Abstract
Objective
To determine the etiology of the loss of epithelial barrier function observed with the administration of total parenteral nutrition (TPN) in a mouse model.
Summary Background Data
Removal of enteral nutrition with the administration of TPN is associated with a loss of intestinal epithelial barrier function. The etiology of this barrier loss is not clear. Because intraepithelial lymphocytes (IELs) produce a number of cytokines that may alter epithelial permeability, the authors investigated IEL cytokine expression in a mouse model of TPN.
Methods
Adult C57BL/6 mice received TPN or enteral diet for 7 days. IELs were subsequently harvested and the mRNA expression of cytokines was measured. Epithelial barrier function was assessed in vitro with 51Cr-EDTA in Ussing chambers and was expressed as the permeability coefficient (Papp).
Results
IEL mRNA expression of interferon-gamma (IFN-γ) rose from 0.14 ± 0.07 in the control (enterally fed) group to 0.44 ± 0.11 attomoles/μL in the TPN group (P < .05). Transforming growth factor-β1 declined slightly but not significantly, from 0.75 ± 0.35 to 0.55 ± 0.18 attomoles/μL in the control and TPN groups, respectively. Epithelial barrier function declined significantly with TPN compared to controls. To assess the relevance of IFN-γ changes, permeability in IFN-γ knockout mice was studied. Barrier function was significantly higher in IFN-γ knockout mice on TPN compared to C57BL/6 mice that received TPN.
Conclusions
IEL cytokine expression changes significantly with TPN administration. The partial correction with IFN-γ knockout mice suggests that an upregulation of IFN-γ expression is one mechanism responsible for the loss of the epithelial barrier associated with TPN.
Epithelial barrier function is essential for the intestine to maintain an effective defense from intraluminal toxins and bacteria, as well as to allow the epithelium to effectively absorb nutrients. 1,2 A common clinical correlate in which a loss of epithelial integrity has been well demonstrated is the administration of total parenteral nutrition (TPN), with the absence of all enteral nutrition. 3–5 The underlying mechanism for this TPN-associated loss of barrier function is unknown. This loss of barrier integrity with the use of TPN, however, has been associated with a high rate of bacterial sepsis. This is thought to be associated with the penetration of endotoxins into the intestinal wall. 6–8 Although some have speculated that this loss of epithelial barrier function is due to a lack of adequate nutritional delivery to intestinal epithelial cells, recent insights with in vitro epithelial cell cultures have shown that cytokines can mediate many epithelial activities. Such actions include the modulation of epithelial cell growth, epithelial cytokine formation, and tight junction integrity. 9,10 One well-characterized cytokine is that of interferon-gamma (IFN-γ). IFN-γ given in vitro to a T84 epithelial monolayer can lead to a loss of tight junction integrity. 1 An even more intriguing fact is that the pretreatment of this cell line with transforming growth factor-β1 (TGF-β1) is able to prevent the IFN-γ-mediated action. 11 Intraepithelial lymphocytes (IELs) are the population of immunocytes within the epithelial layer and are a rich source of such cytokines. 12–14 Based on this, we previously investigated the expression of these cytokines in a mouse model of TPN. 15 This study showed an increased expression of IFN-γ mRNA with TPN administration. The study, however, did not examine the cell populations responsible for this change, nor did we examine the physiologic significance of this IFN-γ expression. The current study hypothesizes that changes in the IELs, during the administration of TPN, would be responsible for the observed loss of epithelial barrier function. The aim of this study was to determine the effect of TPN administration on the expression of cytokines in the IELs, and to determine its relevance in the alteration of epithelial barrier function.
METHODS
Animals
The studies conformed to the guidelines for the care and use of laboratory animals established by the University Committee on the Use and Care of Animals at the University of Michigan, and the protocols were approved by that committee. Male, specific pathogen-free, adult C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were maintained in temperature-, humidity-, and light-controlled conditions. In some experiments, IFN-γ knockout (KO) mice (C57BL/6-Ifngtm1Ts, Jackson Laboratories) were used. During administration of intravenous solutions, the mice were housed in metabolic cages.
Operative Procedures
Administration of TPN was performed as previously described. 15 Mice were infused with a crystalloid solution (dextrose 5% in 0.45 NS with 20 mEq KCl/L) at 4 mL/d. After 24 hours, mice were randomized into two groups. The control group received the same intravenous solution at 7 mL/24 hours, in addition to standard laboratory mouse chow and water ad libitum. The TPN group received a standard TPN solution intravenously at 7 mL/24 hours with no oral intake. The TPN solution contained a balanced mixture of amino acids, lipids, and dextrose in addition to electrolytes and vitamins. 15 Caloric delivery was based on estimates of caloric intake by the control group and from previous investigators, so that caloric delivery was essentially the same in both groups. All animals were sacrificed at 7 days using CO2.
IEL Isolation
IELs were isolated as previously described. 15 Briefly, the small bowel was placed in tissue culture media (RPMI 1640 [Gibco BRL, Gaithersburg, MD], with 10% fetal calf serum). Mesenteric fat and Peyer’s patches were removed. The intestine was then opened longitudinally and agitated to remove mucus and fecal material. The intestine was cut into 5-mm pieces, washed three times in an IEL extraction buffer (l mmol/L EDTA, l mmol/L dithiothreitol in phosphate-buffered saline), and incubated in the same buffer with continuous brisk stirring at 37°C for 30 minutes. The supernatant was then filtered rapidly through a glass-wool column. After centrifugation, the pellets were purified in 40% isotonic Percoll (Pharmacia, Piscataway, NJ) and reconstituted in tissue culture medium. Viability exceeded 95% using trypan blue exclusion staining. After isolation, the cell suspension contained an enriched lymphocyte population, but approximately one third to one half of the cells were epithelial cells.
