Abstract
Objective
To prospectively evaluate the use of 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) in the initial staging of squamous cell head and neck carcinoma.
Summary Background Data
The status of cervical lymph nodes is an important prognostic factor and determinant of management approach in squamous cell head and neck cancer.
Methods
FDG-PET findings were compared with those of computed tomography (CT) before removal of the primary tumor and/or neck dissection. Histopathologic analysis was used as the gold standard for assessment of the sensitivity and specificity of these modalities.
Results
FDG-PET correctly identified the primary tumor in 35 of 40 patients in whom the site of the primary was known clinically and still present (sensitivity 88%). None of four unknown primaries were detected. Tumors not detected by FDG-PET were generally superficial, with depths of less than 4 mm. CT correctly identified 18 of the 35 primary tumors (sensitivity 51%). Eleven of 17 CT false-negative tumors were detected by FDG-PET. The sensitivity and specificity for the presence of metastatic neck disease on FDG-PET were 82% and 100%, respectively; those for CT were 81% and 81%, respectively. FDG-PET was true positive for metastatic neck disease in two of the three CT false-negative patients.
Conclusions
FDG-PET shows promise in the initial staging of head and neck cancer and provides additional accuracy to a conventional staging process using CT.
The presence or absence of cervical malignant adenopathy is the single most important prognostic indicator, along with tumor site and size, in patients with squamous cell carcinoma of the head and neck. 1 Consequently, these features are important determinants of optimal management.
Present noninvasive staging techniques include clinical examination, x-ray computed tomography (CT), and magnetic resonance imaging (MRI). Criteria used in the interpretation of CT and MRI in staging lymph nodes include size of concerned lymph nodes, presence of central lucency reflecting central necrosis, presence of irregular enhancement with a rim of enhancement, indistinct nodal margins, and obliteration of fat or tissue planes. 1–4 The calculated sensitivity of CT and MRI for detecting lymph node metastases ranges from 36% to 94%, respectively, while specificity has ranged from 50% to 98%. 5 Additional staging information can be obtained with ultrasound-guided biopsy of nodes that are borderline for malignant involvement based on criteria used by CT or MRI, but this may be impractical in some cases because of the number of nodes in question. At present, neck dissection with histologic examination is the most reliable staging procedure, providing important prognostic information. There is a need for a noninvasive procedure that provides high-quality prognostic information approaching this gold standard.
18F-Fluorodeoxyglucose (FDG) is a positron-emitting glucose analogue. It competes with glucose for both facilitated transport into cells and phosphorylation intracellularly by hexokinase. Unlike glucose, FDG is not metabolized further in tissues, except in those, such as the liver, that contain glucose-6-phosphatase. Elsewhere, and in tumor tissue, the radiopharmaceutical is trapped in its phosphorylated form in proportion to the rate of glycolysis. Uptake is therefore a reflection of the tissue glucose metabolic rate and can be used to distinguish metabolically active tumor from normal tissues.
18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) has been successfully applied to the evaluation of several different cancer types, including breast, lung, colon, and brain, complementing or simply demonstrating greater accuracy than anatomic imaging modalities. 5,6 Also, there are an increasing number of studies of FDG-PET in head and neck cancer; although the methodology varies, very promising accuracy statistics are emerging. However, data on the accuracy of FDG-PET in comparison to histopathology have been preliminary to date. We present our experience with FDG-PET in the staging of head and neck cancer, with comparison to the definitive gold standard of surgical pathology.
MATERIALS AND METHODS
A total of 48 patients were prospectively recruited into this study (34 men, 14 women; average age 61, range 26–92). Inclusion criteria were a clinical diagnosis of squamous cell head and neck cancer, no prior radiotherapy to the head and neck region, no surgery to the neck region, and that patients were planned for dissection or biopsy of the primary site, if known and if still present, with or without neck dissection. The evaluation of these patients with FDG-PET was performed as part of a PET oncology protocol approved by the Human Ethics Committee of our institution. All management was planned on the basis of the usual decision-making strategy of the patient’s respective head and neck surgical team, and independent of the result of the FDG-PET study.
