Abstract
Objective
To review the current status of pancreatoduodenectomy for pancreatic cancer and chronic pancreatitis using evidence-based methodology.
Summary Background Data
Despite improved results of pancreatoduodenectomy over the recent years, the reputation of the Whipple procedure and its main modifications has remained poor. In addition, the current status of newer modifications of standard pancreatoduodenectomy is still under debate.
Methods
Medline search and manual cross-referencing were performed to identify all relevant articles for classification and analysis according to their quality of evidence. The search was limited to articles published between 1990 and 2001.
Results
The mortality rate of pancreatoduodenectomy has declined to less than 5% for chronic pancreatitis and 3% to 8% for pancreatic cancer. In contrast, overall morbidity rates remain high, ranging between 20% and 70%. Delayed gastric emptying represents almost half of all complications. The overall 5-year survival rate for patients with pancreatic cancer remains poor, ranging between 5% and 15%, with a median survival of 13 to 17 months. Mortality and morbidity are not related to the type of pancreatoduodenectomy; however, patients with pancreatic cancer tend to be at increased risk for complications. Extended lymph node dissection and portal vein resection can be performed with similar mortality and morbidity rates as standard procedures, but without apparent survival benefits in the long term. Major relief of pain is achieved in 70% to 100% of patients with chronic pancreatitis.
Conclusions
Pancreatoduodenectomy and its main modifications are safe and effective treatment modalities, especially in experienced centers with a high patient volume. For chronic pancreatitis, surgical resection provides major relief of pain and thus increased quality of life. Overall survival for patients with pancreatic cancer is determined predominantly by the pathology within the resected specimen.
The first successful pancreatoduodenectomies (PDs) were performed by Walter Kausch 1 in 1912 and Allan Whipple 2 in 1934. For many decades the procedure was associated with high morbidity and mortality rates, and thus the reputation of PD for benign and malignant pancreatic disorders was low among the medical community. 3,4 Surgery produced only minimal survival benefits compared to the natural history of pancreatic cancer, as almost all patients died within a short period. As a result, a quite nihilistic approach was used toward pancreatic cancer. Over the past two decades different medical and interventional therapies have replaced surgery as the first treatment option for chronic pancreatitis. However, the results of PD have gradually improved since the mid-1980s due to better understanding of pancreatic diseases, improvements in surgical techniques and perioperative care, and the appearance of high-volume centers with a large patient load (“centers of excellence”). While the mortality has dramatically decreased to well below 5%, morbidity rates have remained at 30% to 50%. 5
The further technical developments of the standard Whipple operation during recent years can be divided into two main types of modifications. In pancreatic cancer, it has been assumed that a larger resection will prolong survival (e.g., resection of the portal vein and extended lymphadenectomy). On the other hand, preserving anatomic and functional structures was thought to result in decreased postoperative morbidity and a better quality of life. Pylorus-preserving PD and duodenum-preserving pancreatic head resection (Beger and Frey procedures) are now widely used modifications of the Whipple procedure. 6–8 However, the latter procedures can be performed only in patients with chronic pancreatitis, and their use is mainly restricted to the German-speaking parts of Europe.
The increasing need to base clinical decisions on the available scientific evidence has been supported by the emphasis on cost containment, the increased interest in outcomes research, and also patients’ interest in the best available treatment. In addition, the development of evidence-based medicine has been facilitated by the availability of electronic databases, which allow systematic reviews of the clinical and scientific evidence in the published literature. Beyond the simple analysis of the results of different surgical treatment modalities, evidence-based medicine also allows us to assess the current quality of clinical surgical research.
The main goal of this study was to review the current results of PD and its main technical modifications for pancreatic cancer and chronic pancreatitis in order to highlight the advances of surgical treatments for pancreatic diseases. In addition, some basic considerations concerning the quality of surgical research should be discussed. To this end, the literature of the past decade has been analyzed according to its level of evidence, with a focus on peri- and postoperative morbidity, mortality, and survival rates.
METHODS
An electronic search of Medline was undertaken; the terms “pancreaticoduodenectomy,” “pancreatoduodenectomy,” “chronic pancreatitis,” “pancreatic cancer,” and “pancreas resection” were used in various combinations. The search terms were identified in the title, abstract, or medical subject heading (MeSH). There was no restriction concerning study design, but reviews and meta-analyses were excluded from final data analysis. With few exceptions, only original articles published in the English language between 1990 and December 2001 were selected for further analysis. Manual cross-referencing was also performed to find further relevant articles. All articles were classified according to their quality of evidence. The grading system proposed by the U.S. Preventive Services Task Force was used for classification (Table 1). 9 Whenever possible, only the best-ranked studies were used for the final data analysis. Although the year of publication was used as an inclusion criterion, the published case series mostly reflect the time period and its technical standard before the publication date.
Table 1. QUALITY OF EVIDENCE
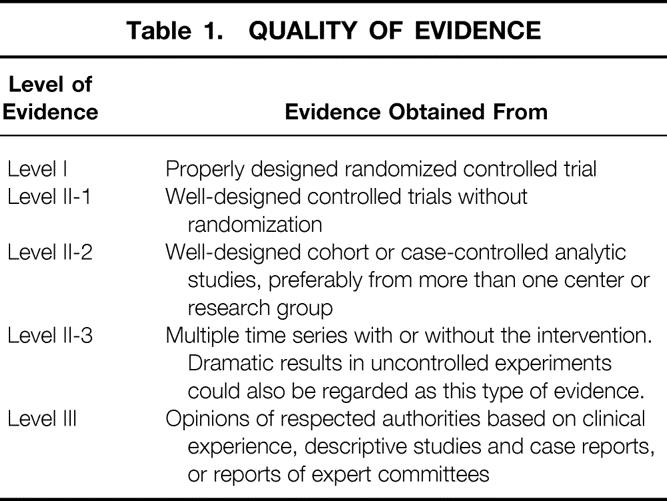
Although evidence-based medicine possibly provides the best methodology, some significant shortcomings remain. Conclusions must often be drawn from lower-ranked studies due to the lack of available level I studies. In addition, study designs in several randomized controlled trials (level I) were questionable; for example, no mention is made regarding sample size calculations and randomization procedures. Moreover, there is no consensus about the definition and severity of complications and the way they are reported. 10 Some authors only list the number of complications, whereas others report the number of patients with complications. Thus, the overall heterogeneity of the available studies severely hampers conclusive comparisons, and therefore interpretation and literature-based statements must be done with caution.
