Abstract
Objective
To determine whether monocyte chemoattractant protein-1 (MCP-1), which initiates subsequent development of burn-associated type 2 T cells, is produced in mice early after thermal injury.
Summary Background Data
A predominance of type 2 T-cell responses is commonly observed in animals and patients with severe thermal injuries. Burn-associated type 2 T cells have been identified as the cells responsible for the increased susceptibility of thermally injured mice to infections with herpes simplex virus type 1 and Candida albicans. Recently, the necessity of MCP-1 for the generation of type 2 T cells was shown in MCP-1 knockout mice. MCP-1 may have an important role in the increased susceptibility of thermally injured mice to various intracellular opportunistic pathogens.
Methods
The production of MCP-1 in sera or in cultures of various cells prepared from thermally injured mice was measured. Dual-chamber transwell cultures were performed to determine the influence of MCP-1-producing cells on the generation of burn-associated type 2 T cells.
Results
Without any stimulation, splenic macrophages from mice (1/2D-Mφ) produced MCP-1 into their culture fluids 12 hours after thermal injury. Interleukin-4 was detected in culture fluids of splenic T cells from normal mice cultured with 1/2D-Mφ in a dual-chamber transwell system; however, this cytokine was not produced by normal T cells cultured with normal macrophages in the transwells. Also, normal T cells cultured with 1/2D-Mφ did not produce interleukin-4 when transwell cultures were performed in the presence of anti-MCP-1 monoclonal antibody. Further, normal T cells directly stimulated with MCP-1 produced interleukin-4 into their culture fluids. Normal T cells, cultured with 1/2D-Mφ for 24 hours in the transwells and recultured with fresh medium for an additional 7 days, produced interleukin-10 (but not interferon-γ) and expressed ST2L mRNA (but not interleukin-12 receptor β2 chain) when they were stimulated with anti-CD3 monoclonal antibody.
Conclusions
Results indicate that MCP-1 is produced in mice within 1 day of thermal injury, and the subsequent development of burn-associated type 2 T-cell responses may be triggered by MCP-1 produced early after thermal injury.
Death in thermally injured patients is significantly associated with infection. 1,2 Herpesviruses and Candida albicans produce severe infections in thermally injured patients, 3–5 but in normal individuals these pathogens are common but rarely cause severe infection. 1,6 A major reason for the increased susceptibility of thermally injured patients to these pathogens appears to be a deficient immune response. 1,7 Among the immunologic abnormalities documented in thermally injured patients, diminished type 1 T-cell responses are common. 8,9 Type 1 T-cell responses are essential for the host defense against these pathogens. 10 Effector cells for type 1 T-cell responses inhibit spreading of intracellular pathogens (herpesviruses and C. albicans) by killing cells infected with pathogens. 10 Type 1 T-cell responses are manifested by the increased production of interleukin (IL)-2 and interferon (IFN)-γ (type 1 cytokines). 11–13 In contrast, type 2 T-cell responses, expressed through the generation of type 2 T cells (T helper type 2 cells, CD8+ type 2 T cells), are manifested by an increased production of IL-4, IL-10, and IL-13 (type 2 cytokines). 11–13 Type 2 cytokines act as inhibitors of type 1 cytokine production from type 1 T cells. 11–13
In our previous studies, 14–17 IFN-γ was not produced in thermally injured mice or in cultures of splenic mononuclear cells from thermally injured mice after stimulation with IFN-γ inducers. As burn-associated type 2 T cells, CD8+ CD11b+ TCRγ/δ+ T cells that produce IL-4 and IL-10 inhibit the generation of type 1 T cells. 15 Burn-associated type 2 T cells have been identified as the cells responsible for the increased susceptibility of thermally injured mice to infections with HSV-1 and C. albicans. 15–17 Further confirming the role of type 2 T cells in the burn-associated deficiency of the immune response is the finding of increased susceptibility of normal mice to these pathogens, to levels observed in thermally injured mice, after the inoculation with cloned burn-associated type 2 T cells. 16,17 Similar results are observed in studies using cells from thermally injured patients. 18 Severe combined immunodeficient (SCID) mice inoculated with peripheral blood lymphocytes (PBLs) from thermally injured patients (patient PBL-SCID chimeras) are susceptible to infection with C. albicans, whereas healthy PBL-SCID chimeras (SCID mice inoculated with PBLs from healthy donors) are resistant to infection. 18 This impaired resistance of patient PBL-SCID chimeras to infection subsequently recovers to levels observed in healthy PBL-SCID chimeras when human SCID chimeras are created with patient PBLs previously depleted of burn-associated type 2 T cells. 18 These facts indicate that the resistance of thermally injured patients to C. albicans infection might be improved through the regulation of burn-associated type 2 T-cell responses. Therefore, studies investigating the generation mechanisms of burn-associated type 2 T cells may provide important information on the immunologic regulation of HSV-1 and C. albicans infections in thermally injured patients.
In the present study, the production of monocyte chemoattractant protein-1 (MCP-1) in sera of thermally injured mice early after thermal injury was shown. Also, MCP-1 induced by the burn stimulation was shown to initiate the burn-associated type 2 T-cell responses. MCP-1 is a member of the β-chemokines and plays a crucial role in the trafficking and recruitment of effector leukocytes to primary sites of immune responses and inflammation. 19,20 MCP-1 also plays a role in the synthesis of IL-4 by ovalbumin-stimulated CD4+ T cells. 21 Recently, the necessity of MCP-1 for the generation of type 2 T cells was shown in MCP-1 knockout mice. 22 MCP-1 may have an important role in the increased susceptibility of thermally injured mice to various intracellular opportunistic pathogens.
METHODS
Animals
Eight-week-old male BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were used in these experiments. Experimental protocols for animal studies were approved by the Animal Care and Use Committee of the University of Texas Medical Branch at Galveston (ACUC approval number: 95–04–039).