PET scanning was performed on a Siemens 951/31R, ECAT scanner (Siemens, Hoffman Estates, IL; CTI, Knoxville, TN). The spatial resolution of this PET system in the axial plane is 6.5 mm. A regional body scan was performed from the level of the orbit to that of the superior mediastinum. Patients abstained from food and drink for 4 hours before injection of [18F]-FDG and gave consent for the procedure. Pre-emission or postemission transmission images were obtained in all patients for 10 minutes per bed position. Emission scans were performed 45 minutes after administration of approximately 400 MBq [18F]-FDG given intravenously. Emission scans were acquired for 10 minutes per bed position. Three bed positions were generally required to cover the area of interest. During the uptake phase the patient lay quietly. The patient was not moved significantly during the whole acquisition period. Both the transmission and emission scans were reconstructed using a 128 × 128-pixel matrix, Hanning filter with a cutoff frequency of 0.4 cycles/pixel, and a zoom factor of 1.5. Decay correction was performed. Final images were reconstructed using transmission data for attenuation correction of the emission scan.
Because of the variety of referral sources to the surgeons concerned, CT scans were performed at various institutions and with slight variations in protocol, but in general contrast-enhanced studies were performed and both soft tissue and bony windows reconstructed, with or without coronal reconstruction in a small number of selected cases. Of the 48 patients included in the study, CT scans were available in 40 for analysis in the study.
Image Analysis
For correlative analysis of the primary site between FDG-PET, CT, and the histopathological reference standard, the upper aerodigestive tract was divided into seven regions: oral cavity, nasopharynx, oropharynx, hypopharynx, supraglottis, glottis, and subglottis. For correlative analysis of nodal staging, the neck was divided into ten levels (five bilaterally) encompassing surgically accessible regional lymph node groups. These were numbered IR to VR on the right and IL to VL on the left (Fig. 1). The surgical specimens were referenced to this schema in terms of the position of malignant histologic foci and normal lymph nodes. Likewise, FDG-PET and CT were interpreted within this schema.
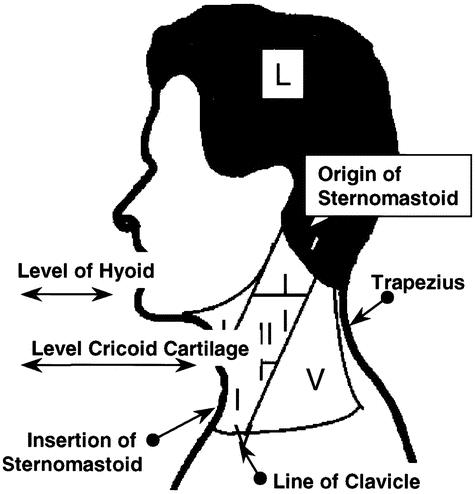
Figure 1. Schema for the location of abnormality when interpreting FDG-PET, CT, and histopathology. Levels are represented bilaterally and abbreviated as IR: level I on the right, IL: level I on the left, etc. They are defined as follows: Level I: (the anterior triangle) bounded by the anterior boarder of sternocleidomastoid, the inferior boarder of the mandible, and the midline anteriorly; level V (the posterior triangle) bounded by the posterior border of sternocleidomastoid, the superior boarder of the clavicle, the superior boarder of trapezius, and the midline posteriorly; and levels II, III, and IV, in relation to the upper, middle, and lower thirds of sternocleidomastoid. Landmarks for separating levels II, III, and IV are the level of the hyoid bone, between the upper and middle third and the level of the cricoid cartilage between the middle and lower thirds of sternocleidomastoid.
PET images were interpreted by two nuclear medicine physicians without knowledge of clinical details and other staging data, such as CT appearance. Both were experienced in the analysis of these studies, and disagreements were resolved by consensus. Abnormal FDG uptake was graded in comparison to background and blood pool activity (grade 0 = background activity, grade 1 = blood pool activity, grade 2 > blood pool activity, and grade 3 ≫ blood pool activity). Each focus of activity that was greater than or equal to grade 1 was assigned a probability of tumor involvement based on our experience in the interpretation of these studies, on a scale of 1 to 5 (1, tumor definitely not present; 2, tumor probably not present; 3, equivocal; 4, tumor probably present; 5, tumor definitely present). In the analysis of this data, a probability of 4 or 5 was regarded as evidence of tumor involvement.
