Abstract
Objective
To characterize factors predictive of improved survival following gastrectomy with additional organ resection for the treatment of gastric cancer.
Summary Background Data
Recent large series have reported significant survival disadvantages to patients who have undergone gastrectomy with splenectomy or pancreaticosplenectomy, and yet gastrectomy with additional organ resection is needed to accomplish an R0 resection in some cases. Gastrectomy with splenectomy and other organ resections has been associated with advanced T-stage, positive resection margins, and higher postoperative morbidity and mortality rather than an absolute predictor of survival.
Methods
The authors reviewed the Department of Surgery prospective gastric database at Memorial Sloan-Kettering Cancer Center from July 1985 to July 2000. During this period, of the 2,112 patients with primary gastric cancer, 1,133 underwent an R0 resection. The R0 resection group included 865 patients who underwent gastrectomy alone and 268 patients who underwent gastrectomy with another organ resection. Clinicopathologic, operative, complication, and survival data were compared between these two groups. Chi-square analysis and the Kaplan-Meier method were used to compare and estimate median survival.
Results
The most common organs resected were the spleen and pancreas, with an even distribution of other organs. Pathologic factors revealed that the gastrectomy with organ resection group had significantly larger lesions, greater T-stage, and a higher incidence of advanced nodal disease than the group who did not undergo additional organ resection. The incidence of pathologically confirmed T4 cancers in the additional organ resection group was only 14%. The overall 5-year survival rate for patients with T3/T4 disease was 27% with additional organ resection. The overall 5-year survival rate for the gastrectomy with organ resection group (32%, median 32 months) was significantly less than the group that did not undergo additional resection (50%, median 63 months) on univariate analysis. However, additional organ resection was not a predictor of survival on multivariate analysis. Multivariate analysis identified advanced T-stage (T3 or greater) and nodal stage (N1 or greater) as adverse predictors of survival in this group.
Conclusions
Long-term survival following gastrectomy with additional organ resection is possible. Depth of invasion and the extent of lymph node metastasis are the most important predictors of survival following gastrectomy with additional organ resection, and a R0 resection has been achieved. Judicious use of additional organ resection for the treatment of advanced gastric cancer must be emphasized, given the increased overall morbidity and infrequent finding of actual T4 disease. Additional organ resection can be performed with minimal morbidity and can improve the chance of overall survival in patients with advanced T-stage disease.
The value of extended organ resection for advanced gastric cancer has been debated for many years. This debate has been escalated recently by the results of both the United Kingdom Medical Research Council and the Dutch trials evaluating the survival benefit of extended lymphadenectomy. Both of these large prospective randomized control trials have reported a significant survival disadvantage in patients who have undergone gastrectomy with splenectomy or pancreaticosplenectomy. 1,2 The Medical Research Council study and the Dutch trial found a higher mortality, higher complication rate, and longer hospital stay associated with extended organ resection.
Some reports have argued that clearance of regional lymphadenopathy is improved by removing adjacent organs. The potential advantage of extended resection for clinical T4N0 gastric adenocarcinoma is necessary to improve the R0 resection rate of these lesions. Arguments against this approach are based on the observed increase in the morbidity and mortality rates, with little objective benefit in survival.
A large retrospective study from Japan found no survival difference when patients undergoing gastrectomy alone were compared to patients with additional organ resection, but again complication rate were greater. 3 This lack of survival disadvantage for gastrectomy with additional organ resection has been demonstrated in other retrospective studies when evaluating outcomes of patients undergoing total gastrectomy alone, with splenectomy, and with pancreaticosplenectomy. 4
The complication rates of additional organ resection with gastrectomy have consistently been reported to be higher when compared to patients undergoing gastrectomy alone. 1–3 In patients undergoing splenectomy, both overall complications and infectious complications have been more common. 5 The increase in overall complications and infectious complications has been implicated as a reason for the decrease in overall survival. For these reasons there has been an unwillingness to perform additional organ resection in patients with T4 disease.
