Abstract
Objective
To establish criteria for venous reconstruction of middle hepatic vein (MHV) tributaries of the right liver graft in adult-to-adult living donor liver transplantation (LDLT).
Summary Background Data
In adult LDLT using the right hemiliver, the MHV is usually separated from the graft, which results in potential venous congestion in the major part of the right paramedian sector (segments 5 and 8). It is controversial whether MHV tributaries should be reconstructed.
Methods
Thirty-nine donors for LDLT were enrolled in the study. After liver transection, temporary arterial clamping was carried out to visualize congestion in the right paramedian sector by occlusion of MHV tributaries. Intra- and postoperative (on postoperative days 3 and 7) Doppler ultrasonography was performed to check the hepatic venous and portal flow in the veno-occlusive area.
Results
In 29 of 37 donors (78%), the liver surface of the veno-occlusive area was discolored with temporary arterial clamping. The discolored area was calculated to represent approximately two thirds of the right paramedian sector on computed tomography volumetry. All of the cases with discoloration exhibited absent venous flow and regurgitated portal flow in the discolored area by intraoperative Doppler ultrasonography. These ultrasonographic findings resolved by postoperative day 7 in 6 of 14 cases (43%).
Conclusions
The state of venous congestion in the right liver graft can be correctly assessed by the temporary arterial clamping method and intraoperative Doppler ultrasonography. If the venocongestive area is demonstrated to be so large that the graft volume excluding this area is thought to be insufficient for postoperative metabolic demand, venous reconstruction is recommended.
While reduced-size liver transplantation, 1 split liver transplantation, 2 and living donor liver transplantation (LDLT) 3 techniques have almost overcome the graft shortage in pediatric patients with end-stage liver diseases, the disparity between supply and demand is still worsening in adult recipients. 4 LDLT 5 and split-liver transplantation 6 using right and/or left hemihepatic grafts have been applied to adult patients to increase the number of adult transplants. In LDLT using the right liver, whether the middle hepatic vein (MHV) should be preserved for the residual left liver 7 or the right liver graft 8 remains to be clarified. Whether MHV tributaries should be reconstructed in the hemiliver without the MHV is another crucial problem, since a congested area in the right liver graft is reported to result in poor liver function, followed by atrophy, unless venous reconstruction is performed. 9
In hepatectomy for hepatic tumors with venous involvement, combined resection of the hepatic veins should be carried out. However, it is still unclear whether the resected hepatic veins should be reconstructed.
In this study, we tried to establish the criteria for venous reconstruction of MHV tributaries in adult LDLT using right liver graft. We prospectively evaluated the state of hepatic congestion and the occurrence of intrahepatic venous anastomoses in the right paramedian sector with occlusion of the MHV by Doppler ultrasonography, temporary clamping of hepatic artery, and near-infrared spectroscopy (NIRS) using donors for LDLT.
PATIENTS AND METHODS
Patients
From January 2000 to December 2000, 39 consecutive LDLT procedures were performed at the University of Tokyo and Matsunami General Hospital: 17 transplantations for child recipients (less than 18 years old) and 22 for adults (18 or older). Demographic data of the donors and recipients are listed in Table 1. The operative procedures were extended left hepatectomy (segments 1, 2, 3, and 4 with MHV) in 15 cases, left hepatectomy (segments 2, 3, and 4 without MHV) in two, extended left lobectomy (segments 2, 3, and part of segment 4) in eight, right hepatectomy (segments 5, 6, 7, and 8 without MHV) in nine, and right lateral sectorectomy (segments 6 and 7) in five.
Table 1. DEMOGRAPHIC DATA
%SLV, percentage of graft weight compared with standard liver volume; MHV, middle hepatic vein; RHV, right hepatic vein.
Data are expressed as mean ± SD [range].