RESULTS
Indications for Pancreatoduodenectomy
The main goals of surgery for chronic pancreatitis are the relief of intractable abdominal pain and decompression of adjacent organs, most commonly the duodenum and common bile duct. Removal of a mass lesion in the pancreatic head, compression of the portal vein, pancreatic duct obstruction, and failure of previous surgery are other accepted indications for surgery. As a principle, all surgical procedures for chronic pancreatitis should preserve as much endocrine and exocrine function as possible to decrease long-term morbidity. The operative procedures fall into two main categories: drainage of a dilated pancreatic duct system and resection of the pancreas. The latter type of operative procedure includes the standard Whipple-Kausch PD, pylorus-preserving PD, duodenum-preserving resection of the pancreatic head (Beger and Frey procedures), and distal and total pancreatectomy. The main goal of this analysis was to compare standard PD, pylorus-preserving PD, and the various forms of duodenum-preserving pancreatic head resection, whereas distal and total pancreatectomy was not evaluated. 6 Although duodenum-preserving pancreatic head resection does not remove the duodenum, and thus might not be considered a true PD, it represents one of the most important technical advances in the surgical armamentarium for chronic pancreatitis.
The incidence of pancreatic cancer ranges between 0.2 and 13.7/100,000. 11 Due to its aggressive tumor growth and high recurrence rate, long-term survival is rare. Ductal adenocarcinoma is by far the most frequent tumor of the pancreas, with a predominant localization within the pancreatic head (78%). Since any other oncologic therapy failed to improve the dismal natural history of this condition, surgical resection has remained the only potentially curative treatment for pancreatic cancer. However, a PD should be performed only in the absence of metastatic disease (e.g., liver metastasis, peritoneal carcinomatosis) and feasible local resectability (e.g., in the absence of encasement of the superior mesenteric vessels). The more conservative pylorus-preserving PD has recently challenged the standard Whipple procedure, and more radical PDs combined with portal vein resection and/or extended lymph node dissection have not reached consensus.
Is the Mortality Rate Dependent on the Type of Surgery and the Underlying Pancreatic Disease?
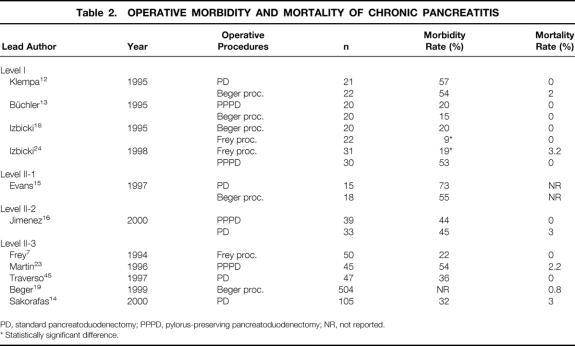
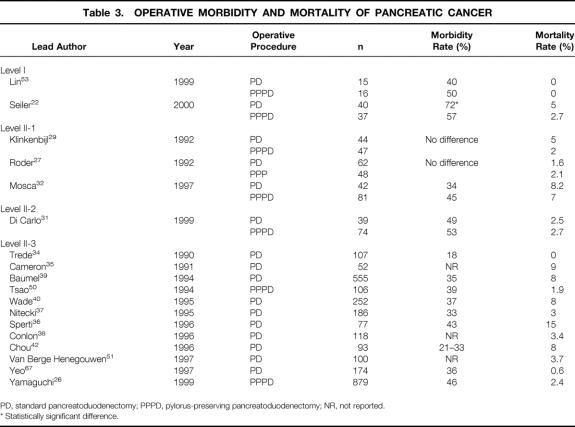
The dramatic decline in mortality after PD represents the most impressive advance of pancreatic surgery during the past two decades. 7–42 While the mortality rates of patients undergoing PD initially exceeded 30%, there are now several single- and multi-institutional series revealing a mortality rate ranging between 0% and 8% (Tables 2 and 3). 4,5,43,44 The definition of mortality usually includes operative and postoperative deaths within 30 days of surgery or before hospital discharge.
Table 2. OPERATIVE MORBIDITY AND MORTALITY OF CHRONIC PANCREATITIS
PD, standard pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; NR, not reported.
* Statistically significant difference.
Table 3. OPERATIVE MORBIDITY AND MORTALITY OF PANCREATIC CANCER
PD, standard pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; NR, not reported.
* Statistically significant difference.
Surgical treatment of chronic pancreatitis using a resectional procedure is associated with a very low mortality of less than 3%. 12–16,18,19,24,45 There is no apparent difference in mortality rates among the three analyzed types of surgical procedures. Despite some recent series from specialized centers reporting mortality rates below 3% (sometimes called “near-zero” mortality), there is strong evidence that current mortality rates after standard and pylorus-preserving PD for pancreatic cancer in many surgical institutions remain significant, ranging between 3% and 15%. 17,27,32,36–39,42,43 The causes of death after PD for pancreatic cancer are not fully elucidated. Possibly, patients with pancreatic cancer carry an increased operative risk due to their malignant disease, advanced age, and comorbidities. Furthermore, pancreatic tissue is soft in pancreatic cancer; thus, pancreaticojejunal anastomosis is more difficult to perform, resulting in a higher incidence of pancreatic fistula. The low number of procedures performed each year in many institutions and the lack of experience may increase mortality rates. The reported major causes of mortality after PD are intraabdominal bleeding, sepsis related to pancreaticojejunal anastomotic leakage, and cardiopulmonary failure. However, a number of studies do not report this information in detail, and often confirmation by autopsy is lacking.
As suggested by many, the most important factor affecting mortality is the emergence of specialized centers focusing on pancreatic surgery. 37 In such centers, not only can a restricted number of surgeons develop high technical skills, but also multidisciplinary experienced teams enable better selection of patients and postoperative care. Several recently published series have shown a distinct association between high patient volume and decreased mortality rates. 38,46–50
Is the Morbidity Rate Dependent on the Type of Surgical Procedure?