CT was interpreted in a blinded fashion, similar to FDG-PET, by pairs of radiologists with experience in this field. Disagreement was resolved by consensus. Factors considered in the interpretation of nodal pathology or normality were size of nodes concerned (pathologic when >1 cm maximal axial diameter for all nodes except the jugulodigastric node, which was considered pathologic when >1.5 cm maximal axial diameter) and the presence of central lucency with or without irregular enhancement. The probability of carcinoma being present in each region was assigned on a scale of 1 to 5, using the same category definitions as for FDG-PET and with values of 4 or 5 being considered as representing tumor for the purposes of analysis. The readers were given the opportunity to exclude studies if they were felt to be of unsatisfactory quality for valid interpretation. This is of particular relevance with the varied sources of CT data used in the study. All available CT studies were included.
Histology
Primary tumor and neck dissection specimens were labeled by the surgeon in such a way that reference could be made to the schema used in the interpretation of the FDG-PET and CT studies. Lymph nodes and tumors were dissected from the specimens and stained with hematoxylin and eosin for histologic analysis. Examination was performed by an experienced anatomical pathologist. The total number of lymph nodes present in the specimen and the presence or absence of tumor within these and at the primary site was also recorded. This information was used as a reference standard for comparison with the imaging modalities.
RESULTS
All 48 patients underwent FDG-PET. In all patients, tumor histology was squamous cell carcinoma (41 oral cavity tumors, 4 of which had been removed before the PET study; 1 oropharynx, 1 supraglottic, 1 hypopharynx, and 4 unknown site on the basis of clinical examination, CT, and panendoscopy). Following the FDG-PET study 37 patients subsequently underwent neck dissection with primary resection and 4 patients underwent neck dissection only, while the remainder underwent primary resection only (n = 6) or biopsy of the primary site (n = 1).
CT was available in 40 of the 48 patients. Valid comparison between CT and FDG-PET for detection of the primary tumor could be performed in 38 (32 patients with oral cavity primaries in situ, 1 hypopharynx, 1 supraglottic, and the 4 patients with unknown primary sites. One patient had an oral cavity primary resected before CT. One patient whose oral cavity primary was resected between the times of the CT scan and the PET scan was included in the group for CT/PET comparison of metastatic disease detection, but not for detection of primary tumor). Thirty-three of the CT group underwent neck dissection.
In the 41 patients who underwent neck dissection, a total of 192 neck levels (882 nodes) were dissected, 43 of which (84 nodes) contained metastatic disease. A total of 23 of the 41 patients demonstrated metastatic neck disease in the levels dissected. Thirteen of the 23 patients had more than one level involved histologically (mean number of levels involved per patient was 0.98 ± 1.2).
Primary Tumors
FDG-PET correctly identified the primary tumor in 35 of the 40 patients in whom the site of the primary was known clinically and still present (Figs. 2 and 3). None of the four unknown primaries was detected. There were therefore five false-negative studies for detection of primary tumor (sensitivity of 88% for detection of known primary tumors). The histopathological dimensions (surface dimensions times depth) and locations of those primary tumors not detected on FDG-PET were as follows: 0.9 × 0.8 × 0.3 cm (patient 17, lateral tongue), 1.5 × 1.2 × 0.5 cm (patient 18, retromolar trigone), 2.8 × 2.0 × 0.2 cm (patient 22, lateral border of tongue), and 1.3 × 0.8 × 0.3 cm (patient 37, floor of mouth). One 15-mm-diameter glossal tumor had been partially removed at biopsy and its depth of invasion was uncertain. None of these PET false-negative primary tumors was identified on CT.
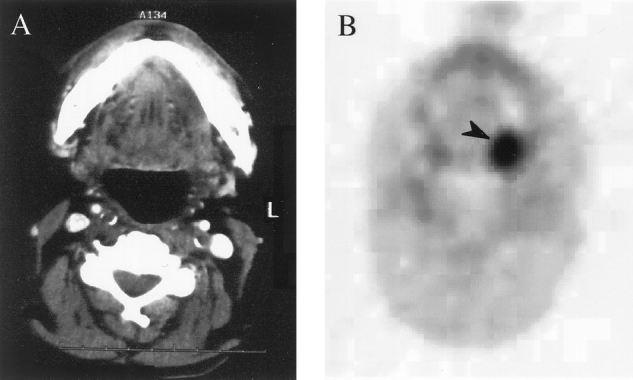
Figure 2. (A) CT scan of patient 25, which is negative for a primary left posterior glossal tumor. (B) FDG-PET transaxial scan showing the glossal primary tumor (black arrow). Pathology from surgery showed a 1.4 × 1.2 × 1.0-cm tumor at this site.