Biomagnetic Separation of IEL Subpopulations
Positive and negative separation of cells was performed with biomagnetic beads as previously described. 16 The purity of the samples was confirmed using flow cytometry and compared to nonseparated IEL samples. Two extraction processes were performed and the purity of the samples ranged from 98% to 99%. Antibodies were obtained from Pharmingen (San Diego, CA) and consisted of (clone): CD8α(53-6.7), CD8β(53-5.8), and CD45 (30-F11). Isotype-matched, irrelevant antibodies were used as negative controls. The CD45+ population was used to define the lymphoid population of the epithelial layer and differentiate this from the epithelial cell population (CD45-). CD8αα+ and CD8αβ+ populations were used to determine the relative contribution of the thymic-independent and thymic-dependent subpopulations of the IELs, respectively. 17
Ussing Chamber Experiments
Full-thickness small bowel segments were mounted in modified Ussing chambers (Physiologic Instruments, Inc. Design, San Diego, CA) as flat sheets on a segment holder. The exposed tissue surface area was 0.3 cm2, and each half-cell was filled with 5 mL of preheated 37°C Krebs buffer, bathing the mucosa specimen on both the mucosa and serosal side. The Krebs buffer contained NaCl 110.0 mmol/L, CaCl2 3.0 mmol/L, KCl 5.5 mmol/L, KH2PO4 1.4 mmol/L, and NaHCO3 29 mmol/L. The buffer was pH-adjusted to 7.4. The Krebs buffer was continuously oxygenated with O2/CO2 (95%/5%) and stirred by gas flow in the chambers. After a 30-minute equilibration period, to achieve steady-state conditions regarding transepithelial potential difference (PD), the Krebs buffer in the serosal compartment was replaced, and marker solution containing 51Cr-EDTA (NEN Life Science Products Inc., Boston, MA) was added in the mucosal compartments. One-milliliter samples were taken every 10 minutes from the serosal compartment for subsequent analysis of 51Cr-EDTA and replaced by fresh Krebs buffer. A 90-minute incubation followed the equilibration. A gamma counter determined the amount of radioactivity. The apparent permeability coefficient (Papp) was calculated according to the following equation:18 EQUATION
Where dC/dt is the change in concentration on the serosal side per unit time (mol/L per second), V is the volume of the chamber (cm3), A is the area of exposed intestine (cm2), and Co is the initial marker concentration in the mucosal reservoir (mol/L).
Reverse Transcriptase and Competitive Polymerase Chain Reactions
Isolation of Total RNA
Total cellular RNA was isolated by a guanidine isothiocyanate/chloroform extraction using TRIzol according to the manufacturer’s instructions (Gibco BRL, Gaithersburg, MD).
Production of cDNA by Reverse Transcriptase
Poly A-tailed mRNA was reverse-transcribed into cDNA by adding 250 ng of total cellular RNA (see changes for competitive PCR) to the following reaction mixture: dATP, dCTP, dTTP, and dGTP nucleotides each at 1 mmol/L (Boehringer Mannheim, Indianapolis, IN); 8 U/μL MMLV (Gibco BRL); and 2.5 μmol/L oligo-dT (New England Biolabs, Beverly, MA); 2 U/μL RNAase (Boehringer Mannheim) and DEPC-treated H2O were added to adjust the volume. Samples (10-μL volume) were incubated at 39°C for 1 hour, and the reaction was terminated by heating to 95°C for 3 minutes.
Competitive PCR
A series of PCRs were performed with oligomers designed for a number of potentially relevant cytokines. Oligomers were designed using an optimization program (OLIGO 4.1, National Biosciences, Plymouth, MN). For IFN-γ (Genebank accession number K00083), the forward primer was 5′-aataagagcaaggcagtgga-3prime; and the reverse primer was 5′-gggatgacagta ggggaacc-3′. For TGF-β1-γ (Genebank accession number M57902), the forward primer was 5′-gcc ctggataccaactat tgc-3′ and the reverse primer was 5′-gcaggagcgcacaatcat gtt-3′. A quantitative PCR assay was established by creating a DNA fragment (Mimic, Clontech Laboratories, Inc., Palo Alto, CA) that competes directly with the cytokine gene transcripts during PCR amplification. The DNA fragment (MIMIC) was comprised of an irrelevant intervening DNA sequence, flanked on each side by the cytokine-specific primer binding sequences, and gave rise to a PCR product approximately 100 bp longer or shorter than the previously studied cytokine product. 19 Accurate quantification of the target PCR product was determined by equating a known concentration of the competing cDNA generated MIMIC product with the generated cytokine cDNA product. Samples were run in duplicate and the mean concentration was determined and expressed as the number of attomoles/μL of mRNA, where each microliter represented 100 μg of total mRNA. PCR was performed using the following reagents (final concentration): 5 μL of RT product added to 5 mmol/L 3′ and 5′ of the cytokine-specific oligomers; 1× AmpliTaq PCR buffer containing 10 mmol/L Tris and 50 mmol/L KCl and 2.5 mmol/L MgCl2 (Perkin-Elmer, Foster City, CA), 0.4 U AmpliTaq polymerase (Perkin-Elmer), and DEPC-treated H2O. The following thermal cycler (PTC-100, MJ Research, Inc., Watertown, MA) settings were used in order: 94°C for 2 minutes; 30 cycles of 94°C for 15 seconds, 55°C for 15 seconds, and 72°C for 30 seconds; 5 minutes final extension time at 72°C; and held at 4°C until assayed. PCR products were electrophoresed on 2% agarose gel containing ethidium bromide for 1 hour at 170 V and then visualized under ultraviolet light. Quantification of cDNA product used image quantification software (ImageQuant, Molecular Dynamics, Sunnyvale, CA).
