Abstract
Objective
To investigate the central regulation of food intake by quantifying neuron activation of the nucleus of the solitary tract (NTS) after injection of cholecystokinin (CCK) or food intake in gastrectomized rats.
Summary Background Data
Total gastrectomy is followed by early satiety, low calorie intake, and weight loss in the majority of patients. The etiology of these effects is unknown. Sixty percent to 70% of patients remain underweight after total gastrectomy, the weight loss averaging 25% of preoperative body weight. About two thirds of gastrectomized patients report early satiety, and about 60% do not reach the recommended daily calorie intake. The NTS is a brain stem center involved in the regulation of food intake; thus, the extent and pattern of neuronal activation provide information on the process involved in the initiation of satiation and the regulation of food intake.
Methods
The authors investigated neuronal activation in the NTS using c-fos immunohistochemistry following CCK injection or food intake in healthy control rats, sham-operated control rats, age-matched control rats, weight-matched control rats, and vagotomized or gastrectomized rats.
Results
Neuronal activation in the NTS after CCK injection was significantly decreased 21 days after total gastrectomy, but increased by up to 51% 3 months and by up to 102% 12 months after surgery compared to age-matched unoperated control rats. Neuronal activation in the NTS in response to feeding was markedly increased up to fivefold in gastrectomized rats. This increase was early in onset and sustained, and occurred despite significantly reduced food intake. Administration of MK329, a CCK-A receptor antagonist, significantly reduced the number of postprandially activated neurons in both gastrectomized and control rats.
Conclusions
The early postprandial activation of NTS neurons after total gastrectomy in rats may correspond to early satiety reported by patients, while the sustained activation of NTS neurons after a meal could contribute to a reduced daily calorie intake. These data suggest that a disturbed central regulation of food intake might contribute to early satiety, reduced food intake, and weight loss after total gastrectomy.
Total gastrectomy is still a mainstay of gastric cancer treatment. 1 It is followed by a variety of symptoms and impairments, commonly referred to as postgastrectomy syndromes. 2 One important feature of the postgastrectomy syndromes is a considerable loss of body weight. 3,4 The loss averages around 25% of preoperative body weight 4,5 and leaves 60% to 70% of patients permanently below ideal body weight. 3,6 While perioperative weight loss due to catabolic metabolism is generally followed by an anabolic phase 3 to 8 days postoperatively with body weight recovery, 7 this is not the case in gastrectomized patients, where weight loss and underweight frequently cause long-lasting morbidity. 4–6,8
There is evidence that pancreatic insufficiency, resulting in malabsorption and impaired food utilization, contributes to weight loss after gastrectomy. 9–11 In addition, several studies have shown a reduced food intake after gastrectomy, 3,12 but the reasons for this are not clear.
Gastrointestinal peptides contribute to the regulation of food intake by activating specific brain centers. 13 We have recently shown that cholecystokinin (CCK) injection or food intake stimulated neurons in the nucleus of the solitary tract (NTS), 14 a brain stem center known to be involved in the regulation of food intake. 15,16 A strong correlation between the CCK dose injected or the amount of food intake and neuronal activation in the NTS was observed, indicating that the activation of these brain stem neurons probably reflects the initiation of satiation. 14
After gastrectomy, gut peptides that inhibit food intake are released at considerably increased amounts in response to a meal. For example, CCK release is increased after gastrectomy, 10,17 and CCK is known to suppress food intake under physiologic conditions. 13 We have shown that CCK receptor blockade increased food intake and body weight after total gastrectomy in rats. 18 However, the mechanisms and pathways leading to an altered regulation of food intake following gastrectomy are not clear.
In the present study, we investigated the central regulation of food intake by quantifying NTS neuron activation after CCK injection or food intake in gastrectomized rats. We obtained evidence that the central regulation of food intake is altered after total gastrectomy; these changes might reflect the disturbances in food intake and satiation clinically observed after total gastrectomy.
METHODS
Animals
Male Sprague-Dawley rats (Charles River, Kieslegg, Germany), weighing 270 to 340 g, were used for experiments. Rats were housed under controlled conditions of illumination (12/12 hours light/dark cycle, dark cycle starting at 10 am), humidity (60–70%), and temperature (21°C). The institutional guidelines for the care and use of laboratory animals were followed throughout the study.
Surgical Procedures
Sham operation was performed by midline laparotomy under ketamine hydrochloride (Ketanest, 100 mg/kg; Parke Davis, Berlin, Germany) and xylazine (Rompun, 15 mg/kg; Bayer, Leverkusen, Germany) anesthesia. The stomach and the intestine were covered with saline-moistened gauze for 45 minutes, which corresponds to the time period required for abdominal vagotomy or total gastrectomy. For subdiaphragmatic vagotomy, the vagus nerves were identified by using a binocular microscope (OP Mi-1, Zeiss, Oberkochen, Germany), ligated, and transected for a distance of approximately 1 cm. A pyloroplasty was added to avoid delayed gastric emptying with gastric distention. Total gastrectomy with Roux-en-Y reconstruction was performed as previously described. 18 In brief, the duodenum was closed by a ligature distal to the pylorus, the stomach was removed, and the jejunum was cut 2 cm distally to the ligament of Treitz. Esophagus and jejunum were anastomosed by single stitches, and an end-to-side jejunal anastomosis (Roux-en-Y) was done about 7 cm distally by a running suture. A recovery period of 21 days was allowed for all operated animals before experiments were commenced.
c-fos Protein Expression in the Nucleus of the Solitary Tract
Rats were fasted for 4 hours with free access to water. Sulfated CCK-8 (Sigma, Deisenhofen, Germany), dissolved in 0.1% bovine serum albumin (Sigma), was injected intraperitoneally 15 minutes before the dark phase. Rats remained in their cages without access to food for another 90 minutes before being euthanized with ketamine (150 mg/kg) and xylazine (20 mg/kg). We have previously shown that CCK-induced c-fos protein expression in the NTS is maximal at this time point. 14 The brain stem was harvested and processed for immunohistochemistry of c-fos protein expression in the NTS as described previously. 14,19
C-fos protein expression in the NTS was investigated in unoperated control rats after injection of CCK at a dose of 4 μg/kg (≈3.5 nmol/kg, n = 4), 8 μg/kg (≈7 nmol/kg, n = 4), and 100 μg/kg (≈87.5 nmol/kg, n = 9), in sham-operated rats 21 days after surgery after injection of CCK at a dose of 100 μg/kg (n = 5), in vagotomized rats 21 days after surgery after injection of CCK at a dose of 4 μg/kg (n = 4), 8 μg/kg (n = 5), and 100 μg/kg (n = 5), and in gastrectomized rats 21 days after surgery after CCK injection at a dose of 4 μg/kg (n = 4), 8 μg/kg (n = 5), and 100 μg/kg (n = 6).