Evaluation of MHV Tributaries Draining the Right Paramedian Sector by Preoperative Computed Tomography
We evaluated the number and diameter of MHV tributaries draining the right paramedian sector (segments 5 and 8) in 39 donors by preoperative dynamic computed tomography (CT). Dynamic CT was carried out in 90 seconds after bolus injection of contrast agents, in a total amount of 2 mL/kg, in a peripheral vein in the upper extremity. The tributaries were classified as V8, which drained the cranial part of the portal trunk of the right paramedian sector, and V5, which drained the corresponding caudal part. After scanning the CT films, the number of MHV tributaries and their diameters were measured on a Macintosh computer using the public-domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Tributaries with a diameter of less than 2 mm were omitted.
Intraoperative Evaluation of Hepatic Venous Congestion
Hepatic venous congestion in the right paramedian sector was investigated intraoperatively after transection of the liver parenchyma in 37 of 39 donors. Two donors, one with extended left hepatectomy and one with right lateral sectorectomy, were excluded due to absence of the examiner (K.S.). In 33 donors, the drainage area of the MHV and its tributaries in the right paramedian sector was evaluated. In the remaining four cases with right lateral sectorectomy, congestion with occlusion of the tributaries of the right hepatic vein (RHV) was assessed.
First, the liver surface in the right paramedian sector was observed to determine whether the surface of the congested area was discolored compared to other sectors in those 37 donors. After the right hepatic artery was clamped for 5 minutes, the surface discoloration was reevaluated. We previously reported that the liver surface of the veno-occlusive area is discolored by temporary clamping of the hepatic artery. 10 In cases with discoloration, We first mapped the diaphragmatic surface of the right paramedian sector and calculated the ratio of discolored and nondiscolored areas. In order to further estimate the congestive liver volume, the right paramedian sector was virtually sliced transversely (i.e., same as CT scan plane), with 1-cm thickness. The width of the discolored and nondiscolored areas on the diaphragmatic surface was measured on each slice. The ratio of the widths was transposed to the liver contour in each corresponding CT slice. CT volumetry was performed in the same manner as described previously. 11
Next, intraoperative Doppler ultrasonography (SSD-2000, or SSD-5500; Aloka, Tokyo, Japan) was performed to evaluate flow in occluded tributaries of the MHV or RHV and in the portal veins in the veno-occlusive area in 34 of 37 donors. Three of the 37 donors were excluded due to the unavailability of Doppler ultrasound modalities. Flow velocity was measured in the expiratory state of controlled respiration under general anesthesia. Portal and venous flows in the veno-occlusive area of the right paramedian sector were first surveyed by color flow mapping, and each flow was diagnosed as either hepatopetal or hepatofugal by fast Fourier transformation of the waveform. If no color signals flow mapping or no waves in fast Fourier transformation were detected in the minimum velocity range, the flow was evaluated as undetectable.
Finally, NIRS was used to monitor hepatic tissue saturation with oxygen in the veno-occlusive area with and without arterial perfusion compared with that in the area with patent venous drainage in 11 donors. The other 26 donors could not undergo NIRS due to unavailability of the equipment. Near-infrared reflectance was measured with a photodetector (PSA-500, Biomedical Science, Co., Ltd., Kanazawa, Japan) connected to a personal computer (Vaio, Sony, Tokyo, Japan).
Postoperative Measurement of Hepatic Venous Flow by Color Doppler Ultrasonography
Twenty (15 cases with extended left hepatectomy and 5 with right lateral sectorectomy) out of 39 donors had venous occlusion in the remnant liver and became candidates for postoperative evaluation of the veno-occlusive area in the right paramedian sector. Doppler ultrasonography (SSD-2000) was carried out to measure the flows of dissected tributaries of the MHV or RHV, and the portal veins in the remnant right paramedian sector in 18 of these 20 donors. Two donors with extended left hepatectomy were excluded due to the lack of Doppler ultrasound modalities. Each flow velocity was measured with breath-holding. The directions of portal and venous flow were evaluated in the same manner as intraoperative measurement on postoperative days 3 and 7.
Statistical Analysis
The significance of differences was assessed using the two-tailed Welch’s t test, the Mann-Whitney test, and the chi-square test. Differences at P < .05 were considered to be statistically significant.