Complication rates for standard and pylorus-preserving Whipple procedures still appear high, ranging between 30% and 55% (see Tables 2 and 3). In contrast, duodenum-preserving PDs, particularly the Frey procedure, are associated with significantly lower morbidity rates, ranging between 9% and 22%. Due to improved perioperative intensive care, myocardial, pulmonary, and thromboembolic complications have dramatically decreased. The current analysis predominantly focused on delayed gastric emptying, pancreatic anastomotic leakage, and abdominal hemorrhage (Table 4).
Table 4. COMPLICATION RATES AFTER PD FOR CHRONIC PANCREATITIS AND PANCREATIC CANCER
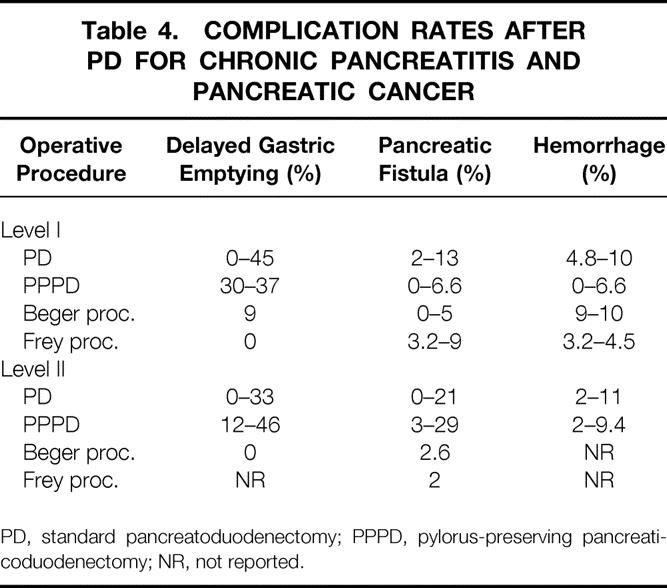
PD, standard pancreatoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy; NR, not reported.
Delayed Gastric Emptying
Delayed gastric emptying (DGE) caused by a prolonged gastroparesis during the first 2 to 4 postoperative weeks accounts for almost half of the overall morbidity after the Whipple procedure, with an incidence ranging between 8% and 45% (see Table 4). 12,14,16,17,24,27,31,32,51–55 It has been suggested that pylorus-preserving PD is associated with a significantly increased risk of DGE compared to the standard PD. There are seven studies (levels I and II) comparing PD and the pylorus-preserving PD. While two studies showed no difference, three favored pylorus-preserving PD, and two showed lower DGE rates after PD compared to pylorus-preserving PD. 16,27,31,32,52,53,56 Two of the three studies (levels I and II) comparing the PD and the Beger procedure revealed a decreased rate of DGE after the duodenum-preserving procedure, 24,52 and one study found no difference. 12 Therefore, the PD has no clear advantage concerning DGE compared to the pylorus-preserving procedure. For chronic pancreatitis with a predominant enlargement of the pancreatic head, duodenum-preserving procedures probably offer significant advantages.
The pathophysiology of DGE has not yet been fully elucidated, but several factors might be involved. Intraabdominal infections, resection of the duodenum with interruption of gastrointestinal neural connections, loss of gastrointestinal hormone production, and local ischemia are some of the reported cofactors in the literature. Moreover, a DGE may herald an otherwise undetected pancreaticoenteric or bilioenteric anastomotic leak.
Pancreatic Leakage
Drainage of the pancreatic remnant to the intestine remains a crucial step after PD. Although pancreaticogastrostomy has been occasionally reported, intestinal drainage of the pancreatic remnant is the standard procedure. The current analysis has been limited to pancreatojejunostomy.
The terms to describe anastomotic leakage at the pancreatojejunostomy vary widely; most of the proposed definitions include the volume of drain output (e.g., >50 mL/d) and increased amylase concentrations in the drained fluid (e.g., >1,00 units/L or three times the upper limit of normal serum values). Some authors additionally use the persistence of postoperative amylase-rich drain fluid production (e.g., >3 days) or radiologic findings of intraabdominal fluid collections. The reported incidence of pancreatic anastomotic leaks varies from 0% to 30%. 12–15,18,24 The large range can be explained by different definitions and reporting of pancreatic leakage, differences in the underlying pancreatic disease (soft vs. fibrotic pancreatic tissue), individual surgical experience, and different surgical techniques. A pancreatic anastomotic leak may clinically be overt as a pancreatic-cutaneous fistula, intraabdominal abscess in association with fever, sepsis, increased leukocytes, and C-reactive protein levels, or only as DGE. Additionally, not every pancreatic anastomotic leak produces clinical symptoms. Even an obvious pancreatic-cutaneous fistula can occur without causing clinical symptoms. The associated mortality of pancreatic leaks has markedly decreased during the past two decades, now ranging between 0% and 5%. Most intraabdominal abscess formation and smaller anastomotic leakage can be detected at an earlier stage by newer and aggressive imaging modalities followed by percutaneous drainage. 57
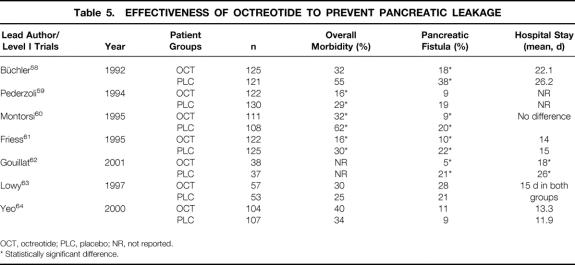
There is an ongoing debate on the prophylactic use of octreotide to decrease the incidence of pancreatic anastomotic leaks after pancreatic surgery 58–64 (Table 5). The synthetic somatostatin analogue octreotide is a powerful inhibitor of gastrointestinal secretion, including pancreatic exocrine secretion. To prevent pancreatic leakage after pancreatic surgery, three daily doses of 100 to 250 μg octreotide are given as subcutaneous injections usually for the first postoperative week, with the first dose given before or during surgery. Continuous intravenous or only postoperative applications are less frequently used. 62,63 Four level I multicenter studies from European centers including patients with pancreatic cancer and chronic pancreatitis treated by different surgical procedures used a protocol with the first dose given preoperatively, followed by three daily doses of 100 μg for 7 days. 58–61 Patients from a recently published French multicenter study who underwent PD for pancreatic malignancy only received a continuous postoperative octreotide infusion for 1 week. 59–62 In contrast, two single-center North American studies (level I) in patients with pancreatic cancer used daily doses of 150 or 250 μg given for 5 or 7 days. In the study reported by Lowy et al, octreotide was started only postoperatively. 63 The series reported by Yeo et al represents the experience of a high-volume center with low pancreatic leak rates. 64
Table 5. EFFECTIVENESS OF OCTREOTIDE TO PREVENT PANCREATIC LEAKAGE
OCT, octreotide; PLC, placebo; NR, not reported.