Additionally, c-fos protein expression in the NTS was investigated in gastrectomized rats 3 months (n = 4) and 12 months (n = 4, age 15 months) after surgery and in 15-month-old unoperated control rats (n = 5) after injection of CCK (8 μg/kg intraperitoneally), since there is evidence that the sensitivity to CCK regarding food intake is increased with increasing age in rats. 20
CCK-induced c-fos protein expression in the NTS of gastrectomized rats 3 weeks and 3 months after surgery was also compared to weight-matched unoperated control rats, since body weight might influence the sensitivity to CCK with respect to food intake 21 (3 weeks after surgery: gastrectomy, n = 5, body weight 274 ± 24 g; weight-matched control rats, n = 5, body weight 273 ± 19 g; CCK dose 100 μg/kg intraperitoneally; 3 months after surgery: gastrectomy, n = 4, body weight 373 ± 8 g; weight-matched control rats, n = 4, body weight 367 ± 7 g; CCK dose 8 μg/kg intraperitoneally).
Food Intake Experiments
Food intake was measured with an automatic recording system (Diet-Scan, Omnitech, Columbus, OH) with a precision of 0.1 g every 15 minutes for a 4-hour period, starting at the beginning of the dark phase. Animals were fasted for 4 hours (food intake and c-fos protein expression after CCK-A receptor blockade) or for 14 hours (postprandial c-fos protein expression) with free access to water. The different fasting periods were chosen due to data obtained from experiments testing for the effects of different fasting periods on CCK-induced inhibition of food intake. 16,21 Food intake in gastrectomized rats was investigated 21 days after surgery after intraperitoneal injection of either the CCK-A receptor antagonist MK329 (MSD, Munich, Germany; 1 mg/kg, n = 10) or vehicle (carboxymethylcellulose 0.1%, Sigma; 1 mL/kg, n = 10) 15 minutes before the start of food intake. Additionally, food intake in gastrectomized rats after MK329 or vehicle treatment was compared to food intake of unoperated control animals after MK329 or vehicle treatment (n = 30).
Postprandial c-fos Protein Expression in the Nucleus of the Solitary Tract
Postprandial c-fos protein expression was investigated 21 days after surgery in gastrectomized rats after a 14-hour fasting period with free access to water. Access to food was given at the beginning of the dark period and rats were euthanized at 0 (n = 3, basal), 30 (n = 4), 60 (n = 4), 90 (n = 4), 120 (n = 4), 150 (n = 5), 180 (n = 4), and 360 (n = 4) minutes. The amount of food intake at that time was recorded for each animal. c-fos protein expression and food intake were compared to data from unoperated control animals. c-fos protein expression in the NTS was also investigated in weight-matched unoperated control animals 60 and 90 minutes after the start of food intake, since body weight is known to influence food intake and might thus alter c-fos protein expression in response to feeding.
Postprandial c-fos Protein Expression in the Nucleus of the Solitary Tract After CCK-A Receptor Blockade
Postprandial c-fos protein expression after CCK-A receptor blockade was analyzed in gastrectomized rats 21 days after surgery. Rats were fasted for 4 hours with free access to water, and the CCK-A receptor antagonist MK329 (1 mg/kg, n = 4) or vehicle (carboxymethylcellulose 0.1%, 1 mL/kg, n = 4) was injected intraperitoneally 15 minutes before the start of food intake. Access to food was given at the beginning of the dark phase and rats were euthanized 90 minutes later. The amount of food intake at that time was recorded for each animal. c-fos protein expression after CCK-A receptor blockade of gastrectomized rats was also compared to vehicle-treated unoperated rats.
Tissue Harvesting and Immunohistochemistry
Tissue harvesting and immunohistochemistry were performed as described previously. 14,19 Rats were perfused with 0.9% saline in 0.1 mol/L phosphate buffer (pH 7.4) via the ascending aorta, followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. The brain stem was removed, postfixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer for 2 hours, and cryoprotected in 25% sucrose in 0.1 mol/L phosphate buffer overnight. Sections were cut with a cryotome (CM 3000, Leica, Bensheim, Germany) at 30 μm and processed as free-floating sections. fos-like immunoreactivity was demonstrated by the avidin-biotin complex method with 3,3′diaminobenzidine serving as the chromagen. Tissue was incubated overnight at 4°C with Fos Ab-5 antibody (Calbiochem, Bad Soden, Germany) at a dilution of 1:2,000, followed by avidin-biotin complex (Vectastain, Camon, Wiesbaden, Germany) and then by 0.05% diaminobenzidine/0.033% hydrogen peroxide (Sigma) for 10 minutes and 5 minutes, respectively. Sections were mounted onto gelatin-coated slides, air-dried, dehydrated, and cover-slipped with mounting medium (Eukitt, Kindler, Freiburg, Germany).
Analysis of c-fos Protein Expression in the Nucleus of the Solitary Tract
Immunohistochemistry was performed on every third section of the brain stem (i.e., approximately every 100 μm of the tissue, 50–60 sections/animal). fos-like immunopositive cells in the NTS were counted by a videocamera- and computer-assisted bright-field microscopy system (Quantimed 500, Leica) at the levels of the obex (interaural about −5.30 mm), the area postrema (interaural about −4.80 mm), and the caudal fourth ventricle (interaural about −4.30 mm), according to a stereotaxic rat brain atlas. 22 In each rat, three sections of each of the three anatomical levels were analyzed. Bright-field microscopy images were captured by a videocamera and transferred to a personal computer. Each image was calibrated to the corresponding objectives, the NTS was marked with a computer mouse, and the area was calculated by the acquisition software.
Fluorescent Labeling Experiments of Efferent Vagal Neurons in Gastrectomized Rats
Efferent vagal neurons, deriving from the dorsal motor nucleus of the vagus nerve (DMN), were labeled by using the fluorescent retrograde tracer fast blue (Sigma, 3 mg/rat, diluted in 0.6 mL NaCl 0.9%, injected intraperitoneally in the upper abdomen at two different sites) in two gastrectomized rats 21 days after surgery. The brain stem was dissected out 3 days later and the DMN was analyzed with a fluorescent microscope (wave length of 360 nm, −4.8 mm interaural), as described by Powley et al. 23
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Differences between groups were determined by analysis of variance, followed by the Student t test, with the help of the software package JMP (Version 3.2.2, SAS Institute Inc., Cary, NC). A probability of P < .05 was taken as significant.