RESULTS
MHV Tributaries Draining the Right Paramedian Sector on Preoperative CT
One tributary was evaluated as V8 in 25 cases, two tributaries in 12, three in 1, and none in 1 case: the mean number of tributaries was 1.33. The mean diameter of V8 was 3.5 ± 1.8 mm (range 2–8). As V5, one tributary was recognized in 28 cases, two in 6, three in 3, and none in 2 cases: the mean number was 1.26. The mean diameter of V5 was 4.5 ± 1.7 mm (range 2–8). The mean total number of tributaries draining the right paramedian sector was 2.7 ± 0.9. In five cases (13%), V5 ran through the right paramedian sector to the caudal part of the right lateral sector (segment 6). The thickest caliber of the tributaries of the MHV was estimated to be not less than 5 mm in 30 cases (77%) by CT.
Liver Surface Discoloration
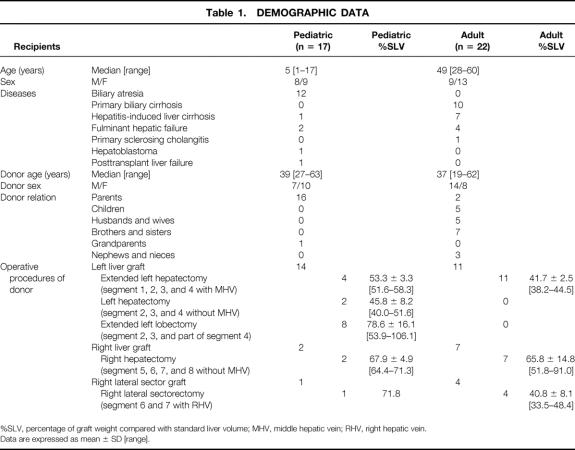
The liver surface was faintly discolored after liver transection in 3 of 37 cases (8%). However, after the right hepatic artery was clamped for 5 minutes, apparent discoloration was seen in 29 of 37 donors (78%) (Fig. 1).
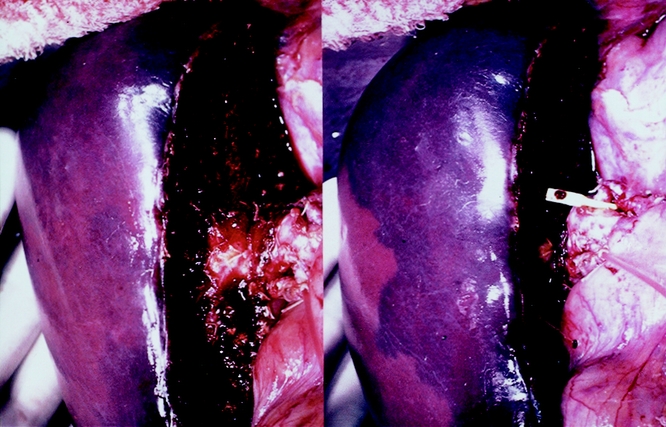
Figure 1. After liver transection for left hepatectomy with the middle hepatic vein, discoloration of the congested area in the right paramedian sector was revealed with temporary arterial occlusion. (Left) Before arterial occlusion. (Right) After arterial occlusion.
In cases with discoloration, the percentage of the area drained by MHV tributaries in the right paramedian sector was 63.0 ± 18.9% (range 28.2–100%). The percent volume of this portion in that sector was estimated to be 60.6 ± 17.4% (range 25.0–100%). The mean total volume of the right liver and the volume without the congested portion were estimated to be 789.6 ± 140.1 mL (range 625.0–1,164.0) and 500.8 ± 142.3 mL (range 253.2–857.8), respectively, by CT volumetry. The ratio of the volume without the congested portion to the total right liver volume was 63.0 ± 11.6%.
Intraoperative Doppler Ultrasonography
In 26 (76%) of 34 cases, the flow was undetectable in the tributaries of the MHV in the right paramedian sector. The flow of the portal branches in the veno-occlusive area was hepatofugal in all of them (Fig. 2). All those 26 donors showed discoloration on the liver surface on temporary clamping of arterial flow. In the other eight cases (24%), the flow in the tributaries was demonstrated by color Doppler ultrasonography as pouring into the right hepatic vein via intrahepatic venous anastomoses (Fig. 3).