The aim of this study was to report our experience with gastrectomy with additional organ resection in patients with T4 gastric carcinoma and to identify factors predictive of improved outcome.
METHODS
From a prospective gastric database, 2,112 patients underwent gastric resection from July 1985 to July 2000, with 1,133 patients undergoing R0 resection. Eight hundred sixty-five patients underwent gastrectomy alone and 268 patients underwent gastrectomy with another organ resection.
The surgical resections were performed by attending surgeons or surgical oncology fellows under direct attending surgeon supervision. Depending on the location of the primary lesion, total, distal subtotal, or proximal gastrectomy was performed. Additional organ resection was performed to facilitate a more extensive lymph node dissection, to gain complete tumor resection, or because of iatrogenic injury to the spleen.
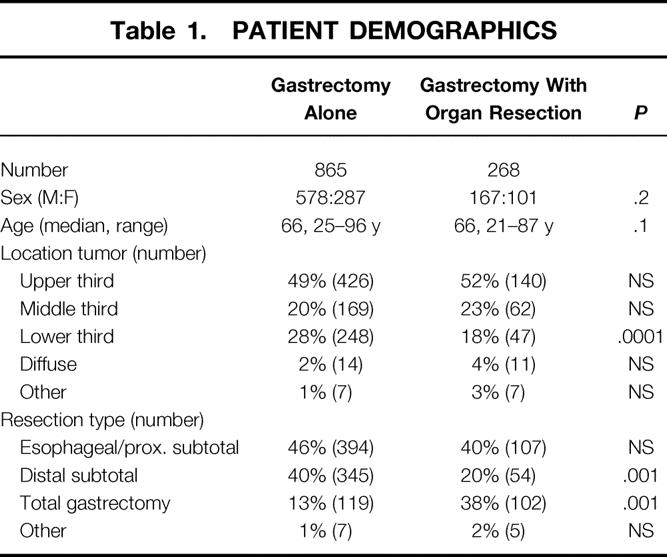
Preoperative laparoscopy as well as laparoscopic ultrasound was performed on these patients, using the technique published earlier. 6 Laparoscopic assessment was performed as stated in Table 1.
Table 1. PATIENT DEMOGRAPHICS
A D2 lymphadenectomy was performed as part of the standard radical gastrectomy. For the purpose of this study, lymphadenectomy was classified by the number of lymph nodes removed and was considered adequate if greater than 15 lymph nodes were removed. 7
Clinicopathologic, operative, and survival data were compared between these two groups. Patients were classified as having additional organ resection when the procedure was reported in the operative note or the pathology report. All staging was assigned using the 1997 American Joint Committee on Cancer TNM staging guidelines. 8
Chi-square analysis was used to compare clinicopathologic data. Parameters influencing survival and recurrence were compared using the Kaplan-Meier method with log-rank comparison. Proportional hazards analysis was performed on all variables found significant by univariate analysis. Differences of P < .05 where considered significant.
RESULTS
From the 2,112 patients identified during this time period, 1,958 (93%) underwent exploration for their primary gastric cancer. One thousand five hundred forty patients underwent gastrectomy alone (79%), and 418 (21%) had a gastrectomy with at least one additional organ resection. An R0 resection was performed in 865 (56%, 865/1,540) patients with a gastrectomy alone and in 268 (64%, 268/418) patients with gastrectomy with additional organ resection.
The groups were similar in age and sex (see Table 1). Gastrectomy with organ resection occurred more commonly in patients with primary lesions in the proximal two thirds of the stomach, with the gastrectomy-alone group having a significant greater incidence of lower third primary lesions (P = .0001). The gastrectomy with organ resection group had a greater number of resections requiring total gastrectomy. The most common organs resected were spleen alone (n = 123), spleen/pancreas (n = 38), spleen/colon (n = 18), pancreas (n = 12), and colon (n = 16) (Table 2).
