thyroid Clinic and Hospital Foundation, 6-33 Noguchi-Nakamachi, Beppu Oita 874-0932, Japan.
E-mail: yama@noguchi-med-or.jp
Accepted for publication December 5, 2001.
References
- 1.Clark OH, Duh QY. Primary hyperparathyroidism. A surgical perspective. Endocrinol Metab Clin North Am 1989; 18: 701–714. [PubMed] [Google Scholar]
- 2.van Heerden JA, Grant CS. Surgical treatment of primary hyperparathyroidism: an institutional perspective. World J Surg 1991; 15: 688–692. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan EL, Yashiro T, Salti G. Primary hyperparathyroidism in the 1990s. Choice of surgical procedures for this disease. Ann Surg 1992; 215: 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruining HA, Birkenha’ger JC, Ong GL, Lamberts SW. Causes of failure in operations for hyperparathyroidism. Surgery 1987; 101: 562–565. [PubMed] [Google Scholar]
- 5.Levin KE, Clark OH. The reasons for failure in parathyroid operations. Arch Surg 1989; 124: 911–914. [DOI] [PubMed] [Google Scholar]
- 6.Clark OH, Okerlund MD, Moss AA, et al. Localization studies in patients with persistent or recurrent hyperparathyroidism. Surgery 1985; 98: 1083–1094. [PubMed] [Google Scholar]
- 7.Thompson GB, Grant CS, Perrier ND, et al. Reoperative parathyroid surgery in the era of sestamibi scanning and intraoperative parathyroid hormone monitoring. Arch Surg 1999; 134: 699–705. [DOI] [PubMed] [Google Scholar]
- 8.Monchik JM, Farrugia C, Teplitz C, et al. Parathyroid surgery: the role of chief cell intracellular fat staining with osmium carmine in the intraoperative management of patients with hyperparathyroidism. Surgery 1983; 94: 877–886. [PubMed] [Google Scholar]
- 9.Welsh CL, Taylor GW. The density test for the intraoperative differentiation of single or multigland parathyroid disease. World J Surg 1984; 8: 522–526. [DOI] [PubMed] [Google Scholar]
- 10.Norton JA, Brennan MF, Saxe AW, et al. Intraoperative urinary cyclic adenosine monophosphate as a guide to successful reoperative parathyroidectomy. Ann Surg 1984; 200: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen I, Pollard A. Ionized calcium in monitoring effective parathyroidectomy: a preliminary report. World J Surg 1988; 12: 630–634. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum SR, Thompson AR, Hutcheson KA, et al. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery 1988; 104: 1121–1127. [PubMed] [Google Scholar]
- 13.McHenry CR, Pollard A, Walfish PG, et al. Intraoperative parathormone level measurement in the management of hyperparathyroidism. Surgery 1990; 108: 801–808. [PubMed] [Google Scholar]
- 14.Robertson GSM, Iqbal SJ, Bolia A, et al. Intraoperative parathyroid hormone estimation: a valuable adjunct to parathyroid surgery. Ann R Coll Surg Engl 1992; 74: 19–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Kao PC, van Heerden JA, Taylor RL. Intraoperative monitoring of parathyroid procedures by a 15-minute parathyroid hormone immunochemiluminometric assay. J Clin Endocrinol Metab 1994; 78: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 16.Sofferman RA, Standage J, Tang ME. Minimal-access parathyroid surgery using intraoperative parathyroid hormone assay. Laryngoscope 1998; 108: 1497–1503. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Sokoll LJ, Udelsman R. Outpatient minimally invasive parathyroidectomy: a combination of sestamibi-SPECT localization, cervical block anesthesia, and intraoperative parathyroid hormone assay. Surgery 1999; 126: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 18.Garner SC, Leight GS, Jr. Initial experience with intraoperative PTH determinations in the surgical management of 130 consecutive cases of primary hyperparathyroidism. Surgery 1999; 126: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita H, Noguchi S, Futata T, et al. Usefulness of quick intraoperative measurements of intact parathyroid hormone in the surgical management of hyperparathyroidism. Biomed Pharmacother 2000; 54: 108S–111S. [DOI] [PubMed] [Google Scholar]
- 20.Irvin G, Dembrow VD, Prudhomme DL. Clinical usefulness of an intraoperative “quick parathyroid hormone” assay. Surgery 1993; 114: 1019–1023. [PubMed] [Google Scholar]
- 21.Libutti SK, Alexander HR, Bartlett DL, et al. Kinetic analysis of the rapid intraoperative parathyroid hormone assay in patients during operation for hyperparathyroidism. Surgery 1999; 126: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 22.Sokoll LJ, Drew H, Udelsman R. Intraoperative parathyroid hormone analysis: A study of 200 consecutive cases. Clin Chem 2000; 46: 1662–1668. [PubMed] [Google Scholar]
- 23.Gordon LL, Snyder WH 3rd, Wians F Jr, et al. The validity of quick intraoperative parathyroid hormone assay: an evaluation in seventy-two patients based on gross morphologic criteria. Surgery 1999; 126: 1030–1035. [DOI] [PubMed] [Google Scholar]
- 24.Weber CJ, Ritchie JC. Retrospective analysis of sequential changes in serum intact parathyroid hormone levels during conventional parathyroid exploration. Surgery 1999; 126: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 25.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 1987; 33: 1364–1367. [PubMed] [Google Scholar]