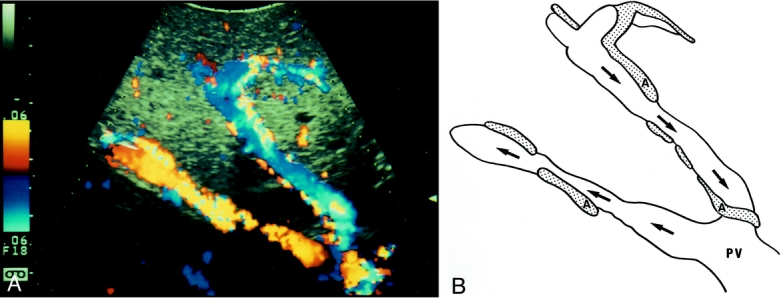
Figure 2. Color flow mapping of intraoperative Doppler ultrasonography after liver transection in extended right hemihepatectomy. Portal flow was hepatofugal in the veno-occlusive area. A; artery, PV; portal vein. Arrows indicate the direction of portal blood flow.
Figure 3. Color flow mapping of intraoperative Doppler ultrasonography after liver transection in extended left hepatectomy. Arrows indicate the direction of portal blood flow. Venous flow was regurgitated in the middle hepatic vein tributaries in S8, while in the right hepatic vein normal hepatofugal flow was observed. A communicating vein (arrowheads) was seen between the middle and right hepatic veins. IVC, inferior vena cava; MHV, middle hepatic vein; RHV, right hepatic vein; P, right paramedian portal pedicles.
Tissue Saturation With Oxygen Measured With Near-Infrared Spectroscopy
NIRS was performed in 11 patients: 7 (4 with right hepatectomy and 1 each with extended left hepatectomy, left hepatectomy without MHV, and extended left lobectomy) in whom venous flow was undetectable by Doppler ultrasonography (group 1) and 4 (3 with extended left hepatectomy and 1 with extended left lobectomy) in whom venous flow was maintained by venous anastomoses (group 2). Tissue saturation with oxygen in the area without venous occlusion in the right paramedian sector was 88.7 ± 10.3% in group 1 and 92.8 ± 3.4% in group 2. Saturation with oxygen in the veno-occlusive area was 74.7 ± 17.0% in group 1 and 92.5 ± 4.2% in group 2, and this difference was significant (P = .03). After 5 minutes of clamping, saturation with oxygen in the veno-occlusive area decreased to 29.6 ± 11.2% in group 1 and 72.8 ± 9.8% in group 2, and this difference was also significant (P < .001) (Fig. 4).
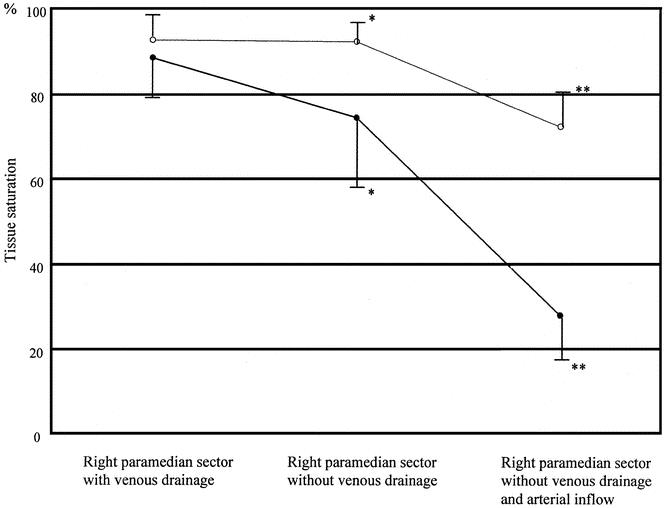
Figure 4. Tissue saturation with oxygen in the normal, veno-occlusive, and veno-arterio-occlusive areas measured by near-infrared spectroscopy. Black circles indicate donors whose hepatic venous flow was undetectable (n = 7). White circles indicate donors with hepatic venous regurgitation (n = 4). *P = .03; **P < .001.