Table 2. ORGANS RESECTED
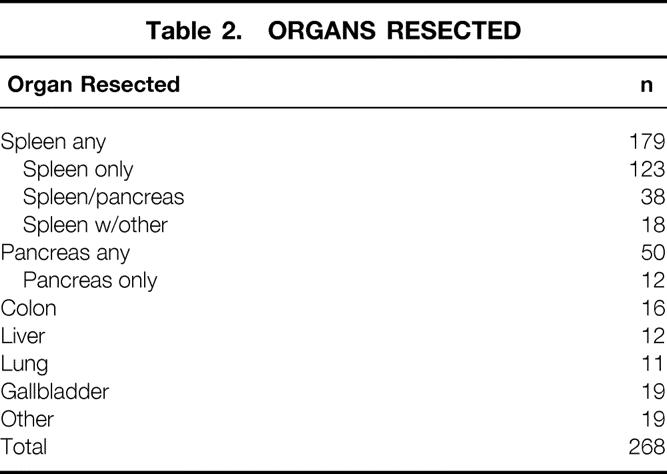
Eleven patients underwent splenectomy because of iatrogenic injury. The gallbladder was removed in 19 patients because of adherence to the primary (n = 16) or because of gallstones (n = 3).
Smaller tumor diameter (P = .0001) and an earlier T-stage were associated with the gastrectomy-alone group. The gastrectomy with organ resection group had a greater percentage of T3 (P = .02) and T4 (P = .0001) lesions (Table 3). The gastrectomy with organ resection group had a greater percentage of N2 (P = .02) and N3 disease. The Lauren types were similar between the groups.
Table 3. STAGING
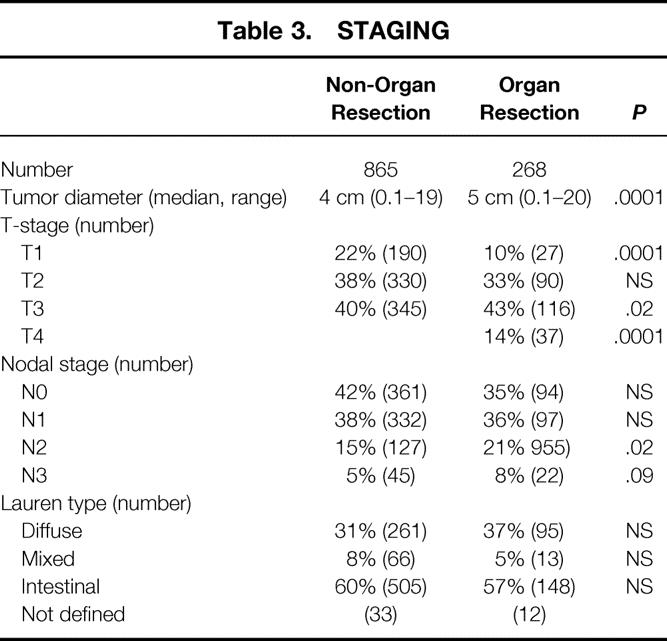
Extent of lymphadenectomy was similar in both groups: the median number of nodes removed in the gastrectomy-alone group (n = 21) was similar to the that in gastrectomy with additional organ resection group (n = 21).
Preoperative laparoscopy was performed in 264 (23%, 264/1,133) patients, with 61 patients undergoing laparoscopic ultrasound. Endoscopic ultrasound was performed in 143 of these patients.
There was no difference in perioperative mortality in the gastrectomy-alone (n = 31, 3.6%) group when compared to the gastrectomy with organ resection group (n = 10, 3.7%).