Enumeration of IFN-γ-Producing Cells
Two methods were utilized to determine whether the observed mRNA expression of IFN-γ was associated with protein expression.
ELISA Analysis
Methods utilized were similar to those previously described. 20,21 Harvested IELs (0.5 × 106 cells per well) were cultured (in complete culture medium that contained RPMI 1640, 2 mmol/L glutamine, penicillin, and streptomycin, 2-mercaptoethanol [5 × 10−5 mol/L], and 10% heat-inactivated fetal calf serum [Gibco BRL]) for 48 hours in 96-well U-bottom plates. To maximize IFN-γ expression, each well was coated with 0.3 μg/mL of anti-CD3 (hybridoma 145–2C11) and 1 μg/mL of anti-CD28 (Pharmingen). 20 Supernatants were then extracted (pooled from three wells of IELs [1.5 × 106 cells total] from each mouse) and measured by ELISA using matched pair antibodies (Pharmingen) according to the supplier’s directions. Cytokine levels were determined graphically using standard curves generated with recombinant murine IFN-γ (Genzyme, Cambridge, MA).
Intracellular IFN-γ Staining Analysis
The cell staining protocol followed that previously described. 22 The cell preparation was suspended in FACS buffer and the cell surface was stained with the appropriate FITC-conjugated antibody at 4°C for 30 minutes. The cells were washed twice and resuspended, and Cytofix/Cytoperm (Pharmingen) was added for 20 minutes at 4°C. The cells were washed twice and resuspended, and PE-conjugated antibody to IFN-γ (2 μg/1 × 106 cells) was added at 4°C for 30 minutes. Cells were washed twice and resuspended in staining buffer before flow cytometry.
Data Analysis
Results were analyzed using unpaired t tests, with SPSS software (SPSS Inc., Chicago, IL). P < 0.05 was considered significant. Unless indicated, results are expressed as the mean ± SD. Power analyses was performed to ensure that a lack of difference between groups had statistical relevance. Based on previous results, we found that at least n = 4 was necessary for detecting differences in cytokine expression. 15 To ensure that lack of statistical difference was meaningful, we typically used n = 6 for most experiments.
RESULTS
General Description
The total amount of intravenous fluid was a mean of 7.3 ± 1.4 mL/d for the TPN group and 7.0 ± 1.8 mL/d for the control group (P > .05). The mean caloric intake of the TPN animals was 9.0 ± 1.7 Kcal/d. Body weight was recorded both before the initiation of the study and after the mice were euthanized. Body weights at the beginning and end of the study were 24.8 ± 1.4 g and 23.8 ± 1.3 g for the TPN group and 25.0 ± 1.7 g and 24.3 ± 1.4 g for the control group (P > .05). For almost all mice, weight loss occurred in the first 3 days after cannulation and plateaued after this point.
Isolated Cell Populations
Cells isolated from the intestinal epithelium made up the IEL population and contained an enriched lymphocyte population as well as epithelial cells. Cell numbers for the lymphocyte population of the IELs were 10.2 ± 6.6 × 106 cells/mL and 8.1 ± 5.6 × 106 cells/mL in the TPN and control groups, respectively (P > .05). Epithelial cell counts were 16.7 ± 12.5 × 106 cells/mL and 10.6 ± 6.9 × 106 cells/mL in the TPN and control groups, respectively (P > .05).
Cytokine mRNA Expression of IFN-γ and TGF-β1
Analysis of Cytokine Expression Within the Mucosa
IFN-γ mRNA expression (Fig. 1) was 0.14 ± 0.07 attomoles/μL and 0.44 ± 0.11 attomoles/μL for the control and TPN groups, respectively (P < .05). TGF-β1 mRNA expression in the IELs was 0.75 ± 0.35 attomoles/μL and declined to 0.55 ± 0.18 attomoles/μL for the control and TPN groups, respectively (P < .05). The ratio of IFN-γ to TGF-β1 mRNA expression rose with TPN, from 0.19 in the control group to 0.80 in the TPN group.