RESULTS
Effects of Gastrectomy on Postprandial c-fos Protein Expression in the Nucleus of the Solitary Tract
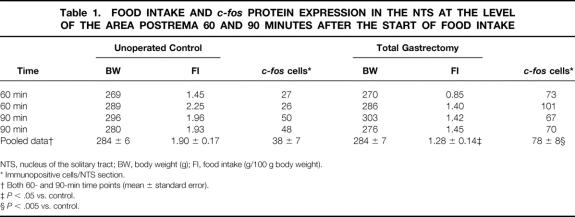
Basal c-fos protein expression in the NTS after a 14-hour fasting period was not significantly different between unoperated and gastrectomized rats. Food intake significantly increased the number of c-fos protein-positive cells in the NTS of gastrectomized rats at 60 minutes compared to basal (68 ± 9 vs. 18 ± 2 positive cells per section at the level of the area postrema, P < .0001), with a further increase at 90 minutes and a c-fos protein expression maintained at a high level afterward (Fig. 1). In unoperated control animals, maximal c-fos protein expression in the NTS was reached 90 minutes after the start of food intake, continuously decreasing thereafter. At every time point investigated, c-fos protein expression in the NTS was significantly increased in gastrectomized animals compared to control animals, reaching a more than fivefold increase at 360 minutes after the beginning of food intake. Gastrectomized rats also expressed a higher number of c-fos protein-positive cells in the NTS in relation to the amount of food intake eaten at each time point investigated (Fig. 2). c-fos protein expression was also significantly increased in gastrectomized rats compared to weight-matched control rats 60 minutes and 90 minutes after the start of food intake, despite a decreased food intake by gastrectomized rats (Table 1).
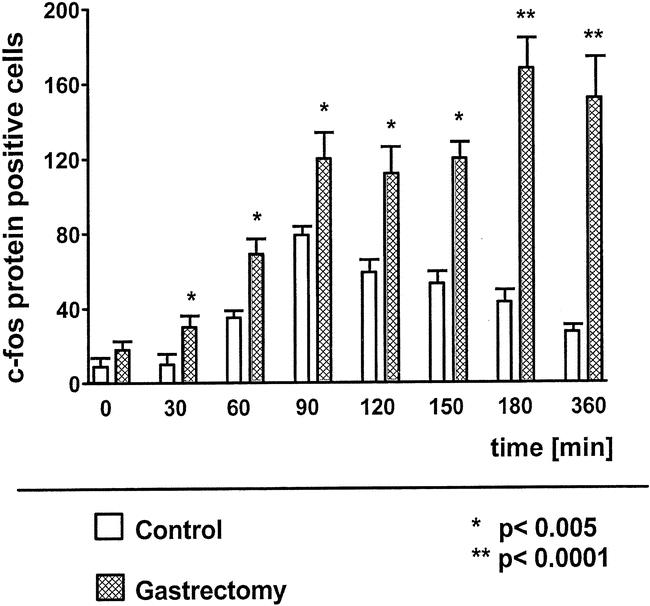
Figure 1. Postprandial c-fos immunopositive cells in the nucleus of the solitary tract at the level of the area postrema in unoperated controls and in gastrectomized rats.
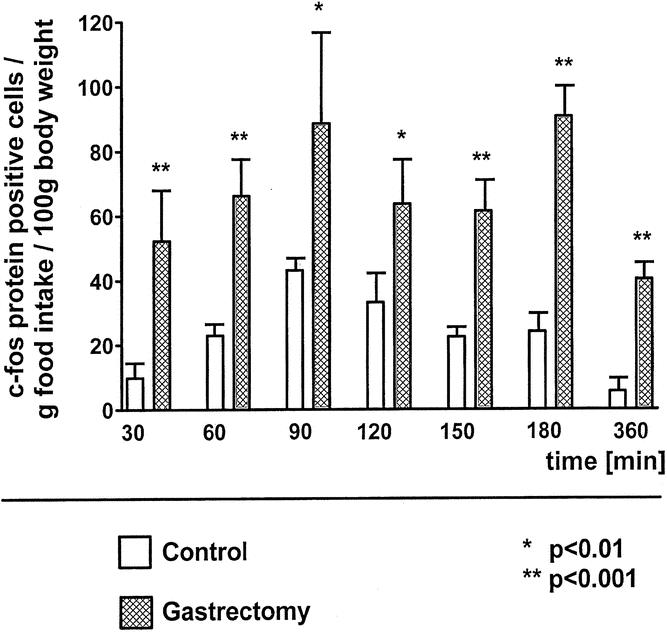
Figure 2. Postprandial c-fos immunopositive cells in the nucleus of the solitary tract at the level of the area postrema in relation to cumulative food intake in unoperated controls and in gastrectomized rats.
Table 1. FOOD INTAKE AND c-fos PROTEIN EXPRESSION IN THE NTS AT THE LEVEL OF THE AREA POSTREMA 60 AND 90 MINUTES AFTER THE START OF FOOD INTAKE
NTS, nucleus of the solitary tract; BW, body weight (g); FI, food intake (g/100 g body weight).
* Immunopositive cells/NTS section.
† Both 60- and 90-min time points (mean ± standard error).
‡P < .05 vs. control.
§P < .005 vs. control.
Effects of Gastrectomy on CCK-Induced c-fos Protein Expression in the Nucleus of the Solitary Tract
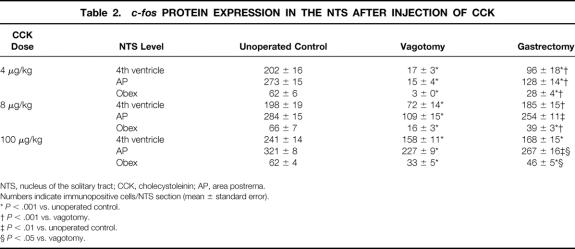
Gastrectomized rats 21 days after surgery expressed significantly fewer c-fos protein-positive cells in the NTS after intraperitoneal injection of CCK at a dose of 4 to 100 μg/kg compared to unoperated control animals (Table 2, Fig. 3). This effect was also observed when gastrectomized and control rats were weight-matched. Weight-matched gastrectomized rats had a significantly reduced c-fos protein expression in the NTS after CCK injection (100 μg/kg) compared to control rats (fourth ventricle: 38% less [173 ± 17 vs. 278 ± 19 cells/section, P < .001]; area postrema: 12% less [288 ± 15 vs. 329 ± 12, P < .01]; obex: 35% less [41 ± 5 vs. 63 ± 4, P < .01]; body weight 273 ± 19 g vs. 274 ± 24 g, NS). However, the pattern of c-fos protein expression in the NTS at the different levels was unchanged.
Table 2. c-fos PROTEIN EXPRESSION IN THE NTS AFTER INJECTION OF CCK
NTS, nucleus of the solitary tract; CCK, cholecystoleinin; AP, area postrema.
Numbers indicate immunopositive cells/NTS section (mean ± standard error).
*P < .001 vs. unoperated control.
†P < .001 vs. vagotomy.
‡P < .01 vs. unoperated control.
§P < .05 vs. vagotomy.
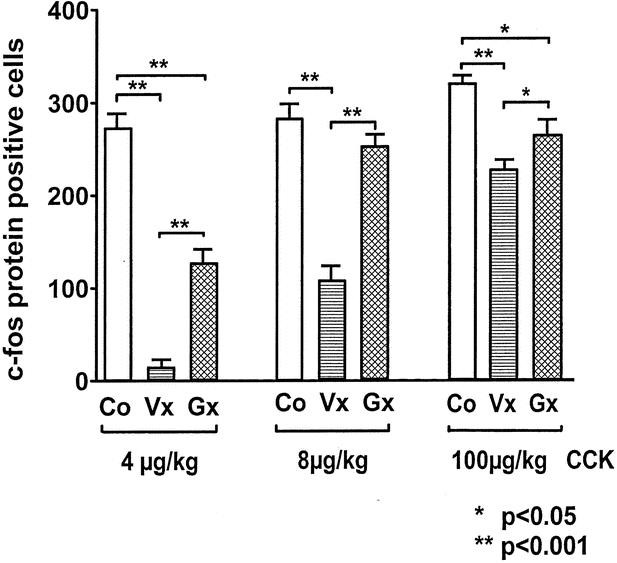
Figure 3. Cholecystokinin-induced c-fos protein expression in the nucleus of the solitary tract at the level of area postrema in unoperated controls (Co), vagotomized animals (Vx), and gastrectomized animals (Gx).