Time to Recurrence
The median follow-up for all patients undergoing an R0 resection was 24 months. Recurrences were seen in 497/1,133 (44%) of the entire patient population: 256 (52%) local recurrences and 241 (48%) distant recurrences. There was a significantly greater incidence of recurrence in the gastrectomy with organ resection group (137/268, 52%) when compared to the gastrectomy-alone group (360/865, 42%, P = .003). The median time to recurrence was 22 months in the gastrectomy with organ resection group, compared to 47 months in the gastrectomy-alone group. When type of organ resected was evaluated for time to recurrence, there was no difference between splenectomy, pancreatectomy, colectomy, or resection of other organs. The number of organs resected was not a predictor of recurrence in this entire patient population. A proportional hazard model was run for predictors and only N2 disease (relative risk [RR] 1.4, confidence interval [CI] 1.2–1.7), T3 or greater (RR 2.0, CI 1.6–2.6), and proximal gastrectomy (RR 1.6, CI 1.2–2.3) were found to significantly increase the recurrence risk in the entire patient population.
When factors of recurrence were evaluated in the 268 patients who had gastrectomy and additional organ resection, the results were similar. Again, only advanced N disease (N2, RR 1.3, CI 1.1–1.8) and depth of invasion (T3, RR 1.8, CI 1.1–3.9) were factors for recurrence, both local and distant. Proximal gastrectomy was not found to be a predictor in this subset analysis.
Survival
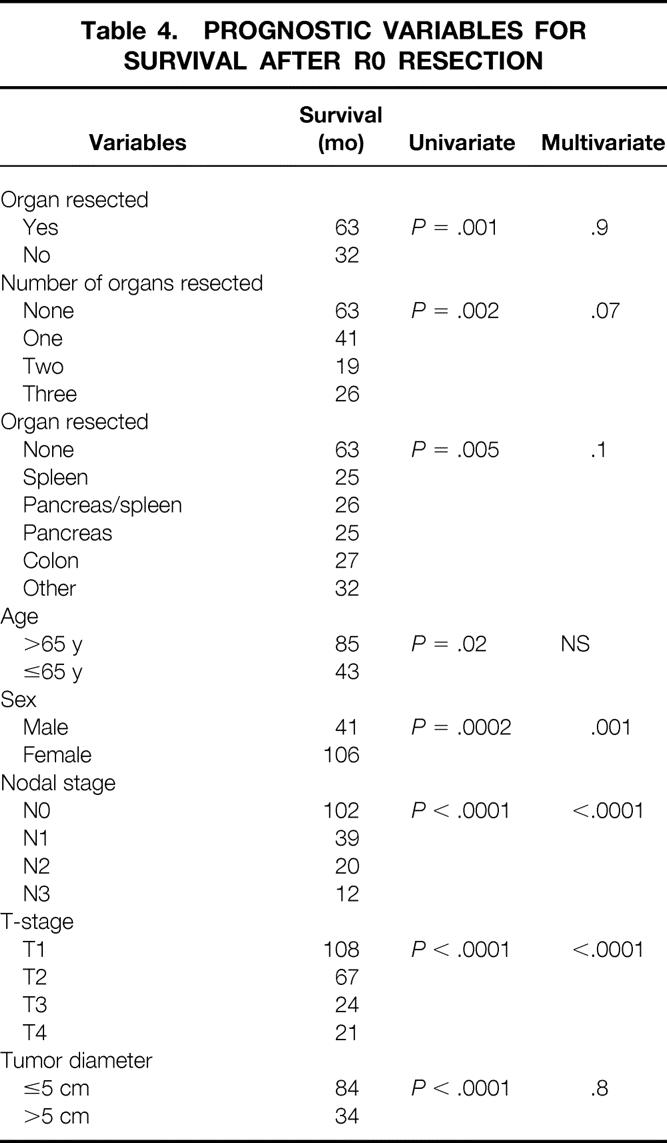
The median survival for the entire group was 54 months. After excluding the 14 patients who had organs removed incidentally, the survival for the gastrectomy-alone group (median 63 months) was significantly better than the gastrectomy with organ resection group (median 32 months) on univariate analysis (Fig. 1). The number of organs resected also influenced survival on univariate analysis (Table 4), but the type of organ resected had no influence on median survival. Patients who had undergone splenectomy had a median survival of 25 months, while those having pancreaticosplenectomy had a median survival of 26 months.