* Statistically significant difference.
Each European study showed a 40% to 50% decrease in overall morbidity rates, including reduction in pancreatic leak rates, whereas both North American studies failed to demonstrate any benefits with octreotide. In agreement with Li-Ling and Irving, who also reviewed this topic recently, octreotide appears to decrease significantly the overall morbidity and pancreatic anastomotic leak rates. 65 To be effective, the first dose must be given 1 to 2 hours preoperatively followed by 3 doses of 100 μg for 5 to 7 days. High-risk anastomoses (soft pancreas, small pancreatic duct) may be better protected, and the benefit is likely to be more apparent in surgical units with higher leak rates. However, further well-designed studies with more patients are still needed to establish the impact of octreotide, particularly in terms of the timing and duration of application.
Hemorrhage
The term “hemorrhage” covers intraperitoneal and gastrointestinal bleeding complications. Intraperitoneal bleeding occurring during surgery is related to the laceration of a vessel and is rarely reported; only data regarding postoperative hemorrhage are usually reported. Such bleeding originates either from an anastomotic source or from an oozing ulcer, which is symptomatic within the first postoperative week. Gastric stress ulceration and anastomotic bleeding caused by local ischemia have been reduced by the use of potent acid secretory inhibition and improved anastomotic techniques (suture materials, better understanding of vascular anatomy). The current reported incidence of bleeding complications varies from 1% to 10%. 12–14,18,23,24,26,27,29,32,34,53–55 A number of series from high-volume centers report a very low bleeding rate (<3%). 13,14,27,53 The incidence of bleeding complications appears to be related to the type of resection. Although the small sample size of level I trials might prevent conclusive statistics, the duodenum-preserving procedures (Beger and Frey) tend to be associated with a slightly increased rate of gastrointestinal hemorrhage, ranging from 5% to 10%. It has been hypothesized that the side-to-side pancreatojejunostomy of the pancreatic head is associated with more bleeding complications. In these cases, the bleeding usually originates from the pancreatic head remnant.
What are the Current Survival Rates for Pancreatic Cancer?
Important methodologic issues hamper the analysis and interpretation of survival data after PD for pancreatic cancer. First, statistical description of survival is often performed as Kaplan-Meier estimates, which represent only a calculated survival time based on the proportion of patients who underwent PD and died during a limited length of follow-up. The mortality rate per time increments is then used to extrapolate the actuarial 5-year survival. In addition, some authors failed to include operative deaths in calculating the actuarial survival. In contrast, the actual survival reflects the real number of patients alive at a certain time point, such as 5 years postoperatively. Second, the accuracy of pathologic diagnosis is of utmost importance regarding interpretation of long-term survival. Since it is known that periampullary, pancreatic, and distal bile duct carcinomas reveal a broad variety of survival, precise histologic diagnosis is mandatory to achieve conclusive tumor-related survival data. Furthermore, due to the relatively low incidence of pancreatic adenocarcinoma, a few misdiagnosed specimens can lead to inaccurate survival rates. Third, extended surgical resection, including portal vein resection and extended lymph node dissection, allows better staging; this creates virtually improved survival rates in some subgroups of patients, while the overall survival remains unchanged. This virtual benefit is known as “stage migration phenomenon” and has been well described in other malignant disorders, such as gastric cancer. 66
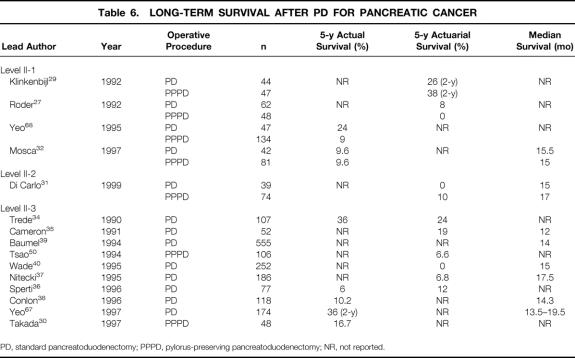
Data regarding survival are available only from nonrandomized or large case series. The median survival reported in the literature shows only a small variation and is ranging between 12 and 18 months for all patients 31,32,35,37–40,67 (Table 6). Both the 5-year actuarial and actual survival rates are poor and range from 7% to 12% and 6% to 10%, respectively. 27,31,32,36,50,68 The issue of whether the pylorus-preserving DP might impair the radicality of tumor resection is not supported by the available data. 29,32,68
Table 6. LONG-TERM SURVIVAL AFTER PD FOR PANCREATIC CANCER
PD, standard pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; NR, not reported.
Extended Lymph Node Dissection and Portal Vein Resection: What Are the Evidence-Based Facts?