Thermal Injury
Thermally injured mice were produced according to our previously reported procedures with minor modifications. 23 Mice were anesthetized with pentobarbital (40 mg/kg) administered by intraperitoneal injection. Electric clippers were used to shave the hair on the back of each mouse from groin to axilla. Thermal injury was produced by pressing a custom-made insulated mold (with a 2.5 × 3.5-cm window) firmly against the shaved back of each mouse and subsequently exposing the area to a gas flame for 9 seconds. The gas flame was produced by a Bunsen burner equipped with a flame-dispersing cap. This procedure produced a third-degree burn on approximately 15% of total body surface area for a 26-g mouse. Immediately after thermal injury, physiologic saline (4 mL per mouse) was administered by intraperitoneal injection for fluid resuscitation.
Reagents, Media, and Cells
Murine recombinant (r)MCP-1, rIFN-γ, and rIL-4 were obtained from PeproTech (Rocky Hill, NJ). Murine rIL-12 and monoclonal antibodies (mAbs) for MCP-1, IFN-γ, IL-2, IL-4, IL-10, IL-12, CD3, and CD28 were purchased from PharMingen (San Diego, CA). For cultivation of macrophages, neutrophils, and lymphocytes, we used RPMI-1640 medium supplemented with 10% FBS, 2 mmol/L l-glutamine, antibiotics, 30 mmol/L HEPES, and 5 × 10−5 mol/L 2-ME (complete medium). For cultivation of fibroblasts, we used Eagle’s MEM supplemented with 10% FBS, 2 mmol/L l-glutamine, and antibiotics (Eagle’s complete medium). The same medium supplemented with 2% FBS was used as maintenance medium.
As previously described, 24,25 standard type 1 T cells (ST1 cells) were generated from naive T cells through stimulation with immobilized anti-CD3/CD28 mAbs (10 μg/mL) in the presence of rIL-12 (2 ng/mL) and anti-IL-4 mAb (200 ng/mL). Standard type 2 T cells (ST2 cells) were generated from naive T cells through stimulation with the same mAbs in the presence of IL-4 (20 ng/mL) and anti-IL-12 mAb (2 μg/mL). 24,25 The resulting cells were cultured in complete medium supplemented with rIL-2 (100 U/mL).
Peritoneal macrophages were prepared from peritoneal cells removed from mice that were injected intraperitoneally with 10 mL phosphate-buffered saline. 23 Splenic macrophages were prepared from single cell suspensions of spleens aseptically removed from both thermally injured and control mice. 23 After red blood cells were lysed by a hypotonic lysing buffer (R&D Systems, Minneapolis, MN), peritoneal exudate cells and splenic cells suspended in maintenance medium were added to fibronectin-coated petri dishes (Protein Polymer, San Diego, CA). Fifteen minutes after incubation at 37°C, the dishes were washed twice with warmed maintenance medium to remove nonadherent cells from the surface of the petri dishes. 23 A macrophage-enriched population (92% pure) was then harvested from the dishes by scraping with a rubber policeman. 23
T and B cells were prepared from the spleens of thermally injured mice through the use of T-cell enrichment columns (R&D Systems) or B-cell immunocolumns (Cytovax, Edmonton, Alberta, Canada), respectively. The purity of these cells was greater than 96%, as described previously. 26 Peripheral blood neutrophils were isolated from heparinized blood by dextran sedimentation followed by Ficoll-Hypaque density gradient centrifugation. 27 Red blood cells were eliminated from the neutrophil preparations by exposure to hypotonic solution. 27 Fibroblasts were obtained from the lungs and kidneys of thermally injured mice by the standard trypsinization technique. 28
Transwell Assay
Cell preparations from thermally injured and control mice were cultured with splenic T cells from normal mice in a dual-chamber transwell culture system. Six hundred microliters of a cell suspension for normal T cells (3 × 105 cells/well) was placed into the bottom chamber of the transwell (0.4- μm micropores) (Costar, Corning NY). One hundred microliters of the cell suspension for macrophages, T cells, B cells, neutrophils, or fibroblasts (7.5 × 104 to 6 × 105 cells/mL) was placed into the upper chamber of the transwell. In some experiments, transwell cultures were performed in the presence of anti-MCP-1 mAb (20 ng/mL). Twenty-four hours after cultivation, culture fluids harvested from the transwell were assayed for IL-4. As required, cells in the bottom chamber were recultured in fresh complete medium for an additional 7 days.
Production and Assay of Cytokines
Culture fluids harvested from the transwell cultures were assayed for IL-4 by enzyme-linked immunosorbent assay (ELISA). Also, culture fluids of normal T cells (2 × 106 cells/mL) were assayed for IL-4 by ELISA after stimulation with 1 to 200 ng/mL of rMCP-1 for 24 hours. To determine the abilities of cells to produce MCP-1, macrophages, B cells, T cells, neutrophils, lung fibroblasts, and kidney fibroblasts (2 × 106 cells/mL), prepared from mice early after thermal injury, were cultured without any stimulation for 24 hours. The harvested culture fluids from these cells were then assayed for MCP-1 by ELISA. To determine circulating IL-4 and MCP-1 levels, serum specimens obtained from mice 2, 6, 12, 18, 24, and 36 hours and 2, 4, 5, 6, 8, 10, and 12 days after thermal injury were assayed by ELISA. In some experiments, anti-MCP-1 mAb (10 μg/mouse) was administered to mice immediately and 3 days after thermal injury. As a control, rat Ig (10 μg/mouse) was injected to thermally injured mice on the same schedules. Serum specimens obtained from mice 6 days after thermal injury were assayed for IL-4 by ELISA. The detection limit for cytokines was 30 to 50 pg/mL in our assay systems. Each assay was performed three times.