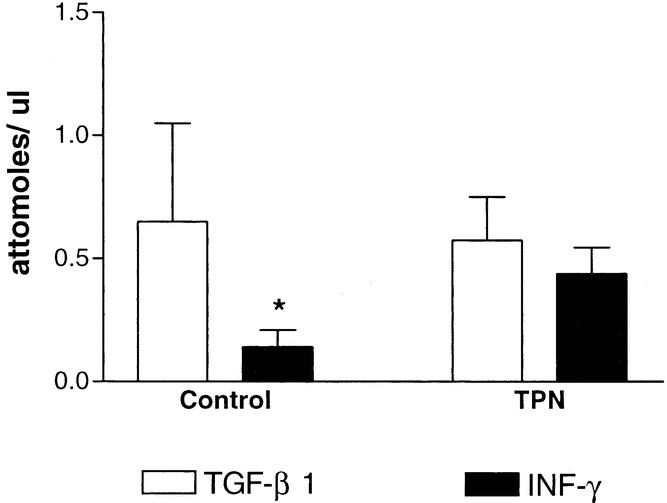
Figure 1. Quantitative expression of interferon gamma (IFN-γ) and transforming growth factor-β1 (TGF-β) mRNA. RNA was isolated from the intestinal mucosa of control mice and mice on TPN (n = 8 per group). *P < .05.
Analysis of Cytokine Expression in Mucosal Subpopulations
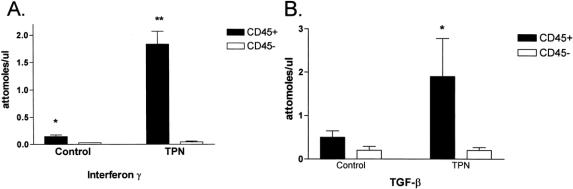
As both lymphoid and epithelial cells can express a number of cytokines, CD45+ and CD45- populations were used to differentiate lymphoid from nonlymphoid cells, respectively. Figure 2 shows the results of these experiments. In the control group, the CD45+ population accounted for the majority of TGF-β1 (72%; i.e., 72% of all TGF-β1 expression was from CD45+ cells) and to a much greater extent for IFN-γ (85%) expression. In the TPN group, the CD45+ population accounted for an increased expression of TGF-β1 (90%) and IFN-γ (98%) mRNA. Both of these changes in cytokine expression for TPN mice were statistically significant (P < .05). Thus, although the relative amount of TGF-β1 declined only slightly, large changes were noted in the population of cells expressing this cytokine.
Figure 2. Quantitative expression of interferon gamma (IFN-γ) (A) and transforming growth factor-β1 (TGF-β) (B) mRNA, as isolated from the intestinal mucosa of control mice and mice on TPN (n = 6 per group). Quantitative analysis was done after the cells in the intestinal mucosa were biomagnetically separated into CD45+ and CD45- groups as markers of lymphoid (IEL) and nonlymphoid (e.g., epithelial cells) populations, respectively. *P < .05 and **P < .01, comparing cytokine expression between lymphoid (IEL) and nonlymphoid (epithelial cell) populations.
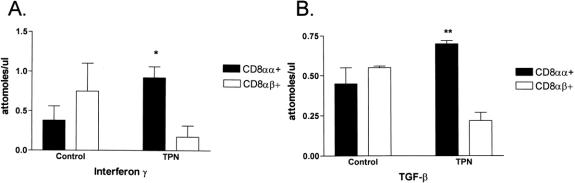
The predominant lymphoid population was CD8+ cells. To better analyze this group of T cells, the subgroups, CD8αα+ and CD8αβ+, were isolated to determine the relative contribution of the thymic-independent and thymic-dependent subpopulations, respectively (Fig. 3). In the control group, the CD8αα+ and CD8αβ+ populations contributed roughly equal amounts to the expression of both IFN-γ and TGF-β1. In the TPN group, the CD8αα+ population accounted for a significantly higher amount of TGF-β1 (72%, P < .01) and IFN-γ (82%, P < .05) mRNA expression. This suggests a greater role for cytokine expression by the thymic-independent population.
Figure 3. Quantitative expression of interferon gamma (IFN-γ) (A) and transforming growth factor-β1 (TGF-β) (B) mRNA, as isolated from the intestinal mucosa of control mice and mice on TPN (n = 6 per group). Quantitative analysis was done after the cells in the intestinal mucosa were biomagnetically separated into CD8αα+ and CD8αβ+ groups. *P < .05 and **P < .01 comparing cytokine expression between thymic-independent (CD8αα+) and thymic-dependent (CD8αβ+) populations.
ELISA Analysis
IFN-γ was targeted for cytokine protein expression as it showed a significant (P < .05) change in cytokine mRNA expression. IEL IFN-γ expression rose from 190 ± 37 pg/mL in the control animals to 455 ± 65 pg/mL in the TPN group (n = 4 per group).
Intracellular IFN-γ Staining Analysis
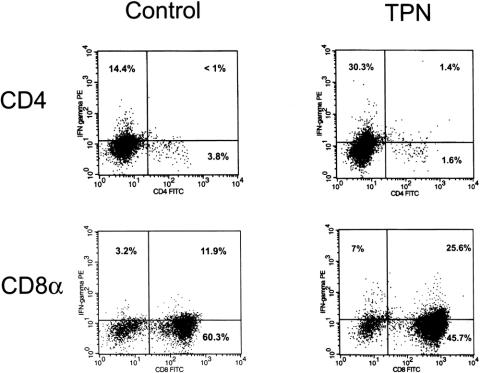
Because of the unique phenotype of the IELs, we elected to further analyze the protein expression of IFN-γ using intracellular staining. This methodology allowed a detailed examination of the specific subpopulations of the IELs that expressed or did not express IFN-γ. Figure 4 shows the results of intracellular staining. IFN-γ expression in the CD8+ subpopulation of the IELs demonstrated a significant (P < .05) rise in the TPN group. The mean percent of the CD8+ IEL subpopulation staining for IFN-γ rose from 11.9 ± 1.6 in controls to 25.6 ± 8.2 in the TPN group. The CD4+ subpopulation of the IELs also showed an increase in IFN-γ expression (control 0.7 ± 0.4 vs. TPN 2.2 ± 1.2); however, the total amount of increase was not significant. Similar to the mRNA data, separate analysis of CD8 subpopulations (CD8 αβ vs. CD8αα) showed that IFN-γ expression in TPN mice was predominately confined to the CD8αα population (CD8 αα23.8 ± 5.8 vs. CD8αβ3.2 ± 1.5).