In gastrectomized rats, c-fos protein expression in the NTS after intraperitoneal injection of CCK at a dose of 8 μg/kg significantly increased 3 months and 12 months after gastrectomy compared to 21 days after surgery, representing an increase of 25%, 44%, and 51%, respectively, at 3 months after surgery (Fig. 4). This effect was also observed when gastrectomized and control rats were weight-matched (fourth ventricle: 15% increase [195 ± 8 vs. 224 ± 12 cells/section, P < .05]; area postrema: 23% increase [289 ± 16 vs. 356 ± 12, P < .005]; obex: 32% increase [49 ± 3 vs. 65 ± 8, P < .05]; body weight 367 ± 7 g vs. 373 ± 8 g, NS). c-fos expression was increased by up to 102% in the NTS at the level of the area postrema in gastrectomized rats 12 months after surgery (age 15 months) compared to 15-month-old unoperated control rats (fourth ventricle: 32% increase [232 ± 19 vs. 176 ± 29 cells/section, P < .01; area postrema: 102% increase [366 ± 21 vs. 181 ± 16, P < .0001]; obex: 59 ± 5 vs. 85 ± 11, NS; body weight 462 ± 56 g vs. 609 ± 4 g, P < .001).
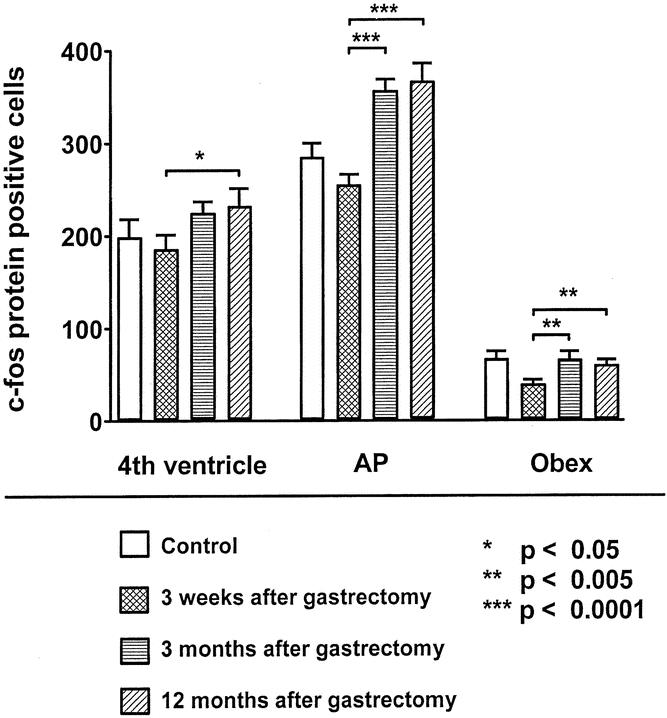
Figure 4. c-fos protein expression in the NTS after intraperitoneal injection of cholecystokinin at a dose of 8 μg/kg 3 weeks (n = 5), 3 months (n = 4), or 12 months (n = 4) after gastrectomy at the level of the fourth ventricle, the area postrema (AP), and the obex.
Intraperitoneal injection of CCK (4–100 μg/kg) dose-dependently increased the number of c-fos protein-positive cells in the NTS in vagotomized rats 21 days after surgery. Vagotomized rats had a significantly reduced c-fos protein expression in the NTS after intraperitoneal CCK injection compared to control rats or gastrectomized rats at all doses investigated (4, 8, and 100 μg/kg). c-fos protein-positive cells in response to CCK injection at a dose of 4 μg/kg were reduced by approximately 90% to 95% at all levels of the NTS compared to control animals and by approximately 80% to 90% at all NTS levels compared to gastrectomized rats (see Table 2, Fig. 5).
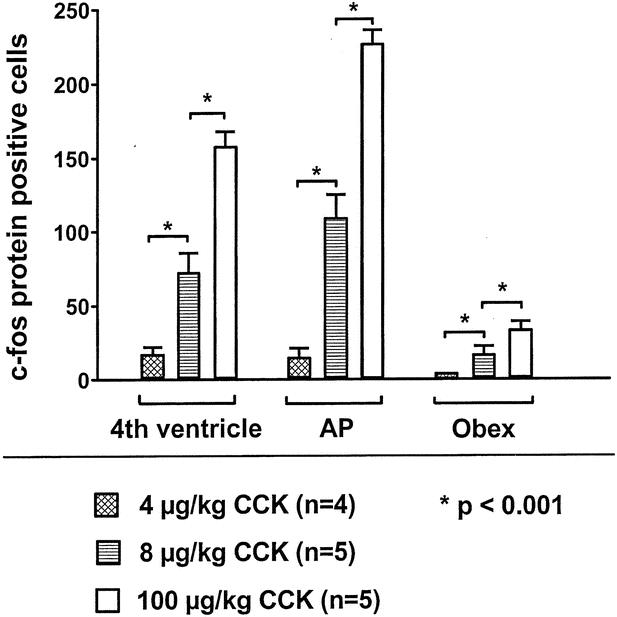
Figure 5. In vagotomized rats, cholecystokinin (CCK) dose-dependently increased the number of c-fos immunopositive cells in the nucleus of the solitary tract at the level of the fourth ventricle, the area postrema (AP), and the obex.
c-fos protein expression after intraperitoneal injection of CCK at a dose of 100 μg/kg was not significantly different between sham-operated and unoperated control animals at all three anatomic levels of the NTS investigated (fourth ventricle: 218 ± 10 vs. 241 ± 14 cells/section; area postrema: 296 ± 14 vs. 321 ± 8 cells/section; obex: 50 ± 4 vs. 62 ± 4 cells/section; NS).
Effects of CCK-A Receptor Blockade on Postprandial c-fos Expression in the Nucleus of the Solitary Tract of Gastrectomized Rats
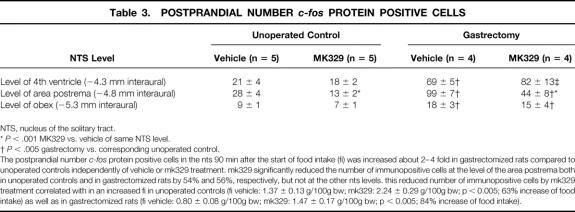
In vehicle-treated gastrectomized rats 21 days after surgery, fasted for 4 hours prior to food intake, the postprandial number of c-fos protein-positive cells was significantly higher than in vehicle-treated unoperated control rats at all NTS levels investigated (Table 3). CCK-A receptor blockade significantly reduced the postprandial number of c-fos protein-positive cells in the NTS at the level of the area postrema in unoperated control rats by 54% and in gastrectomized rats by 56%, while no significant changes could be observed at the level of the fourth ventricle or the obex.