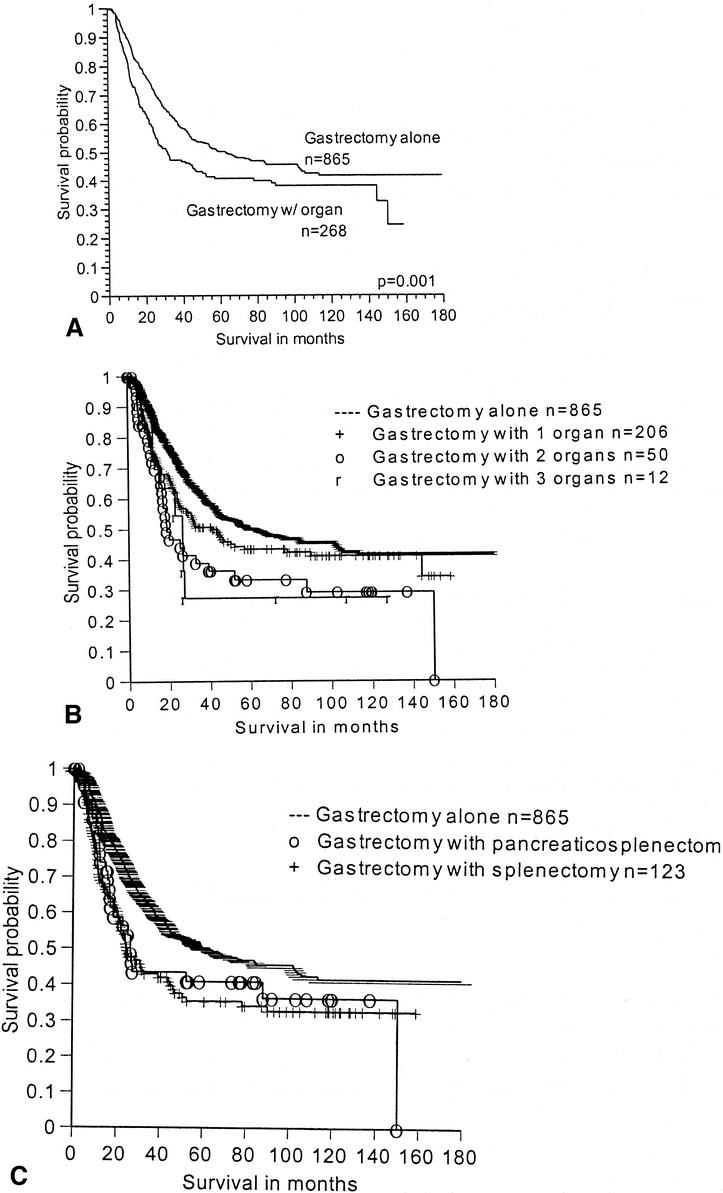
Figure 1. Survival for patients with gastrectomy alone versus the polyvisceral resection group. (A) Survival for patients with gastrectomyalone versus gastrectomy with any additional organs resected. (B) Survival by the number of additional organs resected. (C) Survival by the type of additional organ resected.
Table 4. PROGNOSTIC VARIABLES FOR SURVIVAL AFTER R0 RESECTION
When survival was analyzed within each stage (stage II to IV), resecting additional organs did not significantly decrease survival (Fig. 2). When the specific type of organ resected was evaluated within stage, again survival was not altered.