Postoperative Measurement of Hepatic Flow by Color Doppler Ultrasonography
In four donors with venous flow in the occluded tributaries of the MHV or RHV intraoperatively, the venous flow remained detectable and the portal flow in that area remained hepatopetal by color Doppler ultrasonography on postoperative days 3 and 7. In 14 donors without venous flow in these tributaries intraoperatively, the venous flow remained undetectable and the portal flow remained hepatofugal on postoperative days 3 and 7 in eight cases (57%). Venous flow with intrahepatic venous anastomoses was detectable and hepatopetal portal flow was restored in six cases (43%), two donors on day 3 and four on day 7.
DISCUSSION
In hepatic resections and partial liver transplantations, venous reconstruction has been considered to be dispensable due to anastomoses between adjacent hepatic veins. Thick venous anastomoses have been demonstrated in Budd-Chiari syndrome. 12 By injecting vinyl-polychloride into the hepatic veins of autopsied liver, Couinaud demonstrated hepatic venous anastomosis in 25 of 30 casts, 13 and Lasinski revealed venous anastomoses between the MHV and right hepatic vein in half of the cases examined. 14 However, it is unclear whether venous anastomoses would develop clinically just after occlusion of the hepatic veins. We have previously reported venous reconstruction for discoloration of the liver surface due to liver congestion 15 and for other findings by Doppler ultrasonography. 10 In the present study, intraoperative Doppler ultrasonography revealed that 76% of the cases had no venous anastomoses, and in these cases venous flow was undetectable and portal flow of the relevant congested area was regurgitated. Kaneko et al reported that venous anastomoses were detected by Doppler ultrasonography 6 days after LDLT using a right liver graft without the MHV. 16 In our series, congestion in donor right livers was relieved in only 2 of 14 cases (14%) in 3 days, and in 6 of 14 (43%) in 7 days. In the other eight donors (57%), portal flow remained regurgitated in the congested area. With regard to recipients with congestion in the right liver graft by ligation of MHV tributaries, portal flow remained regurgitated in a half of them 1 week after transplantation. Development of venous anastomoses in a week is not expected in all patients.
In 1951, Rappaport reported that during wedge hepatic venography, the liver parenchyma was first stained and the portal vein was then revealed. 17 Based on this finding, it was considered that hepatic venous blood could be regurgitated to the portal vein through the sinusoid when the hepatic vein was obstructed. Regurgitation of the portal flow in the congested area of the liver has since been described experimentally 18 and clinically. 19,20 Murata et al demonstrated hyperattenuation of the congested area and late attenuation in the adjacent area by CT angiography with balloon occlusion of the hepatic vein, which revealed that portal veins became draining veins and the occluded area was supplied with arterial blood alone. 20 In these previous reports, hepatic hemodynamic evaluation was carried out using contrast medium. We first reported the direct demonstration of regurgitation of the portal flow in the congested area by intraoperative Doppler ultrasonography. 10 Using Doppler ultrasonography, liver congestion can be diagnosed intraoperatively and postoperatively by demonstrating both absent venous flow and regurgitated portal flow.
We reported that clamping the hepatic artery was another available method for diagnosing hepatic congestion intraoperatively. 10 In this study, the liver surface became discolored by temporary arterial clamping in all donors with signs of congestion by Doppler ultrasonography. Therefore, Doppler ultrasonography and temporary arterial clamping are convenient and reliable methods for intraoperative diagnosis of hepatic congestion.
NIRS is a noninvasive and continuous optical method for measuring tissue oxygenation. 21 NIRS is based on two fundamental characteristics: the relative transparency of tissue to light in the near-infrared region, and the oxygenation-dependent changes in absorption in tissue caused by chromophores, mainly oxygenated and deoxygenated hemoglobin. By measuring changes in absorption at different wavelengths (750 and 830 nm), tissue oxygenation can be monitored continuously. The relation between absorption and concentration is given by a modified Lambert-Beer law, 22 which incorporates a path length factor to account for light scattering in tissue. In this study, the distance between the light-emitting diode and the photodiode detector was 5 mm and the sampling time of each scan was 1 second. NIRS revealed that the average tissue saturation with oxygen of the veno-occlusive area with and without arterial flow was 74.7% and 29.6%, respectively. Tissue saturation with oxygen of less than 30% in the area without venous or arterial flow suggests that portal perfusion of that area was also absent or very scarce.