Figure 4. Results of intracellular staining of IFN-γ. The gated subpopulations IEL are shown for both control and TPN groups (n = 6 per group). The percentages of each quadrant are shown, with the right upper quadrant representing IFN-γ-positive cells in the respective cell population. See the “Results” section for the mean percentage of IFN-γ expression in individual cell populations.
Ussing Chamber Assays for Epithelial Barrier Function
To address the physiologic significance of the changes in the cytokines, we next examined alterations in epithelial permeability with TPN by quantifying the permeability coefficient (Papp) for 51[Cr]-EDTA. A significantly higher Papp for 51[Cr]-EDTA (P < .01) was noted in the TPN group (14.7 ± 4.4 × 10−6 cm per second) compared to the control group (6.87 ± 1.25 × 10−6 cm per second) (Fig. 5). Because the only significant change in cytokine expression was a threefold increase in IFN-γ, we next examined the potential physiologic role of IFN-γ on epithelial barrier function. Papp of 51[Cr]-EDTA was studied in IFN-γ KO mice that received either TPN or enteral nutrition. The IFN-γ KO TPN group (9.2 ± 2.73 × 10−6 cm per second) was significantly lower when compared to the wild-type C57BL/6J mice on TPN (P < .05). Additionally, there was no significant difference in epithelial permeability between IFN-γ KO mice on enteral nutrition (6.42 ± 1.26 × 10−6 cm per second) and enterally fed wild-type C57BL/6J mice (P > .05). This suggests that there is at least a partial correction of the loss of epithelial barrier function with the elimination of IFN-γ expression in mice receiving TPN.
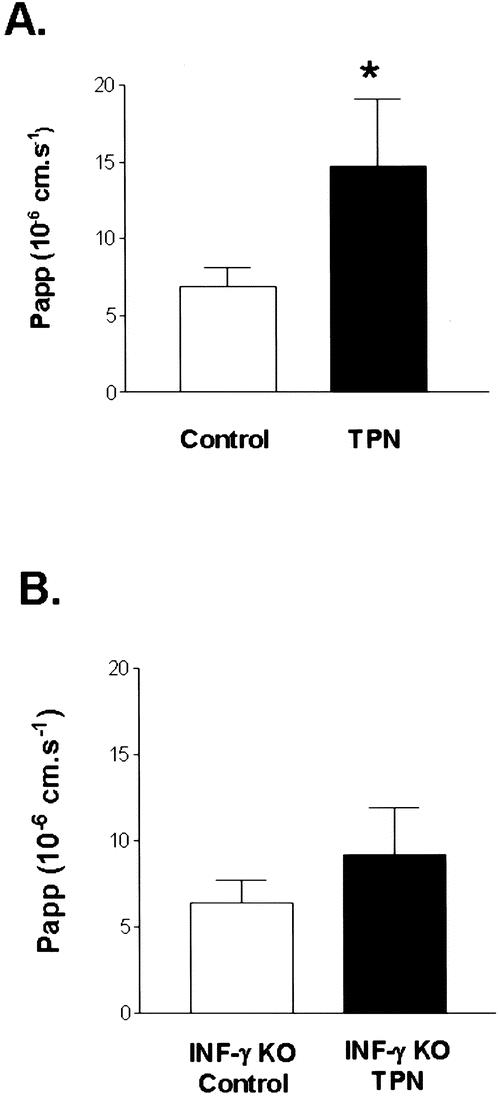
Figure 5. Results of intestinal permeability as measured by the permeability coefficient (Papp) from Ussing chamber experiments (n = 8–10 per group). Study of epithelial barrier function in both C57BL/6J and IFN-γ knockout mice after a 7-day period of enteral nutrition or TPN (P < .05 between groups). Epithelial leak is expressed as the Papp of 51Cr-EDTA. *P < .05 compared with control. The IFN-γ knockout TPN group permeability was also significantly (P < .05) lower than the wild-type TPN mouse group.