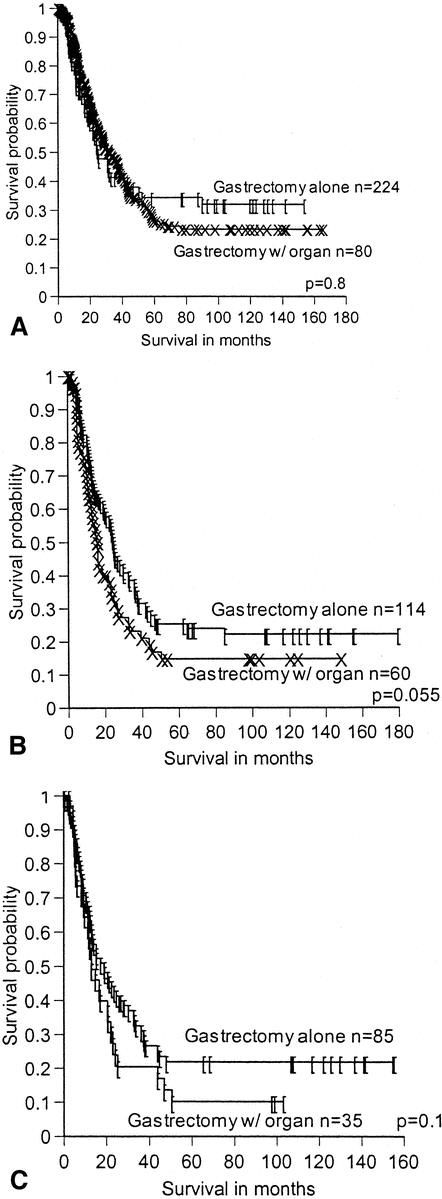
Figure 2. Survival by overall stage, comparing patients undergoing gastrectomy alone to the PVR group. (A) Stage 3a. (B) Stage 3b. (C) Stage 4.
On multivariate analysis of the entire 1,133 patients, neither the organ resected, the number of organs resected, nor the presence of a complication was a predictor of survival (see Table 4). Only gender, nodal stage, and depth of invasion were predictors of poor outcome in this entire patient population. No other factors were demonstrated to be adverse predictors of survival.
In evaluating all 254 patients with gastrectomy with additional organ resection, again T-stage (P < .001), N-stage (P < .001), and overall stage (P < .0001) were the only predictors of survival in this group. Age, site of primary, type of resection, specific organ resected, number of organs resected, tumor diameter, preoperative chemotherapy, external beam radiotherapy, and the presence of a complication were all not predictors of survival in this subset of patients.
When survival was evaluated for the subgroup of patients who had one or more of the three most common organs resected (spleen, pancreas, and colon), the results of the multivariate analysis were the same. Overwhelmingly, the effect of T-stage and N-stage dominated the outcome. The presence of a complication, the type of organ resected, and the number of organs resected did not affect outcome.
DISCUSSION
The disadvantages of gastrectomy with additional organ resection have been recently highlighted in two large prospective randomized control trials. 9,10 Bonenkamp et al 2 reported on 996 patients randomized to a D1 or D2 lymph node dissection. A significant increase in postoperative morbidity, mortality, complications, reoperation, and hospital stay was seen in patients requiring a D2 lymphadenectomy. A disproportionate increase in morbidity and mortality was associated with the addition of splenectomy and/or pancreatectomy to the procedure. Cuschieri et al’s evaluation of 400 patients randomized to a D1 or D2 lymphadenectomy found a significant survival difference between patients with gastrectomy alone compared to patients with gastrectomy and splenectomy or pancreaticosplenectomy, regardless of the extent of lymphadenectomy. 1
This survival difference was not demonstrated in a study from Kasakura et al. 3 This retrospective study of 1,938 patients evaluated patients undergoing gastrectomy alone, gastrectomy with splenectomy, and gastrectomy with pancreaticosplenectomy. They found that gastrectomy with additional organ removal was not a factor of survival in stage II, III, and IV, with stage I having small numbers for the organ resection group. This lack of survival difference has been reproduced in another recent study by Otsuji et al. 4 This report found no survival difference in the same three groups, with a 5-year survival rate of 54.2%.