Criteria for Identification of Type 2 T Cells
Type 2 T cells were generated from normal T cells cultured with 1/2D-Mφ for 24 hours after recultivation in fresh complete medium for an additional 7 days. Type 2 T cells were identified by the following properties: 1) cytokine-producing profile; 2) preferential expression of ST2L mRNA; and 3) loss of IL-12 receptor β2 chain (IL-12Rβ2) mRNA expression. To determine the cytokine-producing profile, cell preparations (2 × 106 cells/mL) were stimulated with immobilized anti-CD3 mAb (10 μg/mL) for 48 hours. The harvested culture fluids were assayed for IFN-γ and IL-10 by ELISA. IL-12Rβ2 antigen has been shown to be expressed on the surface of type 1 T cells. 24 The expression of ST2L antigen has been shown on murine type 2 T cells. 25 Therefore, mRNAs for these antigens were considered as markers for type 1 or type 2 T cells. mRNA extracted from these cells was subjected to reverse transcriptase (RT)-nested polymerase chain reaction (PCR; ST2L) or RT-PCR (IL-12Rβ2). cDNA was synthesized using oligo(dT) primers and AMV reverse transcriptase (Pharmacia, Piscataway, NJ). PCR was carried out using oligonucleotide primers (Genosys, Woodlands, TX). ST2L mRNA was amplified by RT-nested PCR using the following primers: sense, 5′-ACTTTGTTCACCACACTCTGC-3′; antisense, 5′-AACAGATGCCGTCTTGGAGGC-3′. 25 Inner primers were as follows: sense, 5′-CAAATTGTGCATTTATGGAG-3′; antisense, 5′-ATGCTTCCAGAATTTGGAAC-3′ (product of 346 bp). Primers for amplifying IL-12Rβ2 were as follows: sense, 5′-AAAGCCAACTGGAAAGCATTCG-3′; antisense, 5′-AGTTTTGAGTCAGGGTCTCTGC-3′ (product of 466 bp). 24 Using a thermal cycler (GeneAmp PCR System 9600), 35 cycles of PCR were performed at 94°C for 15 seconds, 60°C for 15 seconds, and 72°C for 20 seconds. The products were analyzed by electrophoresis in 2% agarose gel containing ethidium bromide with the PCR Marker (Sigma, St. Louis, MO). As controls, mRNAs from ST1 cells and ST2 cells were also subjected to RT-nested PCR or RT-PCR, respectively.
Statistical Analysis
Data are presented as mean ± standard deviation. Comparisons between experimental and control groups were made by analysis of variance followed by the Fisher protected least significant difference test. Significance was set at P < .05.
RESULTS
Monocyte Chemoattractant Protein 1 and Interleukin-4 Shown in Sera of Mice Various Times After Thermal Injury
Serum specimens were prepared from mice various hours and days after thermal injury. The amounts of MCP-1 and IL-4 in sera were determined by ELISA. Two hours after thermal injury, MCP-1 at a concentration of 420 ± 52 pg/mL was first detected in sera (Fig. 1). Peak serum MCP-1 levels (1,528 ± 135 pg/mL) occurred 1 day after thermal injury and subsequently declined to undetectable levels within 2 days of thermal injury. IL-4 was first detected in sera of mice 4 days after thermal injury; it peaked at 6 days after thermal injury and then gradually declined to undetectable levels by 12 days after thermal injury. In our previous studies, 15–17 CD8+ CD11b+ TCRγ/δ+ T cells detected in spleens of thermally injured mice have been characterized as burn-associated type 2 T cells. IL-4, detected in sera of mice 4 to 10 days after thermal injury, has been confirmed to be released from burn-associated type 2 T cells. 16,17
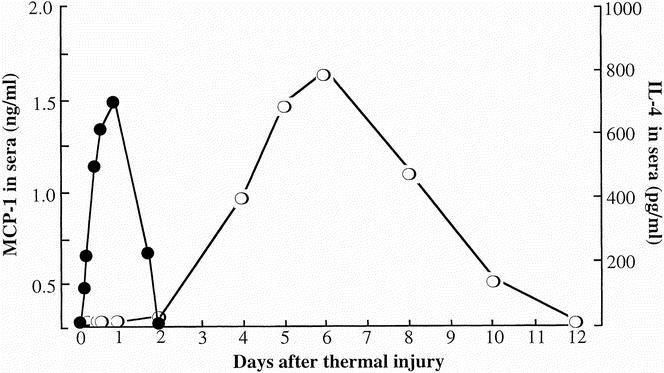
Figure 1. Production of interleukin-4 (IL-4) and monocyte chemoattractant protein 1 (MCP-1) in sera of mice various times after thermal injury. Serum specimens, prepared from mice 2, 6, 12, 18, 24, and 36 hours and 2, 4, 5, 6, 8, 10, and 12 days, were assayed for MCP-1 (solid circle) or IL-4 (open circle) by enzyme-linked immunosorbent assay. Each point is displayed as mean ± standard deviation (n = 5).
Production of Monocyte Chemoattractant Protein 1 In Vitro
The ability of cells to produce MCP-1 was examined in vitro. Cells were prepared from mice 10 to 24 hours after thermal injury. These cells included splenic macrophages, peritoneal macrophages, splenic B cells, splenic T cells, lung fibroblasts, kidney fibroblasts, and peripheral blood neutrophils. These cells were cultured in complete medium at cell densities of 2 × 106 cells/mL for 24 hours at 37°C in CO2 without any stimulation. The harvested culture fluids were assayed for the amount of MCP-1 by ELISA. MCP-1 was not detected in all culture fluids harvested from cell preparations obtained from control mice. Also, MCP-1 was not produced by splenic T cells and splenic B cells from thermally injured mice. The MCP-1 production was detected in cultures of splenic macrophages, lung fibroblasts, and kidney fibroblasts derived from thermally injured mice. MCP-1 was also produced by peritoneal macrophages and peripheral blood neutrophils from thermally injured mice. Representative results showing MCP-1 production in cultures of splenic macrophages are shown in Figure 2A. Splenic macrophage, obtained from mice 24 hours after thermal injury, produced 2.2 ± 0.3 ng/mL MCP-1 into their culture fluids when cultured at a cell density of 2 × 106 cells/mL for 24 hours. In comparison, splenic macrophages from control mice produced less than 30 pg/mL MCP-1 into their culture fluids. The greatest amount of MCP-1 (3.4 ± 0.2 ng/mL) was produced by splenic macrophages from mice 12 hours after thermal injury (see Fig. 2B). Therefore, splenic macrophages from mice 12 hours after thermal injury were abbreviated as “1/2D-Mφ,” and these cells were used in the following experiments.