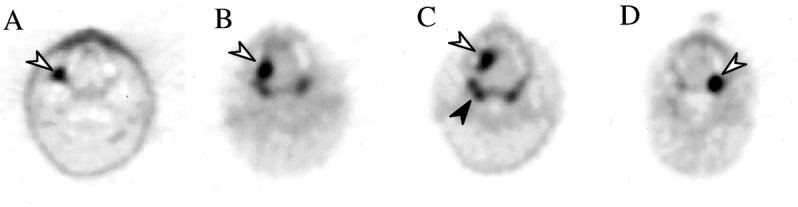
Figure 3. FDG-PET scans of primary tumors that were negative on CT scan. (A) Patient 1, with a right retromolar trigone oral cavity tumor (arrow). (B) Patient 33, with right-sided tongue tumor. (C) Patient 39, with a right anterior tongue tumor. (D) Patient 25, left oral cavity tumor. Normal FDG uptake in Waldeyer’s ring is evident (black arrow in C).
CT correctly identified 18 of the 35 known or present primary tumors (sensitivity of 51%). All of the 17 primary tumor sites confirmed on histology in which CT was negative were oral cavity tumors. Of these 17 tumors, 11 were detected by FDG-PET (see Fig. 3). In one of these patients, CT was falsely positive for primary disease in the vallecular region and falsely negative for the actual primary on the tongue. Artifact on the CT caused by dental metallic filling material obscured the probable site of primary tumor in 7 of the 17 false-negative studies.
Metastatic Neck Disease
On a level-by-level basis, FDG-PET detected sites of disease accurately in 26 of 43 levels (60.5%) (Figs. 4 and 5). PET was falsely positive in five levels in five patients. Inaccuracies could be divided into two types: those relating to location and those relating to lesion detectability. Spatial inaccuracy of PET contributed to nine false-negative levels and all five false-positive levels and explained inaccuracy in eight patients. In four patients the presence of metastatic neck disease was missed on FDG-PET, so that on the basis of detection of metastatic neck disease rather than on a level-by-level basis, PET was truly positive in 19 of 23 patients (sensitivity for the presence of metastatic neck disease on FDG-PET = 82%). One patient (patient 28) was assigned as having neck metastases on the basis of FDG-PET and did not have such histopathologically (specificity for the presence of metastatic neck disease on FDG-PET = 94%). This patient had a large primary that appeared to extend into the adjacent level I on FDG-PET.
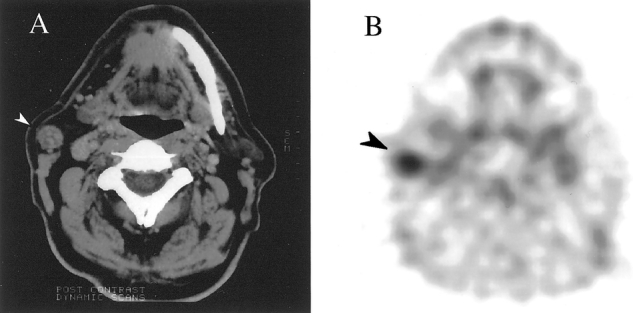
Figure 4. (A) CT scan showing an enlarged lymph node in the right neck (white arrow). (B) Transverse FDG-PET scan showing abnormal FDG uptake in the node in the right neck (black arrow). This node was removed at surgery and was found to contain metastatic squamous cell carcinoma.