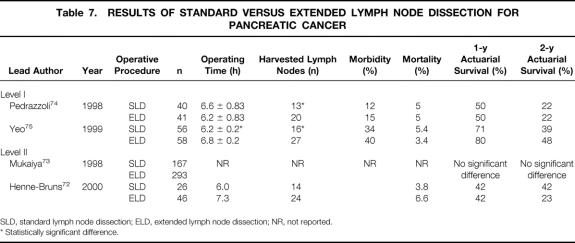
To improve long-term survival in patients with pancreatic cancer, more radical surgical procedures have been proposed. 69,70 Since it is known that perineural tumor invasion and lymph node metastasis occur even at early tumor stages, it was postulated that extending local resection margins might improved long-term survival. 71 Extended lymph node dissection and portal vein resection are the most common surgical procedures used in an attempt to increase survival. Regional pancreatectomy, as described by Fortner, which includes en bloc removal (subtotal or total pancreatectomy) of a pancreatic tumor with large amounts of surrounding soft tissue and resection of the superior mesenteric artery, is no longer used. 69 Extended lymph node dissection includes resection of bilateral paraaortal lymphatic tissue from the diaphragm down to the inferior mesenteric artery and laterally to the hilum of the right kidney. Although the technique of extended lymph node dissection is not yet standardized, there are several recent studies (levels I and II) that allow us to draw some conclusions concerning extended lymph node dissection and its potential benefits (Table 7). 72–75
Table 7. RESULTS OF STANDARD VERSUS EXTENDED LYMPH NODE DISSECTION FOR PANCREATIC CANCER
SLD, standard lymph node dissection; ELD, extended lymph node dissection; NR, not reported.
* Statistically significant difference.
The two level I studies available reported a significantly increased number of removed lymph nodes by extensive retroperitoneal clearing (13 vs. 20, 16 vs. 27 lymph nodes, respectively). 74,75 However, there was only limited information concerning the pathologic workup of the removed specimens. Both studies revealed similar overall morbidity, although one study reported an increased rate of DGE after extended lymph node dissection. 75 A level II study performed by Henne-Bruns et al reported long-term diarrhea in 75% of patients after extended lymph node dissection. 72 This complication could be avoided in subsequent patients by excluding the nerve plexus along the left side of the superior mesenteric artery. 72,76 The operating time for extended lymph node dissection was slightly prolonged, but the differences remained within 20 to 30 minutes. The mortality rates range between 3.5% and 6.5%; thus, increased radicality of lymph node dissection does not seem to be associated with increased mortality rates. Survival rates, the primary endpoints, were not found to be prolonged in any study.
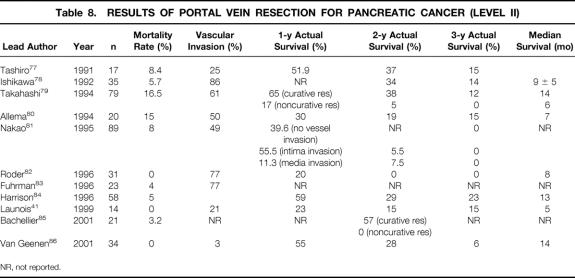
During the last decade, 10 level II studies have been published evaluating mortality rates and long-term survival after partial and segmental portal/mesenteric vein resection 41,77–86 (Table 8). The operative mortality is reported to range from 0% to 16%, but recent studies clearly revealed mortality rates below 5%, similar to those of standard PD. Histologically proven venous tumor infiltration varies widely, from 3% to 80%. 77–84,87 This underlines the difficulty of determining venous tumor invasion before and during surgery, since peritumoral inflammation may simulate true tumor adherence and infiltration. Extended resections including portal vein resection have not been associated with improved long-term survival. Median survival, 1-year actuarial survival rates, and 2-year actual survival rates are reported to range from 4 to 14 months, 20% to 60%, and 0% to 38%, respectively. 41,79–82,84,86 Noncurative resections and histologically confirmed tumor infiltration into the tunica media of the portal vein are consistently associated with limited long-term survival of less than 2 years. 79,81,85
Table 8. RESULTS OF PORTAL VEIN RESECTION FOR PANCREATIC CANCER (LEVEL II)
NR, not reported.
What is the Clinical Effectiveness of Pancreatoduodenectomy for Chronic Pancreatitis?
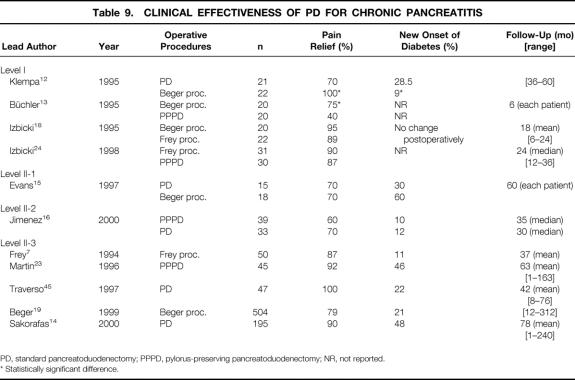
It has clearly been demonstrated that major relief of pain is achieved after standard PD, pylorus-preserving PD, and duodenum-preserving pancreatic head resection 7,8,12–16,18,19,23,24 (Table 9). Studies comparing the PD (standard and pylorus-preserving PD) to the Beger procedure indicate a superiority of the duodenum-preserving procedure. Long-term pain relief has been documented in several studies with a follow-up of over 5 years. Recurrence of pain is often related to recurrent alcohol consumption after surgery or incomplete resection of chronically inflamed pancreatic tissue. 15
Table 9. CLINICAL EFFECTIVENESS OF PD FOR CHRONIC PANCREATITIS
PD, standard pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; NR, not reported.
* Statistically significant difference.
There is an increasing incidence of diabetes mellitus during the natural course of chronic pancreatitis; this eventually requires insulin treatment to achieve normoglycemia. In a large series of alcohol-induced chronic pancreatitis reported by Ammann et al, 75% of all patients developed diabetes mellitus within a median of 6 years. 88–90 Although the Beger procedure preserves the duodenum (and gastric antrum), the endocrine function of the pancreas is not better preserved in the long-term follow-up compared to PD and the pylorus-preserving PD. New onset of diabetes mellitus is reported to occur in 10% to 48% of patients during the postoperative course; the observational time ranges from 3.5 to 7 years. 19 Major relief of pain leads to improved food intake, which may uncover previously undetected diabetes or progression of the disease.