Past reports evaluating the influence of splenectomy on survival after gastrectomy have been mixed. Most reports have come from the Japanese literature: three studies found that splenectomy was an negative predictor of survival in the treatment of gastric cancer, three found no difference in survival, and two studies came to mixed conclusions depending on the stage of disease. Stage IV disease made no difference in survival, and in stage III disease preservation of the spleen was found to have a better outcome. 11–14
Our study supports the more recent reports in that we found that the number and type of organs were not independent predictors of survival. Splenectomy, pancreaticosplenectomy, or any other organ resection was not found to be a poor predictor of survival on multivariate analysis. Our study results are similar to an older report of Brady et al in which splenectomy was not found to be a predictor of poor outcome. 5
Ozaki et al 15 and others 16 have found that an aggressive approach to resection of the stomach with the body and tail of the pancreas or pancreaticoduodenectomy 17 and right hemicolectomy can lead to an acceptable 5-year survival rate of 29%. We found that advanced T- and N-stage had a far more influential effect on survival than the type or number of organs resected. We have demonstrated a similar overall survival in this report, with patients of stage III or greater who have undergone gastrectomy and additional organ resection with a 5-year survival rate of 23%.
This study differs from the previous historical reports in the Japanese literature because we included only patients undergoing R0 resection, as well as patients with distal stomach lesions. The number of organs resected, as well as the spleen, and pancreas and spleen, were factors on univariate analysis, but they were not found to be predictors of poor survival on multivariate analysis. The impact of splenic resection was not a factor of survival primarily because of the overwhelming effect that advanced nodal stage and depth of invasion had on this analysis. Only the number of organs resected (three or more) approached being a significant factor of outcome; however, the small numbers (n = 12) do not allow for any definitive conclusions to be made.
The overall 5-year survival rate for the 286 patients undergoing gastrectomy with additional organ resection was 32%. This is significantly less than the gastrectomy-alone group and reflects the greater incidence of nodal stage as well as depth of invasion when compared to patients with gastrectomy alone. This survival is similar to previous reports of patients treated with extended surgical resection in advanced T-stage gastric cancer. Shchepotin et al 18 demonstrated a 25% 5-year survival rate in 353 patients with T4 gastric cancer treated with additional organ resection. The most common organ resected in this report was the colon, followed by pancreaticosplenectomy, with similar complication rates as reported in this study.
Our study continues to confirm previous reports that patients with advanced T-stage disease can benefit from aggressive en bloc surgical resection, with minimal morbidity, and should not be rendered unresectable by preoperative imaging, endoscopic ultrasound, or intraoperative assessment of adjacent organ involvement. In addition, if organ involvement is suggested on preoperative evaluation but not found on final pathologic analysis, the survival for these patients is the same, stage for stage (see Fig. 2). However, this does not translate into the need for additional organ resection because of only assumed invasion. If additional organ involvement is suspected on one type of imaging modality, attempts should be made to confirm this invasion with another imaging modality because while this data do not demonstrate a survival disadvantage, the number of patients treated with pancreatectomy and with three-organ resection is small and appears to be approaching significance (see Fig. 1). Thus, attempts to confirm serosal and/or adjacent organ involvement cannot be overemphasized before additional organ resection.
Recurrence of gastric cancer after resection is not uncommon and represents an aggressive histology. Patients with polyvisceral resection (PVR) have a greater burden of disease and subsequently a greater incidence of recurrence (52%) when compared to patients with gastrectomy alone (42%). Neither the specific organ type resected nor the number of organs resected was predictive of recurrence or the pattern of recurrence. The most powerful predictors of recurrence were nodal stage, depth of invasion, and the site of the primary lesion (distal esophagus and proximal stomach).