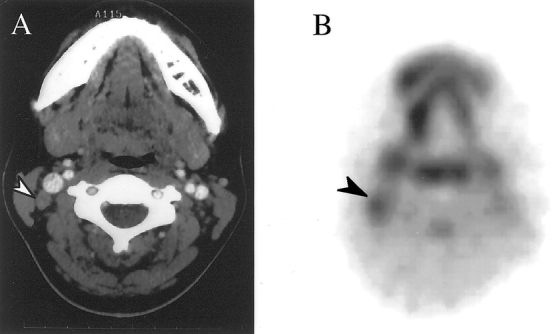
Figure 5. (A) CT scan showing an equivocal lymph node in the right neck (white arrow). (B) Transverse FDG-PET scan showing abnormal FDG uptake in the node in the right neck (black arrow). This node was removed at surgery and was found to contain metastatic squamous cell carcinoma.
Failure of lesion detectability contributed to eight false-negative levels in five patients. In the first patient, although a lymph node within the submandibular gland and two nearby lymph nodes were involved with tumor, the majority of each was occupied by cystic degeneration, surrounded by an intermittent rim of viable tumor whose maximum thickness was only 2.7 mm. In the second patient, while involved nodes measuring 17 mm and 8 mm in maximal dimension and almost replaced by tumor were demonstrated by PET, two other nodes were only 5 mm and approximately 10 mm in maximum dimension, and only 50% of each was occupied by viable tumor. These two nodes were not detected. In the third patient a single involved node measured 15 mm in maximum dimension, but only 30% of the node was involved with viable tumor. In the fourth patient a large, 40 × 40 mm mass, packed with apparently viable tumor histologically, was not seen on FDG-PET, but considerable reconstruction artifact obscured the region concerned. In the fifth patient, findings on FDG-PET were equivocal for the presence of disease (activity slightly greater than blood pool) in the level where this was demonstrated histologically. The involved lymph nodes in this case measured 10.7 mm and 4.5 mm and were largely replaced by viable tumor. These last two patients were the only false-negative cases unexplained by the spatial resolution limitations of the PET system.
In the subgroup of 33 patients who underwent neck dissection and in whom CT was available for analysis, a total of 154 levels were dissected (692 nodes), of which 26 levels (55 nodes) were involved with metastatic disease in a total of 16 patients. On a level-by-level basis, CT detected sites of metastatic disease accurately in 16 of 26 levels (61.5%). CT was falsely positive in seven levels in six patients. Spatial inaccuracy (i.e., relating involved nodes to an incorrect level) contributed less to CT inaccuracy than it did to FDG-PET inaccuracy, as would be expected. Two false-negative and two corresponding false-positive levels were attributable to spatial inaccuracy. The remaining false-negative and false-positive levels related to failure of criteria used to differentiate involved from uninvolved nodes (see Fig. 5).
CT failed to detect any metastatic neck disease in three patients where disease was present. One of these was also negative on FDG-PET; disease was detected by FDG-PET in both of the other patients. Of the four patients where FDG-PET was falsely negative for metastatic neck disease, CT was truly positive in two owing to nodal size and presence of central cavitation respectively (patients 14 and 36, respectively). In another, CT was also falsely negative (patient 39), and in the fourth CT was not included in the study. CT assigned metastatic neck disease to four patients where none was present histopathologically. These data yield a sensitivity for CT of 81% (13/16 patients) and a specificity of 81% for detection of metastatic neck disease.
FDG-PET demonstrated a greater number of levels involved than CT in two patients; conversely, CT demonstrated a greater number of levels than did FDG-PET in four patients.
Results are summarized on a patient-by-patient basis in Table 1.
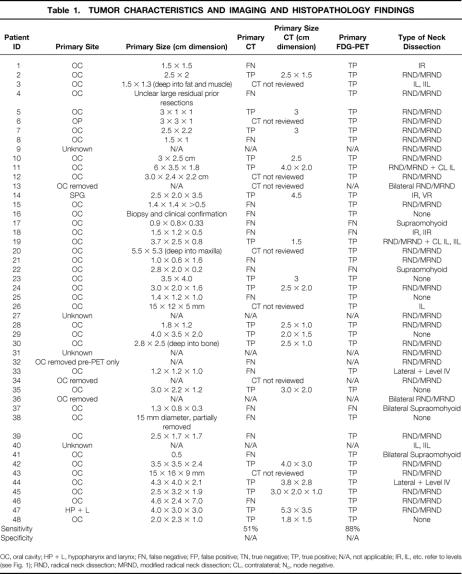
Table 1. TUMOR CHARACTERISTICS AND IMAGING AND HISTOPATHOLOGY FINDINGS
OC, oral cavity; HP + L, hypopharynx and larynx; FN, false negative; FP, false positive; TN, true negative; TP, true positive; N/A, not applicable; IR, IL, etc. refer to levels (see Fig. 1); RND, radical neck dissection; MRND, modified radical neck dissection; CL, contralateral; No, node negative.