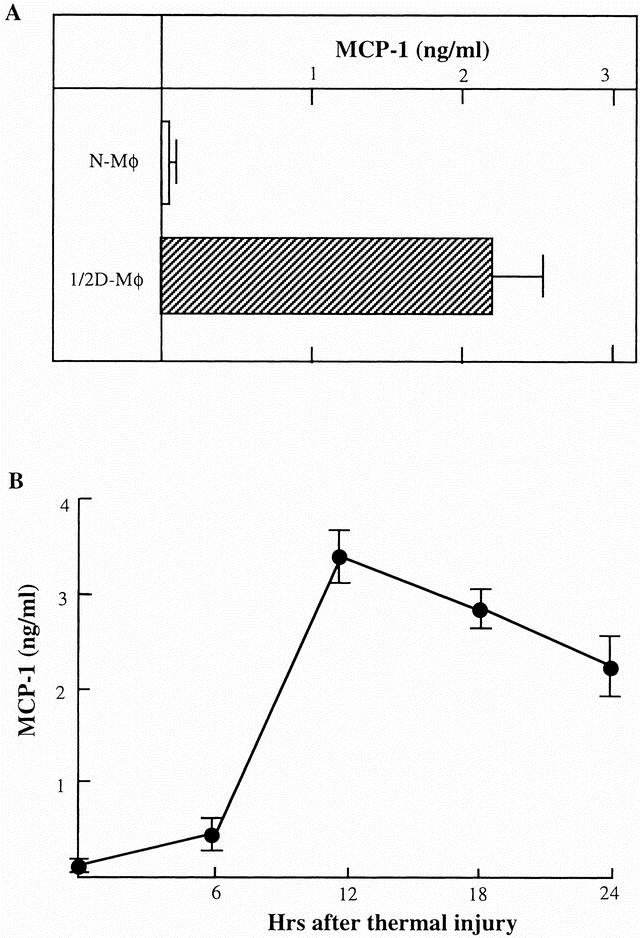
Figure 2. Production of monocyte chemoattractant protein 1 (MCP-1) in cultures of splenic macrophages from mice early after thermal injury. (A) Splenic macrophages (2 × 106 cells/mL) from normal mice or thermally injured mice 12 hours after thermal injury (1/2D-Mφ) were cultured for 24 hours in fresh complete medium without any stimulation. (B) Splenic macrophages, prepared from mice 6, 12, 18, and 24 hours after thermal injury, were cultured for 24 hours without any stimulation. Culture fluids obtained from both experiments were assayed for MCP-1 by enzyme-linked immunosorbent assay. Each result is displayed as mean ± standard deviation (n = 5).
Effect of 1/2D-Mφ on Interleukin-4 Production by Splenic T Cells From Normal Mice
The effect of 1/2D-Mφ on the IL-4 production by normal T cells was examined in a dual-chamber culture system. Normal T cells (3 × 105 cells/well) were placed into the bottom chamber of transwells, and 7.5 × 104 to 6 × 105 cells/well of 1/2D-Mφ were placed into the upper chamber of transwells. These chambers were cultured for 24 hours at 37°C in CO2, and harvested culture fluids were assayed for IL-4 by ELISA. IL-4 at an amount of 388 ± 42 pg/mL was detected in culture fluids of transwells cultured with a bottom cell to upper cell ratio of 2:1 (Fig. 3). The IL-4 production by normal T cells increased in correlation with the increased numbers of 1/2D-Mφ cultured in the upper chamber. As a control, various numbers of splenic macrophages from normal mice placed into the upper chamber were cultured with constant numbers of normal T cells in the same fashion. Significant amounts of IL-4 were not detected in culture fluids of these cells. Also, IL-4 was not produced by normal T cells cultured at a cell density of 3 × 105 cells/well or by 1/2D-Mφ cultured at a cell density of 6 × 105 cells/well. IL-4 was detected in culture fluids of transwell cultures performed with normal T cells (3 × 105 cells/well, placed into the bottom chamber) and peritoneal macrophages, peripheral blood neutrophils, lung fibroblasts, and kidney fibroblasts (6 × 105 cells/well, placed into the upper chamber) derived from thermally injured mice.
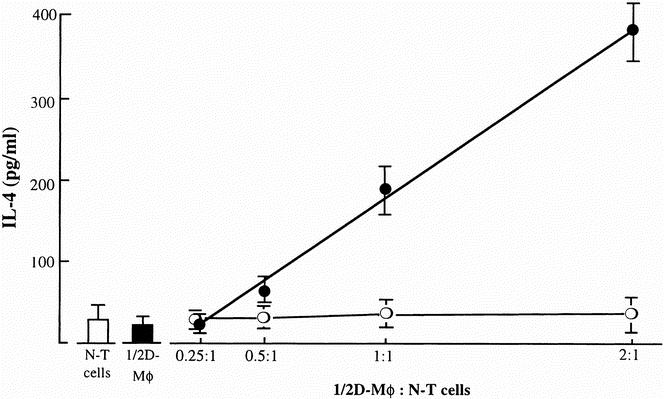
Figure 3. Production of interleukin (IL)-4 by splenic T cells from normal mice previously cultured with 1/2D-Mφ. In the transwell culture system, various numbers of 1/2D-Mφ (upper chamber) were cultured with a constant number of normal T cells (bottom chamber; cell density, 3 × 105 cells/well, solid circle). Controls (normal macrophages) were cultured with normal T cells in the same fashion (open circle). Also, normal T cells alone (3 × 105 cells/well, white bar) or 1/2D-Mφ alone (6 × 105 cells/well, black bar) were cultured and served as controls. Culture fluids harvested 24 hours after cultivation were assayed for IL-4 by enzyme-linked immunosorbent assay. Each point is displayed as mean ± standard deviation (n = 4–6).