This report demonstrates that gastrectomy with additional organ resection for gastric cancer can be achieved with minimal perioperative mortality (4%). Long-term survival can be achieved in this patient subset, with a 3-year survival rate of 47%. Only the biology of the primary lesion (i.e., depth of invasion and nodal stage) was found to predict overall survival in this group of patients. Additional organ resection was not found to be an adverse predictor of survival.
With careful patient selection, gastrectomy with additional organ resection can be done with acceptable morbidity and low mortality. Improvements in preoperative evaluation to confirm T3 and T4 disease are needed to minimize unnecessary organ resections for early-stage disease. Depth of invasion and the presence and extent of lymph node metastasis are the most powerful determinants of survival following an R0 resection. Gastrectomy with additional organ resection should be limited to clinically T4 tumors, with the understanding that the majority will be T3.
Footnotes
Correspondence: Martin Karpeh, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: karpehm@mskcc.org
Supported by a grant from the Richard M. Gelb Foundation.
Accepted for publication February 18, 2002.
References
- 1.Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996; 347: 995–999. [DOI] [PubMed] [Google Scholar]
- 2.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345: 745–748. [DOI] [PubMed] [Google Scholar]
- 3.Kasakura Y, Fujii M, Mochizuki F, et al. Is there a benefit of pancreaticosplenectomy with gastrectomy for advanced gastric cancer? Am J Surg 2000; 179: 237–242. [DOI] [PubMed] [Google Scholar]
- 4.Otsuji E, Yamaguchi T, Sawai K, et al. Total gastrectomy with simultaneous pancreaticosplenectomy or splenectomy in patients with advanced gastric carcinoma. Br J Cancer 1999; 79: 1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady MS, Rogatko A, Dent LL, et al. Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg 1991; 126: 359–364. [DOI] [PubMed] [Google Scholar]
- 6.Conlon KC, Karpeh MS Jr. Laparoscopy and laparoscopic ultrasound in the staging of gastric cancer. Semin Oncol 1996; 23: 347–351. [PubMed] [Google Scholar]
- 7.Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg 2000; 232: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 1997.
- 9.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. Dutch Gastric Cancer Group. N Engl J Med 1999; 340: 908–914. [DOI] [PubMed] [Google Scholar]
- 10.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999; 79: 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimachi K, Kodama Y, Kumashiro R, et al. Critical evaluation of prophylactic splenectomy in total gastrectomy for the stomach cancer. Gann 1980; 71: 704–709. [PubMed] [Google Scholar]
- 12.Orita K, Konaga E, Okada T, et al. Effect of splenectomy in tumor-bearing mice and gastric cancer patients. Gann 1977; 68: 731–736. [PubMed] [Google Scholar]
- 13.Suehiro S, Nagasue N, Ogawa Y, et al. The negative effect of splenectomy on the prognosis of gastric cancer. Am J Surg 1984; 148: 645–648. [DOI] [PubMed] [Google Scholar]
- 14.Koga S, Kaibara N, Kimura O, et al. Prognostic significance of combined splenectomy or pancreaticosplenectomy in total and proximal gastrectomy for gastric cancer. Am J Surg 1981; 142: 546–550. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki H, Kinoshita T, Kosuge T, et al. An aggressive therapeutic approach to carcinoma of the body and tail of the pancreas. Cancer 1996; 77: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 16.Korenaga D, Okamura T, Baba H, et al. Results of resection of gastric cancer extending to adjacent organs. Br J Surg 1988; 75: 12–15. [DOI] [PubMed] [Google Scholar]
- 17.Yonemura Y, Ooyama S, Matumoto H, et al. Pancreaticoduodenectomy in combination with right hemicolectomy for surgical treatment of advanced gastric carcinoma located in the lower half of the stomach. Int Surg 1991; 76: 226–229. [PubMed] [Google Scholar]
- 18.Shchepotin IB, Chorny VA, Nauta RJ, et al. Extended surgical resection in T4 gastric cancer. Am J Surg 1998; 175: 123–126. [DOI] [PubMed] [Google Scholar]