Patients with long-standing chronic pancreatitis often reveal malnutrition caused by both the severe abdominal pain associated with food ingestion and the maldigestion related to the exocrine insufficiency. The incidence of exocrine insufficiency increases up to 60% to 80% of patients after pancreas resection. As a direct consequence, all these patients require oral enzyme substitution. During long-term follow-up (>6 months), most of the studies report a significant increase in body weight, ranging from 2 to 8 kg. 14,15,23 Two randomized studies showed better weight gain and lower rates of exocrine insufficiency after the duodenum-preserving Beger procedure when compared to the standard and pylorus-preserving PD. 12,13
DISCUSSION
For many years PD was characterized as a difficult, hazardous, and somewhat questionable procedure. However, during the past two decades the Whipple operation and its modifications have clearly evolved to become safe and effective procedures for several indications. The aim of this review was to analyze the current results of PD for pancreatic cancer and chronic pancreatitis. To this end, the literature published in the 1990s was analyzed according to the criteria of evidence-based medicine.
The emergence of evidence-based medicine has separated the medical community into enthusiastic advocates and doubting skeptics. Compared to meta-analyses, which generally are restricted to proper randomized trials, evidence-based medicine offers the benefit of a more critical review of all published data. Thus, evidence-based analysis represents a tool to gain deeper scientific insight to answer specific clinical questions and to critically assess current clinical practice. However, the lack of appropriate studies in many cases should call for caution in making definite statements; therefore, evidence-based analysis should not tyrannize daily clinical practice.
Classification systems of evidence-based medicine are mainly influenced by the type of study, favoring randomized and multicenter study designs. With such an approach, single opinions of experts have the lowest rank. However, other criteria are not taken into account, such as sample size calculation and experience with the procedures studied. The lack of a uniform tumor staging system and reporting of complications may further hamper the data analysis. Even well-designed randomized controlled protocols may reveal some weaknesses. As recently outlined by Black, there are four main limitations of randomized controlled trials:91
Experimentation may be unnecessary if the effect of an intervention represents a dramatic improvement of current standard treatments.
Randomization may be inappropriate by limited numbers of patients if complications are very rare and/or occur in the late postoperative course. In addition, results of randomized controlled trials may be negatively influenced by the learning curve for a new surgical procedure with which the participating surgeons are not familiar.
Randomization may be impossible if clinicians refuse to participate, and because of ethical and legal issues.
Randomization may be inadequate by the limited generalizability of randomized controlled trials.
Beyond these methodologic limitations, surgical research reveals a lack of randomized trials with a multicenter setting.
The operative mortality of PD has dramatically declined to 3% to 8% for pancreatic cancer and to less than 5% for chronic pancreatitis. In addition to advances in surgical techniques, numerous improvements in diagnostic and interventional radiology, intensive care, and management of complications have contributed to the currently low mortality of PD. In contrast, overall morbidity rates remain still high, ranging between 20% and 70%; thus, patients with pancreatic cancer tend to be at increased risk for complications. DGE, defined as prolonged gastroparesis for 10 to 30 days postoperatively, represents almost half of all complications. The incidence of DGE is not related to the underlying pancreatic disease. While it has been suggested that pylorus-preserving PD results in an increased incidence of DGE, current studies do not support this statement. Duodenum-preserving procedures, as promoted by Beger and Frey, have a significantly decreased incidence of DGE (10%). Despite varying definitions of pancreatic leakage and fistula, the incidence of clinically relevant pancreatic leakage currently ranges between 5% and 10%. The impact of octreotide has been analyzed in several randomized controlled trials. While most European level I studies found significant decreases in overall morbidity and pancreatic fistula rates, two well-quoted level I North American studies failed to demonstrate any benefits. Administration of the drug before surgery might be key for success.
Pancreatic cancer is still associated with poor long-term survival. Overall 5-year survival rates for all patient groups remain between 5% and 15%, with a median survival of 13 to 17 months. In experienced centers extended resectional procedures (e.g., extended lymph node dissection and portal vein resection) can be performed with similar mortality and morbidity rates as standard procedures. However, survival benefits still need to be demonstrated.
The classic Whipple procedure still represents the gold standard of PD. However, the term “gold standard” is somewhat misleading because there is no clear and uniformly accepted standard procedure. In particular, the type of reconstruction (e.g., pancreaticojejunostomy with or without stents, pancreatogastrostomy, optional construction of a Braun anastomosis) shows a considerable variety among different surgical institutions and countries. Current studies comparing the classic Whipple operation to pylorus-preserving procedures reveal no evident superiority of one procedure over the other. Neither the operating times, the complication rates, particularly the incidence of DGE, or long-term survival appears different. Therefore, the type of PD should be left to local preference. The duodenum-preserving pancreatic head resections for the treatment of chronic pancreatitis are newer modifications preserving the integrity of the upper gastrointestinal tract. Both procedures have not yet gained widespread acceptance, although current studies report favorable complication rates and excellent outcomes.
In case of chronic pancreatitis, partial resection of the chronically inflamed tissue leads to major pain relief, which is closely related to improved nutritional status and quality of life. If the inflammatory process is predominantly localized in the pancreatic head (approximately 35% of all patients), the duodenum-preserving pancreatic head resection provides the best approach. Nevertheless, the natural course of parenchyma destruction can only be delayed, and the incidence of diabetes mellitus and exocrine insufficiency continues to increase in the long term.
In conclusion, the PD and its main modifications are proven and established treatment modalities with a low mortality in pancreatic cancer and chronic pancreatitis. Although the morbidity remains high, most complications can be effectively treated in a multidisciplinary setting. The clinical effectiveness is excellent for chronic pancreatitis. Since any other treatment modalities failed, surgical resection remains the only curative chance for patients with pancreatic cancer. Future breakthroughs may be related more to innovative neoadjuvant or adjuvant strategies rather than surgical treatments.
Footnotes
Correspondence: Pierre-Alain Clavien, MD, PhD, FACS, Department of Visceral and Transplantation Surgery, University of Zürich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
E-mail: clavien@chir.unizh.ch
Presented in part at the 3rd Annual Meeting of SACRE, Hamburg, Germany, November 16–18, 2000, and the Davos postgraduate GI course, Davos, Switzerland, March 4–8, 2001.
Accepted for publication March 1, 2002.