DISCUSSION
An intact intestinal epithelium is critical to maintaining a barrier between the external environment of the bowel lumen and the host organism. A loss of this barrier may allow foreign antigens and endotoxins to enter the bowel wall, with subsequent injury to the bowel and the organism itself. 23 Despite TPN being a life-saving measure, several investigators have documented a loss of the intestinal epithelial barrier in both humans and experimental models of TPN. 24 This was demonstrated by Purandare et al, who showed an increased permeability using fluorinated dextran molecules. 4 Such an increased permeability has been also shown by an increase in tissue electrical conductance. 25 The mechanism by which this TPN-associated epithelial leak occurs has not been determined, but it may be associated with an impairment in the mucosal immune system. 26,27 The presumed etiology of this barrier loss has been attributed to the lack of adequate nutritional support of intestinal epithelial cells, as well as to a loss of critical enteral or hormonal trophic factors causing a loss of epithelial growth. 28 Use of cultured epithelial monolayers has given better insight into the mechanisms required for the maintenance of epithelial barrier function. 29 Madara and Stafford studied epithelial integrity using in vitro cultures of human intestinal epithelial cells (T84 cell line). 1 They demonstrated a loss of epithelial tight junction integrity with exogenous application of IFN-γ. Planchon et al, using a similar model, showed that pretreatment with TGF-β1 can prevent the effects of IFN-γ on these cells and maintain epithelial integrity, 11 suggesting a highly cytokine-dependent system for the maintenance of epithelial barrier function.
IELs represent the layer of the gastrointestinal immune system closest to the intestinal lumen and are a rich source of cytokines. 13 Previous work by our group and others has shown that IELs undergo significant phenotypic changes with the administration of TPN. 15,30 These changes include alterations in the absolute number of lymphocytes as well as a specific decline in the number of CD4+, CD8-, and CD44+ subsets of IELs. Based on this, we hypothesized that TPN would result in altered IEL cytokine expression and that these changes would mediate the observed physiologic changes in the intestinal epithelium. Although some authors have found a decline in the total number of IELs and epithelial cells, this was not seen in our study. 30 It is possible that our method of cell isolation was sufficiently different from these authors that this may account for the stable numbers of epithelial cells and IELs in our study. Importantly, however, our method of isolation is well established by many investigators. 12,31 Based on this, we feel that the cells isolated should sufficiently represent an accurate phenotype and function of the cells in the mucosal layer.
IFN-γ mRNA expression increased over threefold in the TPN group. It is also not clear why this alteration in cytokine expression occurs with TPN. Clearly, there is a close intercommunication between epithelial cells and IELs. 10,32 It may well be the case that a loss of enteral nutrition may result in an alteration of the epithelial production of cytokines or other factors that may influence IEL activity. A problem with the quantification of mRNA expression using standard PCR techniques is that it can lack both precision and reproducibility. 19 Because of this, we determined the mRNA expression for two cytokines (IFN-γ and TGF-β1) using a competitive, quantitative PCR technique. Both of these cytokines have been well documented to influence epithelial barrier function, and thus each had the potential to influence permeability in our TPN model. 1,11 A significant increase in IFN-γ mRNA expression (greater than threefold) was noted in the IELs of mice receiving TPN. A slight, but not significant, decline in TGF-β1 mRNA expression was also noted. By using a series of positive and negative cell selections, we were able to determine the subpopulations of the IELs responsible for the altered expression of these cytokines. This was critical in that both intestinal epithelial cells as well as lymphocytes are known to express a large number of cytokines. 33 When mice are given TPN, the relative amount of IFN-γ and TGF-β1 expression by the lymphoid population significantly increases relative to that expressed by the epithelial cells. Additional segmentation of the IELs between CD8αα+ and CD8αβ+ was done as the CD8+ population is the major T-cell population within the IELs. 31 The CD8 homodimeric form (CD8αα) has been hypothesized to be a thymic-independent population, whereas the heterodimeric form (CD8αβ) is considered the thymic-dependent portion of the IELs. 17 In control mice, we showed that the CD8αα+ and CD8αβ+ subpopulations accounted for approximately equal amounts of IFN-γ or TGF-β1 mRNA expression. However, TPN administration led to a significant increase in the expression of both IFN-γ and TGF-β1 by the CD8αα+ subpopulation. This is important, as the thymic-dependent portion of the IELs has been shown to be the predominant population responsible for IEL proliferative activity, 34 and may suggest other functional implications of altered cytokine expression.
In order to determine a potential physiologic result for the increase in IFN-γ expression, a determination of epithelial barrier function was performed. We utilized EDTA as a marker of epithelial permeability. This is based on previous validation demonstrating EDTA as a valid marker of intestinal permeability because its uptake reflects that of macromolecular protein antigens. 35 Our finding of increased permeability of the small bowel epithelium to EDTA with administration of TPN is in close agreement with that of other investigators. 24,27 Likewise, other investigators have quantified a loss of epithelial barrier function based on an increase in electrical conductance in rodent models of TPN administration. 25 We used IFN-γ KO mice to determine the relevance of the increase in IFN-γ expression in our TPN group. The absence of IFN-γ expression in this group resulted in a marked improvement in epithelial barrier function compared to wild-type mice that also received TPN. Importantly, we confirmed that enterally fed IFN-γ KO mice showed no change in epithelial permeability compared to wild-type C57BL/6J control mice. This suggests that IFN-γ may play a critical role in the mediation of this TPN-associated epithelial leak.