Table 1. Continued
DISCUSSION
Although there are now considerable data in the literature regarding the utility of FDG-PET in the setting of de novo head and neck cancer, to our knowledge this study is the most comprehensive yet reported evaluating FDG-PET in patients with head and neck cancer compared to a histologic gold standard.
It is evident from the literature that FDG-PET is very sensitive in detecting primary tumors in the head and neck, and this is further supported by our data, at least with respect to oral cavity tumors. Specificity is also high, as reflected by the absence of false-positive results for primary tumor. Our data demonstrate a sensitivity of 88% for FDG-PET in the detection of known primary tumors and 51% for CT in this setting. FDG-PET accurately located 11 of 17 CT false-negative primaries. Very superficial tumors were not located by either modality. Tumors of unknown primary were not detected, but this is in keeping with the findings of other investigators. Whether these tumors are still present at the time when neck disease is evident or have undergone involution before this point is still uncertain.
The critical determinant of the utility of an imaging modality in this setting is its ability to detect the presence or absence of metastatic neck disease, particularly where this is not clinically otherwise evident. This information has the potential to alter the treatment plan and morbidity. Our data document the difficulty inherent in attempting to locate sites of abnormality in functional images in relation to normal structures. Even though the landmarks used to delineate different lymph node levels within the neck conveniently use some structures, such as the sternocleidomastoid, which may be metabolically active, difficulty still arises. Our quiet, supine patient preparation tends to minimize uptake in these muscles as this can introduce false-positive findings, especially if muscular uptake is nonuniform. At the same time, it robs us of an important localizing structure much of the time in an area where metabolically active distinct structures are hard to come by.
The most rewarding analysis of FDG-PET data in this setting is performed on a broader basis by identifying the abnormality more approximately than the standard level structure allows—in other words, correctly identifying patients with metastatic neck disease who will require neck dissection. Analyzing our data on this basis achieves a sensitivity of 82% and a specificity of 94%. The sensitivity for detection of neck disease by CT is similar at 81% in our study, with a lower specificity than for FDG-PET of 81%.
Although the numbers are very small, the presence of central cavitation on CT carries a specificity of 100% in this series. In the three FDG-PET false-negative studies for neck metastatic disease where CT was reviewed, nodes with central cavitation were seen in two. As seen histopathologically in these patients, small rims of viable tumor may be present that do not display sufficient FDG retention for detection on functional images. It should be noted that all patients studied in our series had received no prior treatment (e.g., surgery or radiotherapy) to the neck region. Therefore, no confounding factor in lymph node appearance was present in our study.
In all cases where central cavitation was seen on CT, nodal size was compatible with disease involvement on CT criteria, but the specificity of this feature was less, as indicated by the fact that FDG-PET was truly negative in three patients where nodes were pathologic on CT size criteria alone. Likewise, in three other patients, FDG-PET correctly identified disease where nodes were nonpathologic on CT size criteria.
We commenced our study in 1994. Various groups have reported on cohorts of patients ranging from 8 up to 45, and there is clearly growing experience in this setting.
Bailet et al 2 studied eight patients with squamous cell carcinoma of the head and neck who subsequently underwent neck dissection. They compared their FDG-PET findings with those of CT, MRI, and the histologic reference standard. Uptake of FDG greater than or equal to that of salivary gland was considered to be neoplastic. Sensitivity and specificity for FDG-PET were 71% and 98%, respectively. CT and MRI had similar sensitivity and specificity of approximately 58% and 98%, respectively.
Braams et al 7 compared their FDG-PET findings with those of clinical examination, MRI, and the histologic reference standard. They studied 12 patients with squamous cell carcinoma of the oral cavity before neck dissection. No specific comment was made with regard to detection of the primary tumor. The smallest malignant lymph node seen by FDG-PET was 4 mm in diameter; the smallest false-negative lymph nodes measured only 2 mm and 3.5 mm. Sensitivities for PET and MRI were 91% and 36%, respectively; specificities were 88% and 94%, respectively.