References
- 1.Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr Klin Chir 1912; 78: 439–486. [Google Scholar]
- 2.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg 1935; 142: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro TM. Adenocarcinoma of the pancreas: a statistical analysis of biliary bypass vs Whipple resection in good risk patients. Ann Surg 1975; 182: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudjonsson B. Carcinoma of the pancreas: critical analysis of costs, results of resections, and the need for standardized reporting. J Am Coll Surg 1995; 181: 483–503. [PubMed] [Google Scholar]
- 5.Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology 1997; 113: 983–994. [DOI] [PubMed] [Google Scholar]
- 6.Beger HG, Krautzberger W, Bittner R, et al. Duodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis. Surgery 1985; 97: 467–473. [PubMed] [Google Scholar]
- 7.Frey CF, Amikura K. Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis. Ann Surg 1994; 220: 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978; 146: 959–962. [PubMed] [Google Scholar]
- 9.U.S. Preventive Service Task Force Staff. The guide to clinical preventive services: report of the United States Preventive Task Force. Philadelphia: Williams & Wilkins; 1996.
- 10.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992; 111: 518–526. [PubMed] [Google Scholar]
- 11.Parkin DM, Muir CS, Whelan SL, et al. Cancer incidence in five continents. Lyon: International Agency for Research on Cancer; 1992.
- 12.Klempa I, Spatny M, Menzel J, et al. Pancreatic function and quality of life after resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized comparative study after duodenum preserving resection of the head of the pancreas versus Whipple’s operation. Chirurgie 1995; 66: 350–359. [PubMed] [Google Scholar]
- 13.Büchler MW, Friess H, Muller MW, et al. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 1995; 169: 65–69. [DOI] [PubMed] [Google Scholar]
- 14.Sakorafas GH, Farnell MB, Nagorney DM, et al. Pancreatoduodenectomy for chronic pancreatitis: long-term results in 105 patients. Arch Surg 2000; 135: 517–523. [DOI] [PubMed] [Google Scholar]
- 15.Evans JD, Wilson PG, Carver C, et al. Outcome of surgery for chronic pancreatitis. Br J Surg 1997; 84: 624–629. [PubMed] [Google Scholar]
- 16.Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 2000; 231: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–299. [DOI] [PubMed] [Google Scholar]
- 18.Izbicki JR, Bloechle C, Knoefel WT, et al. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized trial. Ann Surg 1995; 221: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beger HG, Schlosser W, Friess HM, et al. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg 1999; 230: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddes EH, Masclee AM, Gooszen HG, et al. Effect of duodenum-preserving resection of the head of the pancreas on endocrine and exocrine pancreatic function in patients with chronic pancreatitis. Am J Surg 1997; 174: 387–392. [DOI] [PubMed] [Google Scholar]
- 21.Muller MW, Friess H, Beger HG, et al. Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg 1997; 173: 257–263. [DOI] [PubMed] [Google Scholar]
- 22.Seiler CA, Wagner M, Sadowski C, et al. Randomized prospective trial of pylorus-preserving vs. classic duodenopancreatectomy (Whipple procedure): initial clinical results. J Gastrointest Surg 2000; 4: 443–452. [DOI] [PubMed] [Google Scholar]
- 23.Martin RF, Rossi RL, Leslie KA. Long-term results of pylorus-preserving pancreatoduodenectomy for chronic pancreatitis. Arch Surg 1996; 131: 247–252. [DOI] [PubMed] [Google Scholar]
- 24.Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg 1998; 228: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zerbi A, Balzano G, Patuzzo R, et al. Comparison between pylorus-preserving and Whipple pancreatoduodenectomy. Br J Surg 1995; 82: 975–979. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Tanaka M, Chijiiwa K, et al. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg 1999; 6: 303–311. [DOI] [PubMed] [Google Scholar]
- 27.Roder JD, Stein HJ, Huttl W, et al. Pylorus-preserving versus standard pancreatico-duodenectomy: an analysis of 110 pancreatic and periampullary carcinomas. Br J Surg 1992; 79: 152–155. [DOI] [PubMed] [Google Scholar]
- 28.Kozuschek W, Reith HB, Waleczek H, et al. A comparison of long-term results of the standard Whipple procedure and the pylorus preserving pancreatoduodenectomy. J Am Coll Surg 1994; 178: 443–453. [PubMed] [Google Scholar]
- 29.Klinkenbijl JH, van der Schelling GP, Hop WC, et al. The advantages of pylorus-preserving pancreatoduodenectomy in malignant disease of the pancreas and periampullary region. Ann Surg 1992; 216: 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada T, Yasuda H, Amano H, et al. Results of a pylorus-preserving pancreatoduodenectomy for pancreatic cancer: a comparison with results of the Whipple procedure. Hepato-Gastroenterology 1997; 44: 1536–1540. [PubMed] [Google Scholar]
- 31.Di Carlo V, Zerbi A, Balzano G, et al. Pylorus-preserving pancreaticoduodenectomy versus conventional Whipple operation. World J Surg 1999; 23: 920–925. [DOI] [PubMed] [Google Scholar]
- 32.Mosca F, Giulianotti PC, Balestracci T, et al. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery 1997; 122: 553–566. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg 2000; 24: 66–70. [DOI] [PubMed] [Google Scholar]
- 34.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 1991; 161: 120–124. [DOI] [PubMed] [Google Scholar]
- 36.Sperti C, Pasquali C, Piccoli A, et al. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg 1996; 83: 625–631. [DOI] [PubMed] [Google Scholar]
- 37.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumel H, Huguier M, Manderscheid JC, et al. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg 1994; 81: 102–107. [DOI] [PubMed] [Google Scholar]
- 40.Wade TP, el Ghazzawy AG, Virgo KS, et al. The Whipple resection for cancer in U.S. Department of Veterans Affairs Hospitals. Ann Surg 1995; 221: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Launois B, Stasik C, Bardaxoglou E, et al. Who benefits from portal vein resection during pancreaticoduodenectomy for pancreatic cancer? World J Surg 1999; 23: 926–929. [DOI] [PubMed] [Google Scholar]
- 42.Chou FF, Sheen-Chen SM, Chen YS, et al. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur J Surg 1996; 162: 477–481. [PubMed] [Google Scholar]
- 43.Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg 1999; 23: 913–919. [DOI] [PubMed] [Google Scholar]
- 44.Rios G, Conrad A, Cole D, et al. Trends in indications and outcomes in the Whipple procedure over a 40-year period. Am Surg 1999; 65: 889–893. [PubMed] [Google Scholar]