- 26.Kao PC, van Heerden JA, Grant CS, et al. Clinical performance of parathyroid hormone immunometric assays. Mayo Clin Proc 1992; 67: 637–645. [DOI] [PubMed] [Google Scholar]
- 27.Brossard JH, Whittom S, Lepage R, et al. Carboxyl-terminal fragments of parathyroid hormone are not secreted preferentially in primary hyperparathyroidism as they are in other hypercalcemic conditions. J Clin Endocrinol Metab 1993; 77: 413–419. [DOI] [PubMed] [Google Scholar]
- 28.Gao P, Fulla Y, Scheibel S, et al. Recognition of the PTH(7–84) fragment by 5 commercial PTH “sandwich” assays. J Bone Miner Res 2000; 15 (suppl): S564. [Google Scholar]
- 29.Gao P, Scheibel S, D’Amour P, et al. Measuring the biologically active or authentic whole parathyroid hormone (PTH) with a novel immunoradiometric assay without cross-reaction to the PTH (7–84) fragment. J Bone Miner Res 1999; 14 (suppl): S446. [Google Scholar]
- 30.Slatopolsky E, Finch J, Clay P, et al. A novel mechanism for skeletal resistance in uremia. Kidney Int 2000; 58: 753–761. [DOI] [PubMed] [Google Scholar]
- 31.Gao P, Scheibel S, D’Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone (1–84): implication for improvement of accurate assessment of parathyroid function. J Bone Miner Res 2001; 16: 605–614. [DOI] [PubMed] [Google Scholar]
- 32.Lepage R, Roy L, Brossard JH, et al. A non-(1–84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 1998; 44: 805–809. [PubMed] [Google Scholar]
- 33.John MR, Goodman WG, Gao P, et al. A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: implications for PTH measurements in renal failure. J Clin Endocrinol Metab 1999; 84: 4287–4290. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita H, Noguchi S, Uchino S, et al. Usefulness of quick intraoperative measurements of intact parathyroid hormone in the surgical management of hyperparathyroidism. J Jpn Surg Assoc 2000; 61: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 35.Wei JP, Burke GJ, Mansberger AJ. Preoperative imaging of abnormal parathyroid glands in patients with hyperparathyroid disease using combination Tc-99m-pertechnetate and Tc-99m-sestamibi radionuclide scans. Ann Surg 1994; 219: 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson GB, Mullan BP, Grant CS, et al. Parathyroid imaging with technetium-99m-sestamibi: an initial institutional experience. Surgery 1994; 116: 966–972. [PubMed] [Google Scholar]
- 37.Caixas A, Berna L, Piera J, et al. Utility of 99mTc-sestamibi scintigraphy as a first-line imaging procedure in the preoperative evaluation of hyperparathyroidism. Calcif Tissue Int 1995; 57: 329–335. [DOI] [PubMed] [Google Scholar]
- 38.Irvin GR, Prudhomme DL, Deriso GT, et al. A new approach to parathyroidectomy. Ann Surg 1994; 219: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miccoli P, Bendinelli C, Conte M, et al. Endoscopic parathyroidectomy by a gasless approach. J Laparoendosc Adv Surg Tech A 1998; 8: 189–194. [DOI] [PubMed] [Google Scholar]
- 40.Norman J, Chheda H. Minimally invasive parathyroidectomy facilitated by intraoperative nuclear mapping. Surgery 1997; 122: 998–1004. [DOI] [PubMed] [Google Scholar]
- 41.Udelsman R, Donovan PI, Sokoll LJ. One hundred consecutive minimally invasive parathyroid explorations. Ann Surg 2000; 232: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita H, Ohshima A, Uchino S, et al. Endoscopic parathyroidectomy using quick intraoperative intact parathyroid hormone assay. J Clin Surg 2000; 55: 767–769. [Google Scholar]
- 43.Proye CA, Goropoulos A, Franz C, et al. Usefulness and limits of quick intraoperative measurements of intact (1–84) parathyroid hormone in the surgical management of hyperparathyroidism: Sequential measurements in patients with multiglandular disease. Surgery 1991; 110: 1035–1042. [PubMed] [Google Scholar]
- 44.Tan P, Leveson SH, Wilkinson H. Limited role for intraoperative intact PTH measurement in parathyroid surgery. Ann R Coll Surg Engl 1995; 77: 28–30. [PMC free article] [PubMed] [Google Scholar]
- 45.Goltzman D, Gomolin H, DeLean A, et al. Discordant disappearance of bioactive and immunoreactive parathyroid hormone after parathyroidectomy. J Clin Endocrinol Metab 1984; 58: 70–75. [DOI] [PubMed] [Google Scholar]
- 46.Lokey J, Pattou F, Mondragon-Sanchez A, et al. Intraoperative decay profile of intact (1–84) parathyroid hormone in surgery for renal hyperparathyroidism-a consecutive series of 80 patients. Surgery 2000; 128: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 47.Aston JP, Wheeler MH, Brown RC, et al. Laboratory comparison of diagnostic specificity of intact hormone and region-specific immunoassays of parathyroid hormone. World J Surg 1990; 14: 419–424. [DOI] [PubMed] [Google Scholar]
- 48.Gao P, Schmidt-Gayk H, Dittrich K, et al. Immunochemiluminometric assay with two monoclonal antibodies against the N-terminal sequence of human parathyroid hormone. Clin Chim Acta 1996; 245: 39–59. [DOI] [PubMed] [Google Scholar]
- 49.Silverberg S, Brown IN, Bilezikian JP, et al. A new highly sensitive assay for parathyroid hormone in primary hyperparathyroidism. J Bone Miner Res 2000; 15 (suppl): S167. [Google Scholar]