The fact that epithelial barrier function was not completely normalized in these KO mice suggests that other factors may also contribute to a TPN-associated epithelial leak. Such factors may include endotoxins, oxidative stress, or other cytokines that may mediate an epithelial leak. 36–38 The relevance of these other factors on epithelial barrier function has yet to be fully determined. Wu et al have recently shown a decline in IL-4 and IL-10 with TPN, which correlated with a decline in the production of IgA. 39 However, in their study, whole gut homogenates were analyzed, which makes extrapolation of these changes to the IELs difficult. Additional cytokines that have been shown to modify epithelial barrier function include IL-10 and IL-4. IL-10 has been shown to dampen the loss of epithelial barrier in T84 monolayers caused by the addition of IFN-γ. 40 IL-4, on the other hand, can attenuate epithelial barrier function in a similar model of cultured human intestinal cells. 41 Additionally, it has been shown that IL-6 may also have an important role in the modulation of gut barrier function in a model of hemorrhagic shock. 42 It is quite possible that such changes may also contribute to the development of the TPN-associated epithelial leak. Because the absence of IFN-γ only partially corrected the loss of epithelial barrier function, additional work needs to be done to determine the relative impact of other cytokine changes in our TPN model.
Other investigators have attempted to simulate IEL–epithelial cell interactions using a series of co-culturing experiments. These studies demonstrated that cytokines expressed by lymphoid cells in such experiments could modulate an inflammatory response on epithelial cell monolayers. 43,44 Unfortunately, in vitro models such as these have several drawbacks that prevent a complete extrapolation to an in vivo setting. An investigation of the IEL–epithelial cell interactions in an in vivo setting has recently been demonstrated. Guy-Grand et al 45 have shown that IFN-γ can mediate a state of small bowel injury in an in vivo setting. In their study, increased expression of IFN-γ by the IELs following IL-12 administration can lead to a state of small bowel enteropathy, with associated cell death and damage to villus epithelial cells. Our series of experiments allowed us to examine a model of epithelial barrier loss (TPN) in an in vivo setting, allowing a more detailed examination of the alteration in the IEL cytokine profile. Such a model confirms in vitro studies that suggest that IFN-γ has a major role in this mediation. Our results also suggest that such IEL-epithelial cell–cytokine interactions have an actual pathophysiologic basis.
In conclusion, administration of TPN in a mouse model resulted in alterations in the increased expression of IFN-γ. Quantitative PCR techniques and use of transgenic mice suggest that a rise in IFN-γ expression may account for a major component of the mechanism by which a loss of epithelial integrity occurs during the administration of TPN. Potential interventions to reverse these changes may be efficacious in reducing the association of sepsis during the administration of TPN.
Footnotes
Correspondence: Daniel H. Teitelbaum, MD, Section of Pediatric Surgery, University of Michigan Hospitals, Mott F3970, Box 0245, Ann Arbor, MI 48109.
E-mail: dttlbm@umich.edu
Supported by NIH grant AI44076-01.
Accepted for publication December 4, 2001.
References
- 1.Madara J, Stafford J. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1989; 83: 724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch E. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg 1990; 125: 403–404. [DOI] [PubMed] [Google Scholar]
- 3.Buchman A, Moukarzel A, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN 1995; 19: 453–460. [DOI] [PubMed] [Google Scholar]
- 4.Purandare S, Offenbard K, Westerom B. Increased gut permeability to fluorescein isothiocyanate-dextran after total parenteral nutrition in the rat. J Gastroenterol 1989; 24: 678–682. [DOI] [PubMed] [Google Scholar]
- 5.Khan J, Iiboshi Y, Nezu R, et al. Total parenteral nutrition increases uptake of latex beads by Peyer’s patches. JPEN 1997; 21: 31–35. [DOI] [PubMed] [Google Scholar]
- 6.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104: 185–90. [PubMed] [Google Scholar]
- 7.Kurkchubasche AG, Smith SD, Rowe MI. Catheter sepsis in short-bowel syndrome. Arch Surg 1992; 127: 21–25. [DOI] [PubMed] [Google Scholar]
- 8.Moore F, Moore E, Jones T. TEN vs. TPN following major abdominal trauma: reduced septic morbidity. J Trauma 1989; 29: 916–923. [DOI] [PubMed] [Google Scholar]
- 9.Hershberg R, Blumberg R. What’s so (Co)stimulating about the intestinal epithelium? Gastroenterology 1999; 117: 726–727. [DOI] [PubMed] [Google Scholar]
- 10.Fujihashi K, Kawabata S, Hiroi T, et al. Interleukin 2 (IL-2) and interleukin 7 (IL-7) reciprocally induce IL-7 and IL-2 receptors on gamma delta T-cell receptor-positive intraepithelial lymphocytes. Proc Nat Acad Sci USA 1996; 93: 3613–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planchon SM, Martins CA, Guerrant RL, et al. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol 1994; 153: 5730–5739. [PubMed] [Google Scholar]
- 12.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol 1993; 5: 247–252. [DOI] [PubMed] [Google Scholar]
- 13.Fujihashi K, McGhee JR, Beagley KW, et al. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods 1993; 160: 181–189. [DOI] [PubMed] [Google Scholar]
- 14.Fujihashi K, Yamamoto M, McGhee JR, et al. Function of alpha beta TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8- and CD4+CD8+ T cells for helper activity. Int Immunol 1993; 5: 1473–1481. [DOI] [PubMed] [Google Scholar]