Table 3. POSTPRANDIAL NUMBER c-fos PROTEIN POSITIVE CELLS
NTS, nucleus of the solitary tract.
*P < .001 MK329 vs. vehicle of same NTS level.
†P < .005 gastrectomy vs. corresponding unoperated control.
THE POSTPRANDIAL NUMBER c-fos PROTEIN POSITIVE CELLS IN THE NTS 90 MIN AFTER THE START OF FOOD INTAKE (FI) WAS INCREASED ABOUT 2–4 FOLD IN GASTRECTOMIZED RATS COMPARED TO UNOPERATED CONTROLS INDEPENDENTLY OF VEHICLE OR MK329 TREATMENT. MK329 SIGNIFICANTLY REDUCED THE NUMBER OF IMMUNOPOSITIVE CELLS AT THE LEVEL OF THE AREA POSTREMA BOTH IN UNOPERATED CONTROLS AND IN GASTRECTOMIZED RATS BY 54% AND 56%, RESPECTIVELY, BUT NOT AT THE OTHER NTS LEVELS. THIS REDUCED NUMBER OF IMMUNOPOSITIVE CELLS BY MK329 TREATMENT CORRELATED WITH IN AN INCREASED FI IN UNOPERATED CONTROLS (FI VEHICLE: 1.37 ± 0.13 G/100G BW; MK329: 2.24 ± 0.29 G/100G BW; P < 0.005; 63% INCREASE OF FOOD INTAKE) AS WELL AS IN GASTRECTOMIZED RATS (FI VEHICLE: 0.80 ± 0.08 G/100G BW; MK329: 1.47 ± 0.17 G/100G BW; P < 0.005; 84% INCREASE OF FOOD INTAKE).
Effects of Gastrectomy on Body Weight
Gastrectomized rats had significantly reduced body weights 21 days after surgery compared to preoperative weights (249 ± 11 g vs. 296 ± 5, P < .01). Body weight increased to 373 ± 8 g at 3 months and to 462 ± 56 g at 12 months after surgery. At the age of 15 months, gastrectomized rats had a reduced body weight compared to age-matched unoperated control rats (462 ± 56 vs. 609 ± 4 g, P < .001).
Food Intake in Gastrectomized Rats
Unoperated control rats, fasted for 14 hours with free access to water, ate most during the first 30 minutes of the recording period, with little feeding activity thereafter. Gastrectomized rats ate significantly less during the first 30 minutes compared to unoperated control animals, but continued to feed for 120 minutes (Table 4). Compared to vehicle injection, CCK-A receptor blockade by MK329 significantly increased the cumulative food intake in gastrectomized rats (Fig. 6). In addition, CCK-A receptor blockade increased food intake in gastrectomized rats to approximately the same levels as in unoperated control animals injected with vehicle.
Table 4. CUMULATIVE FOOD INTAKE
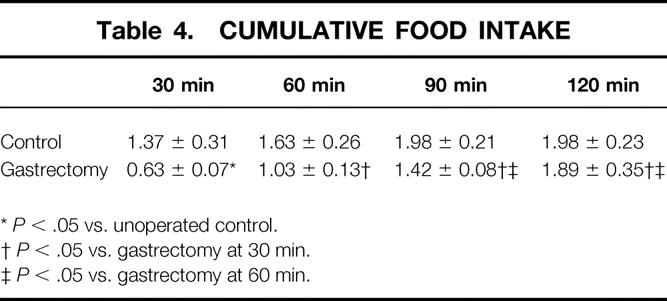
*P < .05 vs. unoperated control.
†P < .05 vs. gastrectomy at 30 min.
‡P < .05 vs. gastrectomy at 60 min.
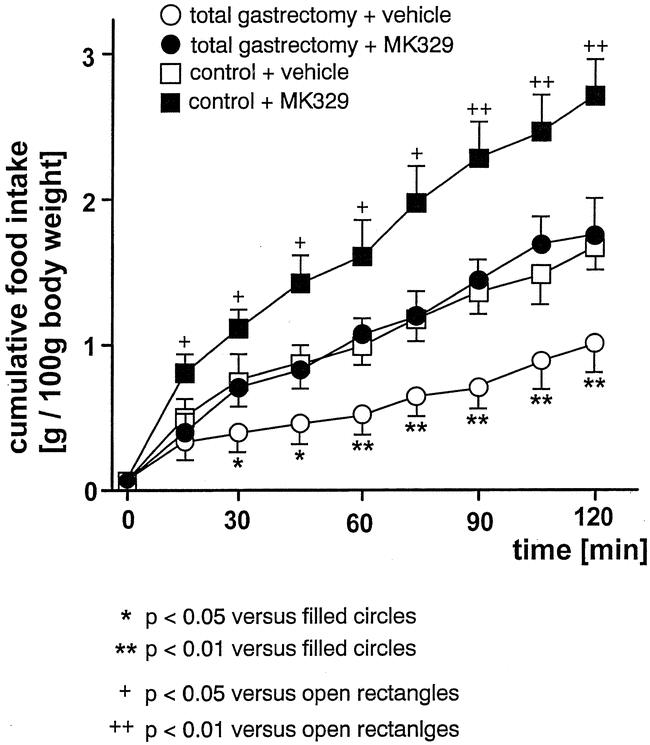
Figure 6. Cumulative food intake (g/100 g body weight) after vehicle injection or CCK-A receptor blockade by MK329 (1 mg/kg). In gastrectomized rats, food intake significantly increased after CCK-A receptor blockade to approximately the same level as in unoperated vehicle-treated controls.
Abdominal Efferent Vagal Neurons in Gastrectomized Rats
In gastrectomized rats 21 days after surgery, only cell bodies of efferent celiac and hepatic vagal neurons were labeled by the retrograde tracer fast blue in the DMN of the brain stem at the level of the area postrema. The ventral and the dorsal branches of the subdiaphragmatic vagal nerve were transected by gastrectomy, as no tracer could be observed in areas of the DMN where its cell bodies are located (Fig. 7).