Laubenbacher et al 8 studied not only the accuracy of staging using FDG-PET, but also its ability to grade malignancy. They studied 22 patients with squamous cell carcinoma of either the oropharynx or hypopharynx. All primary tumors were detected by FDG-PET. They demonstrated a tendency for tumors of lesser differentiation to display higher FDG uptake, where FDG uptake was estimated, using standardized uptake values. The sensitivity for PET was 90% and specificity was 96%. MRI analysis resulted in a sensitivity of 72% and specificity of 56% for metastatic nodal disease. This is out of keeping with sensitivity and specificity figures for MRI in other studies and may relate to the diagnostic criteria used.
McGuirt et al 4 used the presence or absence of metastatic neck disease as the criteria by which CT, FDG-PET, and clinical examination were reported and compared with a histologic reference standard in 45 patients. They did not comment on involvement of individual lymph node groups. It was their experience, and we too have found, that the resolution of FDG-PET where there is a large focus of abnormal FDG accumulation is often insufficient to delineate individual nodes. No comment was made on the detection of primary tumors. For the detection of nodal disease they calculated a sensitivity of 83% and specificity of 82% for FDG-PET.
Also using the presence or absence of nodal involvement as the endpoint and with histopathological correlation, Paulus et al 9 from Belgium reported a surprisingly high proportion of false-negative FDG-PET studies (50%) in a group of 25 patients, 10 of whom had histologically proven neck metastases, which was explained by microscopic or smaller than 1-cm macroscopic invasion of lymph nodes in these patients. This is out of keeping with the data outlined above and may relate to the use of a suboptimal attenuation correction method on their camera system, or unique patient characteristics. On an anecdotal basis, we have found attenuation correction to be of great importance in these studies. Nevertheless, camera resolution is highlighted as an important issue in the study.
A relatively large experience with FDG-PET in the de novo setting has been reported by Keyes et al. 10 They reported accurate determination of the presence or absence of metastatic disease in 84% of patients with various primary head and neck tumors in a group of 45 patients. This group has also reported on the detection of occult primary malignancies of the head and neck. 11 They reported successful detection in 1 of 3 patients where panendoscopy and biopsy detected tumor, and false detection of a primary in 6 of 13 patients. The remaining five patients had negative PET studies, panendoscopy, and CT and MRI examinations.
The present management of head and neck cancer consists of resection of or radiotherapy to the primary tumor, which may then be coupled with subsequent neck surgery or radiotherapy. The decision as to whether therapy aimed at metastatic disease in the neck is undertaken is dependent on factors such as the presence of disease and the size and site of the primary tumor. The incidence of metastatic disease varies with primary tumor location. Tumors involving the paranasal sinuses or laryngeal glottis are the least likely to metastasize to local nodes, with tumors of the nasopharynx, oropharynx, oral cavity, hypopharynx, and supraglottic larynx (in descending order of likelihood) most likely to have metastatic spread. 12 These treatment methods have resulted in a substantial improvement in control of local disease within the head and neck region, but they also involve the resection of large amounts of nondiseased tissue from the neck. For example, of the 192 neck levels dissected in our study, only 43 (22%) contained metastatic disease, and of 41 patients undergoing neck dissection, in only 23 (56%) did the resected neck tissue contain metastatic disease. The improved specificity that FDG-PET can add to conventional imaging intuitively may lead to more limited initial surgery, based on imaging findings and observation for future recurrence with FDG-PET and CT or MRI. The availability of simultaneous, coregistered anatomical and functional imaging with hybrid machines may improve comparative analysis of these two modalities in the near future.
Although FDG-PET has resolution limitations and is not likely to be able to detect small-volume disease (i.e., <0.5 cm), size is less of a limiting factor than for the other available staging modalities, since metabolic function contributes a significant part to its power of detection. In the setting of absent metastatic neck disease (N0 disease) by present staging techniques, it is likely that FDG-PET will contribute to the upstaging of disease in some cases, resulting in more aggressive intervention. Thus, it may result in a further improvement in local and regional control. Similarly, it may, in the future, also provide sufficient information to limit surgical or radiotherapy treatment fields in the neck, reducing morbidity of patients with this disease.