To determine the role of 1/2D-Mφ−released MCP-1 on the production of IL-4 by normal T cells, these two cell preparations were cultured in transwells supplemented with or without anti-MCP-1 mAb (20 ng/mL). When the transwell cultures were supplemented with 20 ng/mL anti-MCP-1 mAb, the production of IL-4 was markedly reduced compared with that of transwell cultures not supplemented with the mAb (Fig. 4). The similar effect of anti-MCP-1 mAb on the IL-4 production was shown in vivo (Table 1). When IL-4 was not found in sera of normal mice (<30 pg/mL), 780 to 825 pg/mL of this cytokine was detected in sera of mice 6 days after thermal injury. At this time, only 45 to 70 pg/mL IL-4 was detected in day 6 sera of mice treated with anti-MCP-1 mAb immediately and 3 days after thermal injury (P < .001).
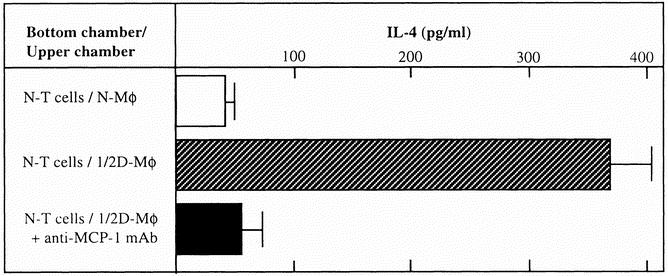
Figure 4. Effect of anti-monocyte chemoattractant protein 1 (MCP-1) monoclonal antibody on the interleukin (IL)-4 production by normal T cells cultured with 1/2D-Mφ. In a dual-chamber culture system, normal T cells (bottom chamber; cell density, 3 × 105 cells/well) were cultured with 1/2D-Mφ (upper chamber, 6 × 105 cells/well) in the presence or absence of anti-MCP-1 monoclonal antibody (20 ng/mL). As a control, normal T cells were cultured with normal macrophages in the same fashion. Culture fluids harvested 24 hours after cultivation were assayed for IL-4 by enzyme-linked immunosorbent assay. Each bar indicates mean ± standard deviation (n = 4–6).
Table 1. EFFECT OF ANTI-MCP-1 MAB ON THE IL-4 PRODUCTION IN THERMALLY INJURED MICE
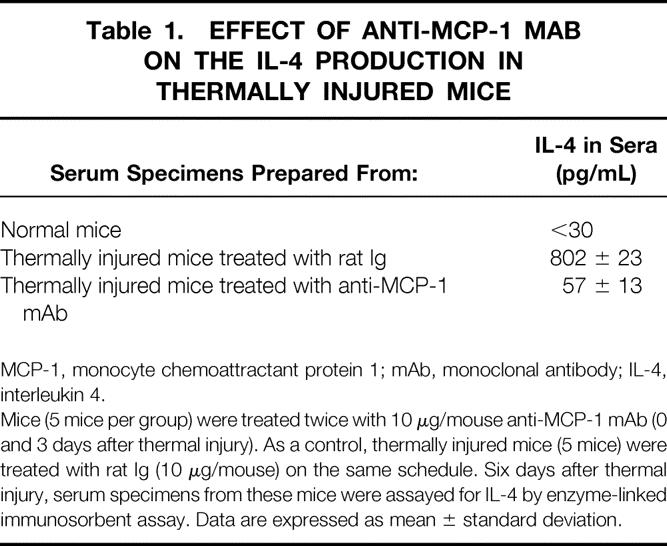
MCP-1, monocyte chemoattractant protein 1; mAb, monoclonal antibody; IL-4, interleukin 4.
Mice (5 mice per group) were treated twice with 10 μg/mouse anti-MCP-1 mAb (0 and 3 days after thermal injury). As a control, thermally injured mice (5 mice) were treated with rat Ig (10 μg/mouse) on the same schedule. Six days after thermal injury, serum specimens from these mice were assayed for IL-4 by enzyme-linked immunosorbent assay. Data are expressed as mean ± standard deviation.
Based on these results, we next examined the ability of MCP-1 to induce IL-4 in cultures of normal T cells. When normal T cells were stimulated with 100 ng/mL rMCP-1 for 24 hours, IL-4 at an amount of 645 ± 52 pg/mL was found in their culture fluids (Fig. 5). The concentrations of IL-4 detected in culture fluids increased proportionally to the dose of rMCP-1 (10–100 ng/mL) added to the normal T-cell cultures. These results indicate that MCP-1 is a key substance in the production of IL-4 by normal T cells in transwell cultures.
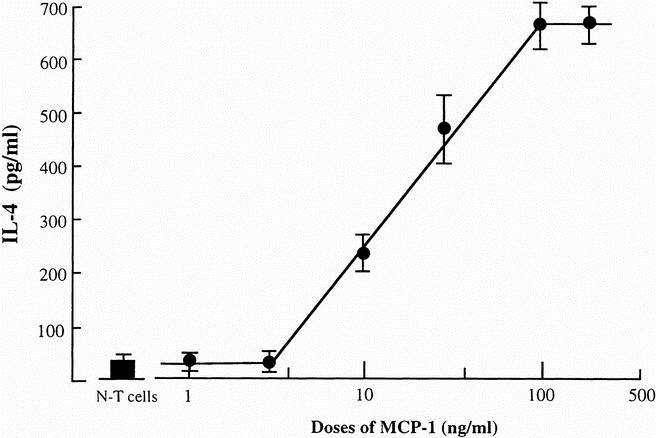
Figure 5. The production of interleukin (IL)-4 in cultures of normal T cells stimulated with recombinant monocyte chemoattractant protein 1 (rMCP-1). Normal T cells (cell density, 2 × 106 cells/mL) were cultured for 24 hours in the presence of various concentrations of rMCP-1. Culture fluids harvested from these cell cultures were assayed for IL-4 by enzyme-linked immunosorbent assay. Each point indicates the mean ± standard deviation of triplicate wells.