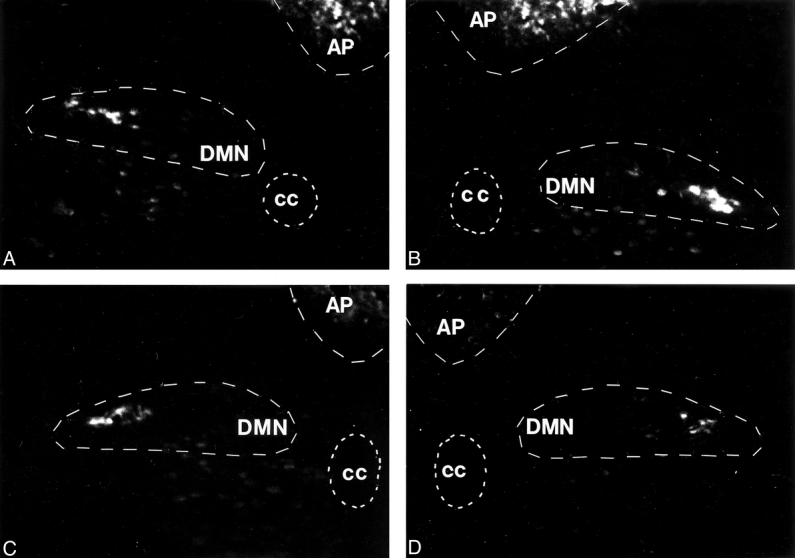
Figure 7. Photomicrographs of the brain stem at the level of the dorsal motor nucleus of the vagus nerve (DMN, −4.8 mm interaural) after retrograde labeling of efferent vagal neurons after total gastrectomy in two rats (A, B: brainstem of rat 1; C, D: brainstem of rat 2). Only cell bodies with intact efferent fibers are labeled in the DMN, as labeling results in a white appearance of cell bodies under the fluorescent microscope. The anatomic distribution of labeled neurons indicated that the celiac and the hepatic branches of the vagus nerve were preserved after total gastrectomy. Areas where the cell bodies of the ventral and the dorsal branches of the vagus nerves are located showed no labeling, indicating severed nerve fibers and corresponding to a transected subdiaphragmal vagal innervation after total gastrectomy. AP, area postrema; cc = central canal.
Area Analysis of Nucleus of the Solitary Tract Sections
No area differences in the NTS sections at the anatomic levels of the obex (interaural about −5.30 mm), the area postrema (interaural about −4.80 mm), and the level of the caudal fourth ventricle (interaural about −4.30 mm) were found between all groups investigated (data not shown).
DISCUSSION
It is well established that feeding and other associated stimuli, such as CCK, activate neurons in the NTS and that this area of the brain stem is in the pathway mediating visceral signals to the “feeding” centers in the hypothalamus. In this study, we have demonstrated for the first time that total gastrectomy results in a markedly altered activation of NTS neurons following a meal. The number of NTS neurons activated was significantly increased in absolute numbers, but most importantly the numbers were also increased relative to the amount of food ingested.
There was a more than fivefold and sustained increase in NTS activation in gastrectomized rats after feeding compared to unoperated controls. This increase was preserved, although to a lesser degree, if gastrectomized rats were compared to unoperated weight-matched controls, indicating that the increase of postprandial neuronal activation in the NTS is not solely an effect of a reduced body weight in gastrectomized rats. We have previously shown a correlation between the amount of food intake and the number of NTS neurons activated in unoperated control rats. 14 Our results suggest that an increased postprandial activation of NTS neurons might represent a neuronal substrate for early satiety, reduced food intake, and eventually body weight loss described after total gastrectomy.
After gastrectomy, the number of NTS neurons activated by CCK increased over time by 25% to 100% compared to unoperated rats of same age, and this effect was again largely preserved if gastrectomized rats were compared to weight-matched unoperated control rats. This finding is particularly important since postprandial CCK levels are increased following gastrectomy. 10,17
Chronic CCK infusions alter the meal pattern in rats by decreasing the meal size but do not alter total food intake. 24 However, CCK-A receptor-deficient rats show an altered feeding pattern and become obese, 25 indicating that CCK-A receptor function does influence body weight. In gastrectomized rats, increased postprandial CCK levels might result in a reduced meal size, but it is unknown whether this alters daily food intake after gastrectomy. CCK-A receptor blockade reduced c-fos protein expression in the NTS and increased food intake in unoperated control rats as well as in gastrectomized rats in this study, and chronic CCK-A receptor blockade increased food intake and body weight after gastrectomy in a previous study. 18 Therefore, it is conceivable that an increased postprandial CCK release might contribute to a reduced body weight after gastrectomy.
Three weeks after gastrectomy, c-fos protein expression in the NTS in response to exogenous CCK was reduced compared to control rats. Possibly, the severing of vagal afferent nerve fibers, which terminate in the NTS and mediate CCK effects on food intake, 26 might have temporarily reduced the stimulatory input of CCK to NTS neurons.
Underweight after total gastrectomy has not gained wide acceptance as a significant clinical problem, although it continues to be present. 2–6,8,12,27–30 Underweight poses risks to patients in various ways. For example, it increases mortality, 31,32 it increases the risk of death from all cardiovascular causes in women, 33 it increases the risk of hip fractures, 34 and it increases the rate of men receiving a disability pension. 35 Further, underweight increases the risk of endocrine underfunction of the pituitary, the thyroid, the gonads, and the adrenals, and underweight may cause the loss of energy and increase susceptibility to injury and infection, as well as being responsible for a distorted body image and psychological problems. 36 Underweight gastrectomized patients suffered significantly more often from symptoms than gastrectomized patients with minor weight loss, 37 and weight loss correlated with a reduced quality of life in gastrectomized patients. 28 Gastrectomized patients who were permanently out of work had a significantly lower body mass index than those that returned to work. 8
Under physiologic conditions, the stomach empties food into the small intestine in a fine-tuned way, mainly depending on the calorie load of the stomach. 38 Gastric emptying is regulated by afferent nerve fibers and CCK, 39 resulting in the release of food into the small intestine adapted to the absorptive capacity of the small intestine. 38 After total gastrectomy, food enters the upper small intestine immediately, where it activates signal pathways to the brain that initiate satiation and inhibit food intake. For example, CCK is released prematurely and at a greater rate after gastrectomy, 10,17 while the inhibitory effects on food intake are preserved after total gastrectomy. 18 Since the number of activated NTS neurons in response to CCK or food is increased after total gastrectomy, we would assume that the food-stimulated neuroendocrine response of the proximal small intestine is decisive for the early initiation of satiety after gastrectomy.
Subdiaphragmal vagotomy resulted in a reduced number of activated NTS neurons in response to CCK. In contrast, after total gastrectomy, which included a subdiaphragmal vagotomy, significantly more NTS neurons were activated in response to CCK. By labeling efferent vagal neurons in the DMN after total gastrectomy, we could show that celiac and hepatic vagal nerve fibers were preserved after total gastrectomy. Either the remaining vagal fibers signal at an increased rate to the NTS, or additional extravagal pathways to the NTS are activated after gastrectomy, possible candidates being spinal afferent nerve fibers. These nerve fibers follow the blood supply and were not transected by gastrectomy. Increased electrical activity of spinal mesenteric nerve fibers after intravenous injection of CCK or after perfusion of the gut lumen with nutrients has been demonstrated previously, 40,41 and spinal pathways are in synaptic contact with NTS neurons. 42 Possibly, a rearrangement of visceral afferent pathways results in the changes of food intake and NTS neuron activation that we observed after total gastrectomy.