- 45.Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg 1997; 226: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neoptolemos JP, Russell RC, Bramhall S, et al. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg 1997; 84: 1370–1376. [PubMed] [Google Scholar]
- 48.Lieberman MD, Kilburn H, Lindsey M, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995; 222: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg 1998; 228: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsao JI, Rossi RL, Lowell JA. Pylorus-preserving pancreatoduodenectomy. Is it an adequate cancer operation? Arch Surg 1994; 129: 405–412. [DOI] [PubMed] [Google Scholar]
- 51.Berge Henegouwen MI, Akkermans LM, van Gulik TM, et al. Prospective, randomized trial on the effect of cyclic versus continuous enteral nutrition on postoperative gastric function after pylorus-preserving pancreatoduodenectomy. Ann Surg 1997; 226: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Büchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg 2000; 87: 883–889. [DOI] [PubMed] [Google Scholar]
- 53.Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg 1999; 86: 603–607. [DOI] [PubMed] [Google Scholar]
- 54.Huang JJ, Yeo CJ, Sohn TA, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg 2000; 231: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg 1992; 127: 945–949. [DOI] [PubMed] [Google Scholar]
- 56.Berge Henegouwen MI, van Gulik TM, DeWit LT, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg 1997; 185: 373–379. [DOI] [PubMed] [Google Scholar]
- 57.Gervais DA, Fernandez-del Castillo C, O’Neill MJ, et al. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics 2001; 21: 673–690. [DOI] [PubMed] [Google Scholar]
- 58.Büchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 1992; 163: 125–130. [DOI] [PubMed] [Google Scholar]
- 59.Pederzoli P, Bassi C, Falconi M, et al. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Italian Study Group. Br J Surg 1994; 81: 265–269. [DOI] [PubMed] [Google Scholar]
- 60.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery 1995; 117: 26–31. [DOI] [PubMed] [Google Scholar]
- 61.Friess H, Beger HG, Sulkowski U, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg 1995; 82: 1270–1273. [DOI] [PubMed] [Google Scholar]
- 62.Gouillat C, Chipponi J, Baulieux J, et al. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br J Surg 2001; 88: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 63.Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997; 226: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000; 232: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fistulas: a systematic review of randomized controlled trials. Br J Surg 2001; 88: 190–199. [DOI] [PubMed] [Google Scholar]
- 66.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985; 312: 1604–1608. [DOI] [PubMed] [Google Scholar]
- 67.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortner JG, Mulcare RJ, Solis A, et al. Treatment of primary and secondary liver cancer by hepatic artery ligation and infusion chemotherapy. Ann Surg 1973; 178: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg 1984; 199: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birk D, Beger HG. Lymph-node dissection in pancreatic cancer—what are the facts? Langenbecks Arch Surg 1999; 384: 158–166. [DOI] [PubMed] [Google Scholar]
- 72.Henne-Bruns D, Vogel I, Luttges J, et al. Surgery for ductal adenocarcinoma of the pancreatic head: staging, complications, and survival after regional versus extended lymphadenectomy. World J Surg 2000; 24: 595–601. [DOI] [PubMed] [Google Scholar]
- 73.Mukaiya M, Hirata K, Satoh T, et al. Lack of survival benefit of extended lymph node dissection for ductal adenocarcinoma of the head of the pancreas: retrospective multi-institutional analysis in Japan. World J Surg 1998; 22: 248–252. [DOI] [PubMed] [Google Scholar]
- 74.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998; 228: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 1999; 229: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imaizumi T, Hanyu F, Harada N, et al. Extended radical Whipple resection for cancer of the pancreatic head: operative procedure and results. Dig Surg 1998; 15: 299–307. [DOI] [PubMed] [Google Scholar]
- 77.Tashiro S, Uchino R, Hiraoka T, et al. Surgical indication and significance of portal vein resection in biliary and pancreatic cancer. Surgery 1991; 109: 481–487. [PubMed] [Google Scholar]
- 78.Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg 1992; 215: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi S, Ogata Y, Tsuzuki T. Combined resection of the pancreas and portal vein for pancreatic cancer. Br J Surg 1994; 81: 1190–1193. [DOI] [PubMed] [Google Scholar]
- 80.Allema JH, Reinders ME, van Gulik TM, et al. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. Br J Surg 1994; 81: 1642–1646. [DOI] [PubMed] [Google Scholar]
- 81.Nakao A, Harada A, Nonami T, et al. Clinical significance of portal invasion by pancreatic head carcinoma. Surgery 1995; 117: 50–55. [DOI] [PubMed] [Google Scholar]
- 82.Roder JD, Stein HJ, Siewert JR. Carcinoma of the periampullary region: who benefits from portal vein resection? Am J Surg 1996; 171: 170–174. [DOI] [PubMed] [Google Scholar]
- 83.Fuhrman GM, Leach SD, Staley CA, et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg 1996; 223: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg 1996; 224: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bachellier P, Nakano H, Oussoultzoglou PD, et al. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg 2001; 182: 120–129. [DOI] [PubMed] [Google Scholar]
- 86.van Geenen RC, ten Kate FJ, de Wit LT, et al. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery 2001; 129: 158–163. [DOI] [PubMed] [Google Scholar]
- 87.Clavien PA, Rudiger HA. A simple technique of portal vein resection and reconstruction during pancreaticoduodenectomy. J Am Coll Surg 1999; 189: 629–634. [DOI] [PubMed] [Google Scholar]
- 88.Ammann RW, Akovbiantz A, Largiader F, et al. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology 1984; 86: 820–828. [PubMed] [Google Scholar]
- 89.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology 1996; 111: 224–231. [DOI] [PubMed] [Google Scholar]
- 90.Ammann RW, Muellhaupt B, Meyenberger C, et al. Alcoholic nonprogressive chronic pancreatitis: prospective long-term study of a large cohort with alcoholic acute pancreatitis (1976–1992). Pancreas 1994; 9: 365–373. [PubMed] [Google Scholar]
- 91.Black N. Evidence-based surgery: A passing fad? World J Surg 1999; 23: 789–793. [DOI] [PubMed] [Google Scholar]