Effect of Monocyte Chemoattractant Protein 1 on the Subsequent Development of Type 2 T Cells
Type 2 T-cell characteristics were examined in normal T cells that were cultured with 1/2D-Mφ and recultured in fresh complete medium for an additional 7 days. Transwell cultures were performed with normal T cells (3 × 105 cells/well) placed into the bottom chamber and 1/2D-Mφ (6 × 105 cells/well) placed into the upper chamber. Twenty-four hours after cultivation, the upper chambers were removed from transwells, and cells in the bottom wells were recultured for an additional 7 days in fresh complete medium supplemented with IL-2 (100 U/mL). As a control, transwell cultures and recultivation of cells in the bottom chamber were performed with normal T cells (placed into the bottom chamber) and splenic macrophages from normal mice.
To induce the production of cytokines, cells harvested after the recultivation were stimulated with 10 μg/mL immobilized anti-CD3 mAb for 48 hours. Freshly isolated normal T cells were stimulated with the same mAb for 48 hours and served as a control. Culture fluids were harvested and assayed for IL-10 and IFN-γ by ELISA. As shown in Figure 6A, IL-10 was produced by recultured T cells harvested from the bottom chamber of transwells cultured with 1/2D-Mφ in the upper chamber. However, IFN-γ was not produced by these T cells (see Fig. 6B). Conversely, IFN-γ was produced by recultured T cells that were harvested from the bottom chamber of transwells cultured with splenic macrophages from normal mice in the upper chamber. However, IL-10 was not produced by these T cells. These results suggest that type 2 T cells were generated during the recultivation of normal T cells previously cultured with 1/2D-Mφ in the transwell cultures.
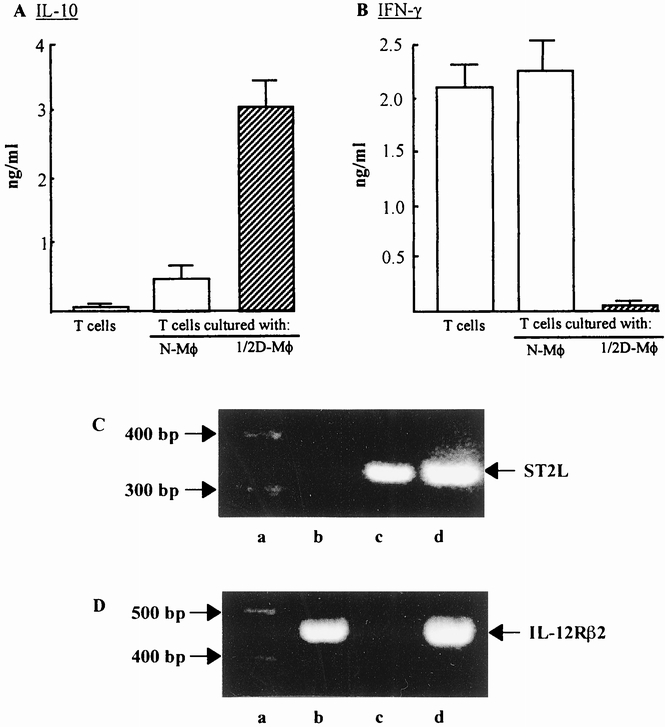
Figure 6. Type 2 T cells generated from normal T cells cultured with 1/2D-Mφ. Normal T cells (bottom chamber, 3 × 105 cells/well) were cultured with 1/2D-Mφ (upper chamber, 6 × 105 cells/well) in a dual-chamber transwell. Twenty-four hours after cultivation, the upper chamber was removed. Cells in the bottom chamber were recultured for an additional 7 days in fresh complete media supplemented with interleukin (IL)-2 (100 U/mL). The production of IL-10 (A) and interferon (IFN)-γ (B) by these cells is shown. For the cytokine production, cells harvested were stimulated with immobilized anti-CD3 monoclonal antibody (10 μg/mL) for 48 hours. The amount of cytokines in culture fluids was measured by enzyme-linked immunosorbent assay. Each bar indicates the mean ± standard deviation (n = 4–6). For the detection of ST2L and IL-12Rβ2 mRNAs, cells cultured with 1/2D-Mφ and recultured an additional 7 days were further stimulated by immobilized anti-CD3 monoclonal antibody for 12 hours. The expressions of ST2L mRNA (C) and IL-12Rβ2 mRNA (D) on these T cells were analyzed by reverse transcriptase-nested polymerase chain reaction (ST2L) or reverse transcriptase–polymerase chain reaction (IL-12Rβ2). As controls, ST2 cells (C), ST1 cells (D), and normal T cells cultured with normal macrophages were stimulated with the same monoclonal antibody. Lanes a, b, c, and d show the polymerase chain reaction marker, normal T cells, 1/2D-Mφ and ST2 (C) or ST1 cells (D), respectively.
It was further confirmed that the recultured T cells derived from transwells cultured with 1/2D-Mφ were shown to be type 2 T cells by the expression of ST2L mRNA (see Figs. 6C, 6D). Thus, after the stimulation with immobilized anti-CD3 mAb, ST2L mRNA was extracted from T cells that were cultured with 1/2D-Mφ in transwells and then recultivated in fresh complete medium. As controls, mRNAs were extracted from standard type 1 (ST1 cells) and type 2 T cells (ST2 cells) 27,28 and subjected to RT-nested PCR (ST2L) or RT-PCR (IL-12Rβ2). These ST1 and ST2 cells exhibited typical cytokine-producing profiles for type 1 (ST1 cells) and type 2 T cells (ST2 cells) when they were stimulated with anti-CD3 mAb. When T cells, cultured with 1/2D-Mφ in the bottom chamber of the transwells followed by the recultivation for an additional 7 days in fresh complete medium, were stimulated with immobilized anti-CD3 mAb, the mRNA expression for ST2L antigen was detected. However, these cells stimulated with anti-CD3 mAb did not express IL-12Rβ2 mRNA. At this time, ST2 cells expressed ST2L mRNA and ST1 cells expressed IL-12Rβ2 mRNA. These results indicated that type 2 T cells were generated from normal T cells through the cultivation with 1/2D-Mφ followed by the recultivation in fresh complete medium. In other words, the generation of type 2 T cells was initiated in cultures of normal T cells by the stimulation with 1/2D-Mφ (or MCP-1 released from 1/2D-Mφ).