Of those neurons stimulated in the NTS by feeding, about 50% were activated via the CCK-A receptor, as CCK-A receptor blockade reduced the number of activated NTS neurons about 50%. This is in agreement with a recent study showing a 50% decrease of NTS neurons activated by feeding in CCK-A receptor-deficient rats. 19 The remaining NTS neurons postprandially might be activated by other gastrointestinal hormones such as serotonin or glucagon that are released postprandially and inhibit food intake.
About two thirds of patients suffer from underweight after total gastrectomy. 3,6,28 This is not due to residual cancer, since all studies investigating food intake or body weight after gastrectomy have excluded patients not cured from cancer. Based on food intake, patients can be divided into those with high calorie intake (≥30 kcal/kg body weight) and those with low calorie intake (<30 kcal/kg body weight). It has been shown that those with low calorie intake experience early satiety more often (76% vs. 50%), 28 report a higher severity of early satiety, 28 and lose more weight postoperatively. 29,43 These data indicate that early satiety is likely to contribute to a reduced daily calorie intake and weight loss. About 60% of gastrectomized patients do not reach the recommended daily calorie intake, 3,28 which is about 40 kcal/kg body weight. 44 In contrast, two studies reported a normal daily calorie intake after total gastrectomy, but patients remained considerably underweight. 45,46 Possibly, a reduced efficiency of the intestinal tract to utilize food requires an increased daily calorie intake in gastrectomized patients to achieve a normal body weight. 11
In the past, it has been proposed that a lack of reservoir is responsible for reduced food intake after gastrectomy. 2 However, the small intestine could be viewed as a much larger reservoir for food than the stomach. Further, creating an intestinal reservoir after total gastrectomy, a so-called pouch reconstruction, did not increase postoperative food intake and body weight 3,30,47,48 and did not improve symptoms of eating dysfunction after gastrectomy. 30 Therefore, based on the data presented, not a lack of reservoir but rather early satiety due to a disturbed central regulation of food intake should be considered as a key factor for reduced food intake and body weight loss after total gastrectomy.
In summary, our study suggests that severe alterations of the central regulation of food intake exist after total gastrectomy. Early satiety and the disruption of a regular pattern of feeding and nonfeeding periods are likely to contribute to a reduced food intake and weight loss after total gastrectomy. As the feeding system is characterized by redundancy and adaptability, 37,49,50 the organism can adapt after gastrectomy to a certain extent, but probably at the expense of a reduced quality of life. 28,30,51,52 Rather than looking for a perfect reconstruction method, it might be worth looking at pathways that regulate food intake after total gastrectomy. The manipulation of these pathways by defined receptor agonist or antagonist treatments might be an alternative way to increase body weight after total gastrectomy, 18,43,53 possibly alleviating the sequelae of total gastrectomy for these patients.
Footnotes
Correspondence: Tilman T. Zittel, MD, University Hospital, Department of General and Transplantation Surgery, Hoppe-Seyler-Str. 3, 72076 Tübingen, Germany.
E-mail: tilman.zittel@med.uni-tubingen.de
Supported by a grant of the Deutsche Forschungsgemeinschaft, Bonn, Germany (Zi 415/2–1).
Accepted for publication December 17, 2001.
References
- 1.Alexander HR, Kelsen DP, Tepper JE. Cancer of the stomach. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer. Principles and Practice of Oncology, 5th ed. Philadelphia: Lippincott-Raven; 1997: 1021–1054.
- 2.Becker HD, Caspary WF. Postgastrectomy and Postvagotomy Syndromes. New York: Springer-Verlag; 1980: 51–162.
- 3.Bradley EL, Isaacs JT, Hersh T, et al. Nutritional consequences of total gastrectomy. Ann Surg 1975; 182: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristallo M, Braga M, Agape D, et al. Nutritional status, function of the small intestine and jejunal morphology after total gastrectomy for carcinoma of the stomach. Surg Gynecol Obstet 1986; 163: 225–230. [PubMed] [Google Scholar]
- 5.Zelnick R, Auguste L-J, Wise L. Nutritional effects of postgastrectomy reconstruction: a clinical evaluation. J Surg Oncol 1989; 40: 219–222. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini CA, Deveney CW, Patti MG, et al. Intestinal transit of food after total gastrectomy and Roux-Y esophagojejunostomy. Am J Surg 1986; 151: 117–124. [DOI] [PubMed] [Google Scholar]
- 7.Lowry SF. Nutrition in the surgical patient. In: Schwartz SL, Shires GT, Spencer FC, Storer EH, eds. Principles of Surgery, 4th ed. Singapore: McGraw-Hill; 1985: 68–70.
- 8.Miholic J, Meyer HJ, Müller MJ, et al. Nutritional consequences of total gastrectomy: The relationship between mode of reconstruction, postprandial symptoms and body composition. Surgery 1990; 108: 488–494. [PubMed] [Google Scholar]
- 9.Bradley EL, Isaacs JT, Del Mazo J, et al. Pathophysiology and significance of malabsorption after Roux-en-Y reconstruction. Surgery 1977; 81: 684–690. [PubMed] [Google Scholar]
- 10.Friess H, Bohm J, Muller MW, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol 1996; 91: 341–347. [PubMed] [Google Scholar]
- 11.Maier GW, Zittel TT, Becker HD. Reduced food intake and impaired food utilization result in weight loss after total gastrectomy in pigs. Dig Surg 1996; 13: 19–25. [Google Scholar]
- 12.Braga M, Zuliani W, Foppa L, et al. Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg 1988; 75: 477–480. [PubMed] [Google Scholar]
- 13.Smith GP. Satiation: From Gut to Brain. Oxford: Oxford University Press; 1998.
- 14.Zittel TT, Glatzle J, Kreis ME, et al. C-fos protein expression in the nucleus of the solitary tract correlates with cholecystokinin dose injected and food intake in rats. Brain Res 1999; 846: 1–11. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Schwaber JS. Abolition of the behavioral effects of cholecystokinin following bilateral radiofrequency lesions of the parvocellular subdivions of the nucleus of the solitary tract. Brain Res 1984; 295: 289–299. [DOI] [PubMed] [Google Scholar]
- 16.Edwards GL, Ladenheim EE, Ritter RC. Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am J Physiol 1986; 251: R971–R977. [DOI] [PubMed] [Google Scholar]
- 17.Büchler M, Malfertheimer P, Friess H, et al. Cholecystokinin influences pancreatic trophism following total gastrectomy in rats. Int J Pancreatol 1989; 4: 261–271. [DOI] [PubMed] [Google Scholar]
- 18.Zittel TT, Von Elm B, Teichmann RK, et al. Cholecystokinin is partly responsible for reduced food intake and body weight after total gastrectomy in rats. Am J Surg 1995; 169: 265–270. [DOI] [PubMed] [Google Scholar]
- 19.Glatzle J, Kreis ME, Kawano K, et al. Postprandial neuronal activation in the nucleus of the solitary tract is partly mediated by CCK-A receptors in rats. Am J Physiol 2001; 281: R222–R229. [DOI] [PubMed] [Google Scholar]
- 20.Voigt J-P, Huston JP, Voits M, et al. Effects of cholecystokinin octapeptide (CCK-8) on food intake in adult and aged rats under different feeding conditions. Peptides 1996; 17: 1313–1315. [DOI] [PubMed] [Google Scholar]
- 21.Voits M, Forster S, Rodel S, et al. Obesity induced by unspecific early postnatal overfeeding in male and female rats: hypophagic effects of CCK-8S. Naunyn Schmiedebergs Arch Pharmacol 1996; 354: 374–378. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th ed. San Diego: Academic Press; 1998.