CONCLUSIONS
FDG-PET demonstrates greater sensitivity for the detection of primary head and neck tumors than CT and as such may have a role in the detailed evaluation of patients before invasive assessment. Our results with respect to the detection of primary head and neck tumors of unknown origin, although limited, parallel those of other investigators, whose findings have been disappointing. This area lacks a gold standard, however, making the interpretation of these findings problematic.
Although sensitivity for the detection of metastatic neck disease is similar in our study for both FDG-PET and CT, the relatively high sensitivity demonstrated for CT is higher than that reported by other investigators. This raises the possibility that the sample we studied differs in some ways from that of other reported groups and emphasizes the need for further evaluation of large study populations. The higher specificity of FDG-PET findings over CT in relation to the detection of neck metastatic disease shows great promise for FDG-PET in the initial staging of patients with head and neck cancer.
Footnotes
Correspondence: Anthony Hannah, FRACP, Centre for Positron Emission Tomography, Austin & Repatriation Medical Centre, Heidelberg, Victoria, 3084, Australia.
E-mail: kiwi@austin.unimelb.edu.au
Reprints: Andrew M. Scott, FRACP, DDU, Centre for Positron Emission Tomography, Austin & Repatriation Medical Centre, Heidelberg, Victoria, 3084, Australia.
Supported in part by the Garnett Passe and Rodney Williams Foundation.
Accepted for publication January 7, 2002.
References
- 1.Snow GB, Patel P, Leemans CR, Tiwari R. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol 1992; 249: 187–194. [DOI] [PubMed] [Google Scholar]
- 2.Bailet JW, Abemayor E, Jabour BA, et al. Positron emission tomography. A new, precise imaging modality for detection of primary head and neck tumors and assessment of cervical adenopathy. Laryngoscope 1992; 102: 281–288. [DOI] [PubMed] [Google Scholar]
- 3.Don DM, Anzai Y, Lufkin RB, et al. Evaluation of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Laryngoscope 1995; 105: 669–674. [DOI] [PubMed] [Google Scholar]
- 4.McGuirt WF, Williams DW 3rd, Keyes JW Jr, et al. A comparative diagnostic study of head and neck nodal metastases using positron emission tomography. Laryngoscope 1995; 105: 373–375. [DOI] [PubMed] [Google Scholar]
- 5.Conti PS, Lilien DL, Hawley K, et al. PET, [F-18]-FDG in oncology. A clinical update. Nucl Med Biol 1996; 23: 717–735. [DOI] [PubMed] [Google Scholar]
- 6.Scott AM. Current status of positron emission tomography in oncology. Int Med J 2001; 31: 27–36. [DOI] [PubMed] [Google Scholar]
- 7.Braams JW, Pruim J, Freling NJ, et al. Detection of lymph node metastases of squamous-cell cancer of the head and neck with FDG-PET and MRI. J Nucl Med 1995; 36: 211–216. [PubMed] [Google Scholar]
- 8.Laubenbacher C, Saumweber D, Wagner-Manslau C, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI, endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med 1995; 36: 1747–1757. [PubMed] [Google Scholar]
- 9.Paulus P, Sambon A, Vivegnis D, et al. [F-18]FDG-PET for the assessment of primary head and neck tumors. Clinical, computed tomography, and histopathological correlation in 38 patients. Laryngoscope 1998; 108: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 10.Keyes JW, Watson NE, Williams D. FDG-PET in head and neck cancer. AJR Am J Roentgenol 1997; 169: 1663–1669. [DOI] [PubMed] [Google Scholar]
- 11.Greven K, Keyes JW, Williams DW. Occult primary tumors of the head and neck: Lack of benefit from positron emission tomography imaging with 2-[F-18]fluoro-2-deoxy-D-glucose. Cancer 1999; 86: 114–118. [DOI] [PubMed] [Google Scholar]
- 12.Madison MT, et al. Radiologic diagnosis: Staging of head and neck squamous cell carcinoma. Radiol Clin North Am 1994; 32: 163–181. [PubMed] [Google Scholar]