DISCUSSION
In the present study we found the production of MCP-1 in sera of mice early after thermal injury. MCP-1 was detected in sera of mice 2 to 24 hours after thermal injury. Without any stimulation, splenic macrophages, peritoneal macrophages, peripheral neutrophils, lung fibroblasts, and kidney fibroblasts, derived from mice 12 hours after thermal injury, produced MCP-1 into their culture fluids. Among these cells, splenic macrophages from mice 12 hours after thermal injury were the best producer cells for MCP-1. Therefore, splenic macrophages from mice 12 hours after thermal injury were designated as 1/2D-Mφ and used for the experiments thereafter. IL-4 was detected in culture fluids of splenic T cells from normal mice cultured with 1/2D-Mφ in the dual transwell culture system; however, this cytokine was not produced by normal T cells cultured with splenic macrophages from normal mice in the same transwells. IL-4 was not produced into the culture fluids when anti-MCP-1 mAb was added to transwells cultured with normal T cells and 1/2D-Mφ. These results indicate that the IL-4 production by normal T cells is stimulated by MCP-1, which is released from 1/2D-Mφ. Further confirming this conclusion is the fact that IL-4-producing T cells were produced when normal T cells were cultured with rMCP-1, without any cells derived from mice early after thermal injury. Type 2 T cells, identified by their cytokine-producing profile and the mRNA expression for ST2L, were obtained when normal T cells cultured with 1/2D-Mφ in the transwells were recultured in fresh complete medium supplemented with IL-2 for an additional 7 days. Also, normal T cells, which were stimulated with 30 ng/mL rMCP-1 for 24 hours and recultured in fresh complete medium with IL-2 for an additional 7 days, produced IL-4 and IL-10 into their culture fluids and expressed ST2L mRNA (data not shown). These results indicate that MCP-1 initiates the generation of type 2 T cells and is released from macrophages, fibroblasts, and neutrophils, all of which appear early after thermal injury. Burn-associated type 2 T-cell responses may be subsequently established through the function of these type 2 T cells.
Multiple pathways appear to be involved in the initiation of the type 2 T-cell responses. 29–31 IL-4, IL-10, and IL-13 have all been found to be initiators of type 2 T-cell generation. 32 Modulation of the signaling chain of the IL-12 receptor (IL-12Rβ2) also results in the generation of type 2 T cells. 31 More recently, Gu et al. 22 reported that mice lacking MCP-1 have impaired Th2 responses. Lymph node cells from immunized MCP-1-/- mice synthesized extremely low levels of IL-4, IL-5, and IL-10, but normal amounts of IFN-γ and IL-2. 22 Also, MCP-1-/- mice did not accomplish the immunoglobulin subclass switch that is characteristic of Th2 responses and were resistant to Leishmania major. 22
High levels of corticosteroids and prostaglandin E2 are routinely observed in sera of patients immediately after thermal injury. 33,34 These substances are considered to be stimulators for the type 2 T-cell generation. Exposure of Con A-activated CD4+ T cells to dexamethasone or prostaglandin E2 increases mRNA levels for IL-4, IL-10, and IL-13. 35,36 Also, type 2 T-cell responses are enhanced by these substances through the inhibition of IL-12 production. 37 However, the direct role of corticosteroids and prostaglandin E2 on the generation of burn-associated type 2 T cells remains unclear. In the present paper, MCP-1 was found in sera of mice early after thermal injury. Also, type 2 T cells were established from splenic T cells from normal mice when they were cultured with 1/2D-Mφ and subjected to recultivation for an additional 7 days. In addition, the levels of IL-4 in sera of thermally injured mice were attenuated when they were treated with anti-MCP-1 mAb. In consideration of the study reported by Gu et al., 22 our data indicate that MCP-1 found early after thermal injury initiates the generation of IL-4-producing T cells.
Systemic inflammatory response syndrome (SIRS) and compensatory antiinflammatory response syndrome (CARS) have been described in patients with surgical trauma and severe burn injuries. 38–40 Inflammatory responses increase systemically early after injuries (SIRS). After the appearance of SIRS, however, immune responses are severely suppressed (CARS). 39 In the first stage of SIRS, proinflammatory cytokines (TNF-α, IL-1) are produced in response to the insult. 39,40 If the original insult is severe, proinflammatory mediators appear in the systemic circulation. Then, antiinflammatory cytokines quickly downregulate the initial inflammation. If mechanisms to regulate the inflammatory response are not optimized, a massive systemic inflammatory reaction causes early multiple organ failure. 39,40 In the stage of CARS, immune suppression in these patients may ultimately occur if large amounts of antiinflammatory mediators (eg, IL-4, IL-10, steroids) are produced. 39 This indicates that CARS may be strongly developed in thermally injured patients when burn-associated type 2 T-cell responses are established. Therefore, the susceptibility of thermally injured patients to certain infections may be strongly influenced by CARS. In our study, MCP-1, able to initiate the development of burn-associated type 2 T-cell responses, was detected in sera of burned mice. MCP-1 may play an important role in the development of CARS in thermally injured patients. Further studies are required to explore the relationship between the production of MCP-1 and the development of SIRS and CARS associated with thermal injury.
Footnotes
Supported by National Institutes of Health Grant R01 AI44218-01A2 and Shriners of North America Grant #8560.
Correspondence: Fujio Suzuki, MD, PhD, Department of Internal Medicine, The University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-0435.
E-mail: fsuzuki@utmb.edu
Accepted for publication August 27, 2001.