- 23.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol 1987; 253: R361–370. [DOI] [PubMed] [Google Scholar]
- 24.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol 1984; 246: R776–R787. [DOI] [PubMed] [Google Scholar]
- 25.Moran TH, Katz LF, Plata-Salaman CR, et al. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol 1998; 274: R618–R625. [DOI] [PubMed] [Google Scholar]
- 26.Garlicki J, Konturek PK, Majka J, et al. Cholecystokinin receptors and vagal nerves in control of food intake in rats. Am J Physiol 1990; 258: E40–E45. [DOI] [PubMed] [Google Scholar]
- 27.Zittel TT, Zeeb B, Maier GW, et al. High prevalence of bone disorders after gastrectomy. Am J Surg 1997; 174: 431–438. [DOI] [PubMed] [Google Scholar]
- 28.Brägelmann R, Armbrecht U, Rosemeyer D, et al. Nutrient malassimilation following total gastrectomy. Scand J Gastroenterol 1996; 31 (Suppl 218): 26–33. [DOI] [PubMed] [Google Scholar]
- 29.Bae J-M, Park J-W, Yang H-K, et al. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg 1998; 22: 254–261. [DOI] [PubMed] [Google Scholar]
- 30.Svedlund J, Sullivan M, Liedman B, et al. Long-term consequences of gastrectomy for patients’ quality of life: the impact of reconstructive techniques. Am J Gastroenterol 1999; 94: 438–445. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds MW, Fredman L, Langenberg P, et al. Weight, weight change, mortality in a random-sample of older community-dwelling women. J Am Geriatr Soc 1999; 47: 1409–1414. [DOI] [PubMed] [Google Scholar]
- 32.Visscher TL, Seidell JC, Menotti A, et al. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: the Seven Countries study. Am J Epidemiol 2000; 151: 660–666. [DOI] [PubMed] [Google Scholar]
- 33.Selmer R, Tverdal A. Body mass index and cardiovascular mortality at different levels of blood pressure: a prospective study of Norwegian men and women. J Epidemiol Community Health 1995; 49: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cumming RG, Klineberg RJ. Case-control study of risk factors for hip fractures in the elderly. Am J Epidemiol 1994; 139: 493–503. [DOI] [PubMed] [Google Scholar]
- 35.Mansson NO, Eriksson KF, Israelsson B, et al. Body mass index and disability pension in middle-aged men—non-linear relations. Int J Epidemiol 1996; 25: 80–85. [DOI] [PubMed] [Google Scholar]
- 36.Mahan LK, Escott-Stump S. Weight management and eating disorders. In: Mahan LK, Escott-Stump S, eds. Food, Nutrition, Diet and Therapy, 9th ed. Philadelphia: WB Saunders; 1996: 451–488.
- 37.Jentschura D, Winkler M, Strohmeier N, et al. Quality-of-life after curative surgery for gastric cancer: a comparison between total gastrectomy and subtotal gastric resection. Hepato-Gastroenterology 1997; 44: 1137–1142. [PubMed] [Google Scholar]
- 38.Stricker EM, McCann MJ. Visceral factors in the control of food intake. Brain Res Bull 1985; 14: 687–692. [DOI] [PubMed] [Google Scholar]
- 39.Raybould HE, Zittel TT, Hölzer HH, et al. Gastroduodenal sensory mechanisms and CCK in inhibition of gastric emptying in response to a meal. Dig Dis Sci 1994; 39: 41S–43S. [DOI] [PubMed] [Google Scholar]
- 40.Kreis ME, Zittel TT, Raybould HE, et al. Prolonged intestinal afferent nerve discharge in response to cholecystokinin 58 compared to cholecystokinin 8 in rats. Neurosci Lett 1997; 230: 89–92. [DOI] [PubMed] [Google Scholar]
- 41.Sharma KN, Nasset ES. Electrical activity in mesenteric nerve after perfusion of gut lumen. Am J Physiol 1962; 202: 725–730. [DOI] [PubMed] [Google Scholar]
- 42.Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol 1996; 42: 29–51. [DOI] [PubMed] [Google Scholar]
- 43.Sategna-Guidetti C, Bianco L. Malnutrition and malabsorption after total gastrectomy. J Clin Gastroenterol 1989; 11: 518–524. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler JG. Ernährungsverhalten und Diät nach Gastrektomie. In: Armbrecht U, Stockbrügger RW, eds. Der operierte Patient-interdisziplinär. Der gastrektomierte Patient, Vol. 2. Basel: Karger; 1992: 79–89.
- 45.Curran FT, Hill GL. Failure of nutritional recovery after total gastrectomy. Br J Surg 1990; 77: 1015–1017. [DOI] [PubMed] [Google Scholar]
- 46.Bisballe S, Buus S, Lund B, et al. Food intake and nutritional status after gastrectomy. Hum Nutr Clin Nutr 1986; 40C: 301–308. [PubMed] [Google Scholar]
- 47.Auguste LJ, Mavor E, Citrin P, et al. Nutritional effects of postgastrectomy reconstructions. Am J Surg 1985; 150: 537–542. [DOI] [PubMed] [Google Scholar]
- 48.Nakane Y, Akehira K, Okumura S, et al. Jejunal pouch and interposition reconstruction after total gastrectomy for cancer. Surg Today 1997; 27: 696–701. [DOI] [PubMed] [Google Scholar]
- 49.Deutsch JA. The stomach in food satiation and the regulation of appetite. Prog Neurobiol 1978; 10: 135–153. [DOI] [PubMed] [Google Scholar]
- 50.Novin D. The integration of visceral information in the control of feeding. J Auton Nerv Syst 1983; 9: 233–246. [DOI] [PubMed] [Google Scholar]
- 51.Buhl K, Lehnert T, Schlag P, et al. Reconstruction after gastrectomy and quality of life. World J Surg 1995; 19: 558–564. [DOI] [PubMed] [Google Scholar]
- 52.Thybusch-Bernhardt A, Schmidt C, Küchler T, et al. Quality of life following radical surgical treatment of gastric carcinoma. World J Surg 1999; 23: 503–508. [DOI] [PubMed] [Google Scholar]
- 53.Zittel TT, Glatzle J, Weimer T, et al. Influence of serotonin receptor blockade on food intake and body weight after total gastrectomy in rats. J Surg Res (in press). [DOI] [PubMed]