- 15.Kiristioglu I, Teitelbaum, DH. Alteration of intestinal intraepithelial lymphocytes (IEL) during total parenteral nutrition. J Surg Res 1998; 79: 91–96. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum D, Reyas B, Merion R, et al. Intestinal intraepithelial lymphocytes: identification of an inhibitory sub-population. J Surg Res 1996; 63: 123–127. [DOI] [PubMed] [Google Scholar]
- 17.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of the gut intraepithelial lymphocyte populations in mice. J Exp Med 1994; 180: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grass G, Sweetana S. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharmaceut Res 1988; 6: 372–376. [DOI] [PubMed] [Google Scholar]
- 19.Siebert PD, Larrick JW. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques 1993; 14: 244–249. [PubMed] [Google Scholar]
- 20.Eisenbraun M, Mosley R, Teitelbaum D, et al. Altered development of intestinal intraepithelial lymphocytes in P-glycoprotein-deficient mice. Dev Comp Immunol 2000; 24: 783–795. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi T, McGhee JR, Coffman RL. Analysis of Th1 and Th2 cells in murine gut-associated tissue: frequencies of CD4+ and CD8+ T cells that secrete IFN-γ and IL-5. J Immunol 1990; 145: 68–77. [PubMed] [Google Scholar]
- 22.Carter L, Swain S. Single cell analyses of cytokine production. Curr Opin Immunol 1997; 9: 177–182. [DOI] [PubMed] [Google Scholar]
- 23.Anderson J, Van Itallie C. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 1995; 269: G467–G475. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Hulst R, Von Meyenfeldt M, Van Kreel B, et al. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 1998; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- 25.Peterson CA, Carey HV, Hinton PL, et al. GH elevates serum IGF-I levels but does not alter mucosal atrophy in parenterally fed rats. Am J Physiol 1997; 272 (5 Pt 1):G1100–1108. [DOI] [PubMed] [Google Scholar]
- 26.Mainous M, Xu DZ, Lu Q, et al. Oral-TPN-induced bacterial translocation and impaired immune defenses are reversed by refeeding. Surgery 1991; 110: 277–284. [PubMed] [Google Scholar]
- 27.Li J, Langkamp-Henken B, Suzuki K, et al. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN 1994; 18: 303–307. [DOI] [PubMed] [Google Scholar]
- 28.Helton WS. The pathophysiologic significance of alterations in intestinal permeability induced by total parenteral nutrition and glutamine. JPEN 1994; 18: 289–290. [DOI] [PubMed] [Google Scholar]
- 29.Madara J, Stafford J, Barenberg D, et al. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol 1988; 17: G416–G423. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 1995; 39: 44–52. [DOI] [PubMed] [Google Scholar]
- 31.Mosley RL, Klein JR. A rapid method for isolating murine intestine intraepithelial lymphocytes with high yield and purity. J Immunol Methods 1992; 156: 19–26. [DOI] [PubMed] [Google Scholar]
- 32.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 1994; 266: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 33.Fujihashi K, Yamamoto M, McGhee JR, et al. Alpha beta T cell receptor-positive intraepithelial lymphocytes with CD4+, CD8- and CD4+, CD8+ phenotypes from orally immunized mice provide Th2-like function for B cell responses. J Immunol 1993; 151: 6681–6691. [PubMed] [Google Scholar]
- 34.Kenai H, Matsuzaki G, Nakamura T, et al. Thymus-derived cytokine(s) including interleukin-7 induce increase of T cell receptor αβ+ CD4-CD8- T cells which are extrathymically differentiated in athymic nude mice. Eur J Immunol 1993; 23: 1818–1825. [DOI] [PubMed] [Google Scholar]
- 35.Ramage J, Stanisz A, Scicchitano R, et al. Effect of immunological reactions on rat intestinal epithelium. Correlation of increased gut permeability to chromium 51-labeled ethylenediaminetetraacetic acid and ovalbumin during inflammation and anaphylaxis in the rat. Gastroenterology 1988; 94: 1368–1375. [PubMed] [Google Scholar]
- 36.Helton WS, Rockwell M, Garcia RM, et al. TPN-induced sympathetic activation is related to diet, bacterial translocation, and an intravenous line. Arch Surg 1995; 130: 209–214. [DOI] [PubMed] [Google Scholar]
- 37.Zareie M, McKay D, Kovarik G, et al. Monocyte/macrophages evoke epithelial dysfunction: indirect role of tumor necrosis factor-α. Am J Physiol 1998; 275: C932–C939. [DOI] [PubMed] [Google Scholar]
- 38.Bulger EM, Helton WS, Clinton CM, et al. Enteral vitamin E supplementation inhibits the cytokine response to endotoxin. Arch Surg 1997; 132: 1337–1341. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg 1999; 229: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen KL, Lewis SA, Tavernini MM, et al. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997; 113: 151–159. [DOI] [PubMed] [Google Scholar]
- 41.Colgan SP, Resnick MB, Parkos CA, et al. IL-4 directly modulates function of a model human intestinal epithelium. J Immunol 1994; 153: 2122–2219. [PubMed] [Google Scholar]
- 42.Wang WY, Smail N, Wang P, et al. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res 1998; 79: 39–46. [DOI] [PubMed] [Google Scholar]
- 43.McKay DM, Croitoru K, Perdue MH. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol 1996; 270: C418–428. [DOI] [PubMed] [Google Scholar]
- 44.Taylor C, Murphy A, Kelleher D, et al. Changes in barrier function of a model of intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut 1997; 40: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guy-Grand D, DiSanto JP, Henchoz P, et al. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol 1998; 28: 730–744. [DOI] [PubMed] [Google Scholar]