References
- 1.Mandell LA. Infections in the compromised host. J Int Med Res 1990; 18: 177–190. [DOI] [PubMed] [Google Scholar]
- 2.Klein DG, Fritsch DE, Amin SG. Wound infection following trauma and burn injuries. Critical Care Nursing Clin North Am 1995; 7: 627–642. [PubMed] [Google Scholar]
- 3.Kagan RJ, Naraqi S, Matsuda T, et al. Herpes simplex virus and cytomegalovirus infections in burned patients. J Trauma 1985; 25: 40–45. [DOI] [PubMed] [Google Scholar]
- 4.Pensler JM, Herndon DN, Ptak H, et al. Fungal sepsis: an increasing problem in major thermal injuries. J Burn Care Rehab 1986; 7: 488–491. [PubMed] [Google Scholar]
- 5.Byers RJ, Hasleton PS, Quigley A, et al. Pulmonary herpes in burns patients. Eur Respir J 1996; 9: 2313–2317. [DOI] [PubMed] [Google Scholar]
- 6.Odds FC. Ecology and epidemiology of Candida. In: Candida and candidosis. Baltimore: University Park Press, 1979: 50–74.
- 7.Rubin RH. Fungal and bacterial infections in immunocompromised host. Eur J Clin Microbiol Infect Dis 1993; 12: S42–S48. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 1995; 222: 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker D, Schondorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119: 316–325. [DOI] [PubMed] [Google Scholar]
- 10.Romagnani S. Understanding the role of Th1/Th2 cells in infection. Trends Microbiol 1996; 4: 470–473. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7: 145–173. [DOI] [PubMed] [Google Scholar]
- 12.Scott P, Kaufmann SHE. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today 1991; 12: 346–348. [DOI] [PubMed] [Google Scholar]
- 13.Del Prete G, Romagnani S. The role of Th1 and Th2 subsets in human infectious diseases. Trends Microbiol 1994; 2: 4–6. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki F, Pollard RB. Mechanism for suppression of γ-interferon responsiveness in mice after thermal injury. J Immunol 1982; 129: 1811–1815. [PubMed] [Google Scholar]
- 15.Kobayashi M, Herndon DN, Pollard RB, Suzuki F. CD4+ contrasuppressor T cells improve the resistance of thermally injured mice infected with HSV. J Leukocyte Biol 1995; 58: 159–167. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo R, Kobayashi M, Herndon DN, et al. Interleukin-12 protects thermally injured mice from herpes simplex virus type 1 infection. J Leukocyte Biol 1996; 59: 623–630. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Mori K, Kobayashi H, et al. The regulation of burn-associated infection with herpes simplex virus type 1 or Candida albicans by a non-toxic aconitine-hydrolysate, benzoylmesaconine: part 1. Antiviral and antifungal activities in thermally injured mice. Immunol Cell Biol 1998; 76: 202–208. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Kobayashi H, Herndon DN, et al. Burn-associated Candida albicans infection caused by CD30+ type 2 T cells. J Leukocyte Biol 1998; 63: 723–731. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med 1998; 338: 436–445. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol 1999; 65: 6–15. [DOI] [PubMed] [Google Scholar]
- 21.Karpus WJ, Lukacs NW, Kennedy KJ, et al. Differential CC-chemokine-induced enhancement of T helper cell cytokine production. J Immunol 1997; 158: 4129–4136. [PubMed] [Google Scholar]
- 22.Gu L, Tseng S, Horner RM, et al. Control of Th2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000; 404: 407–411. [DOI] [PubMed] [Google Scholar]
- 23.Takagi K, Suzuki F, Barrow RE, et al. Growth hormone improves immune function and survival in burned mice infected with herpes simplex virus type 1. J Surg Res 1997; 69: 166–170. [DOI] [PubMed] [Google Scholar]
- 24.Szabo SJ, Dighe AS, Gubler U, et al. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 1997; 185: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Chan WL, Leung BP, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med 1998; 188: 1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi H, Kobayashi M, Utsunomiya T, et al. Therapeutic protective effect of IL-12 combined with soluble IL-4 receptor against established infections of herpes simplex virus type 1 in thermally injured mice. J Immunol 1999; 162: 7148–7154. [PubMed] [Google Scholar]
- 27.Romani L, Mencacci A, Tonnetti L, et al. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol 1997; 158: 2356–2362. [PubMed] [Google Scholar]
- 28.Pechhold K, Patterson NB, Craighead N, et al. Inflammatory cytokines IFN-γ plus TNF-α induce regulated expression of CD80 (B7–1) but not CD86 (B7–2) on murine fibroblasts. J Immunol 1997; 158: 4921–4929. [PubMed] [Google Scholar]
- 29.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998; 8: 275–283. [DOI] [PubMed] [Google Scholar]
- 30.Szabo SJ, Jacobson NG, Dighe AS, et al. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity 1995; 2: 665–675. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan MH, Grusby MJ. Regulation of T helper cell differentiation by STAT molecules. J Leukoc Biol 1998; 64: 2–5. [DOI] [PubMed] [Google Scholar]
- 32.Kopf M, Le Gros G, Bachmann M, et al. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 1993; 362: 245–248. [DOI] [PubMed] [Google Scholar]
- 33.Calvano SE, Chiao J, Reaves LE, et al. Changes in free and total levels of plasma cortisol and thyroxine following thermal injury in man. J Burn Care Rehab 1984; 5: 143–151. [Google Scholar]
- 34.Grbic JT, Mannick JA, Gough DB, et al. The role of prostaglandin E2 in immune suppression following injury. Ann Surg 1991; 214: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez F, Fowell DJ, Puklavec M, et al. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol 1996; 156: 2406–2412. [PubMed] [Google Scholar]
- 36.Demeure CE, Yang LP, Desjardins C, et al. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol 1997; 27: 3526–3531. [DOI] [PubMed] [Google Scholar]
- 37.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 1998; 160: 2231–2237. [PubMed] [Google Scholar]
- 38.Wu CY, Wang K, McDyer JF, et al. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol 1998; 161: 2723–2730. [PubMed] [Google Scholar]
- 39.Bone RC. Sir Isaac Newton, sepsis, SIRS, CARS. Crit Care Med 1996; 24: 1125–1128. [DOI] [PubMed] [Google Scholar]
- 40.Fry DE. Sepsis syndrome. Am Surgeon 2000; 66: 126–132. [PubMed] [Google Scholar]