A lack of portal blood perfusion of the liver parenchyma would induce liver dysfunction in the congested area, since metabolism of the liver is mainly dependent on the portal blood from the intestine and the pancreas. 23 If the congested area is suspected to be large, the remnant liver or the liver graft could not support the metabolic demand due to the absence of portal blood flow in the relevant area. Under such conditions, venous reconstruction should be indicated in liver resection or partial liver transplantation.
In partial liver transplantation, the MHV is included in either the right or left hemiliver. Especially in adult-to-adult LDLT, right liver graft with the MHV sometimes induces postoperative liver failure in donors due to the insufficient remnant liver volume. 8 When the MHV is included in the left liver, the area drained by tributaries of the MHV in the right paramedian sector is a problem, since the MHV is known to be the major drainage vein for this sector. 24 The MHV was preserved in donors who underwent left hepatectomy in 6 of 66 left liver grafts among our 139 LDLTs, due to a very thick MHV and its tributaries draining the right liver. 25 However, the area drained by the MHV has not yet been accurately clarified. In this study, we visualized the area drained by the MHV using the intraoperative arterial clamping method and calculated the proportion in the right paramedian sector. The MHV was shown to drain approximately two thirds of the right paramedian sector using both the diaphragmatic surface area and the volume calculated by CT volumetry. The right liver graft volume without the MHV was estimated to be 63% of the total graft volume when the congested portion was excluded. Lee et al reported two cases of severe congestion of the graft without the MHV; one resulted in sepsis due to congestive infarction and the other patient developed prolonged massive jaundice. 9 Therefore, we have to consider venous reconstruction when the right liver graft without the congested area is estimated to be insufficient compared to the standard liver volume of the recipient.
An absolute indication for venous reconstruction is when a single hepatic vein does not remain and when the liver surface is apparently discolored with mere venous occlusion, as noted by Kakazu et al. 15 The latter condition was rarely encountered in our hepatectomy series of more than 1,000 cases; however, it has been suggested that arterial blood may not perfuse the sinusoidal space of the liver, resulting in necrosis of the congested area. 9 In almost all of the patients with hepatic venous congestion, the color of the liver surface does not change, so that relative criteria for venous reconstruction are needed.
We previously proposed relative criteria for venous reconstruction. 10 With occlusion of the hepatic artery, apparent discoloration soon appeared on the liver surface. The relevant congested volume of the liver was subtracted from the remnant liver or the graft liver volume. When the remaining liver volume was less than 30% of the standard liver volume in liver resection for the normal liver and less than 40% in liver transplantation, reconstruction of the hepatic vein or its tributaries should be considered.
In our institutions, donor hepatectomy procedures are selected to leave at least 35% liver volume with intact afferent and efferent blood circulation to avoid the need for venous reconstruction of their remnant liver. In the present series, 15 of 39 donors had occluded MHV tributaries, but none of them underwent venous reconstruction. Their postoperative courses were all uneventful. On the other hand, 11 recipients, 9 with right liver graft and 2 with left liver graft, had interrupted MHV tributaries. Three of them underwent venous reconstruction of these tributaries. The first patient was a 62-kg male who was transplanted with a right liver graft from his 44-kg sister. Although the preoperative estimated graft volume was 52% of his standard liver volume, the majority of the right paramedian sector became congested after temporary clamping of the MHV during the donor operation. Graft volume, recalculated excluding this congestive area, was 27% of the recipient’s standard liver volume. Thus, we decided to carry out reconstruction of MHV tributaries. The second and the third patients received left liver grafts. In these cases, the MHV trunk was preserved to the donor’s remnant right liver, since the major part of the right liver was thought to be drained by MHV tributaries. Although grafts were preoperatively estimated to be 52% and 40% of their standard liver volume, intraoperatively calculated noncongestive graft volume was 32% and 28%. Based on these findings, MHV tributaries were reconstructed. All the reconstructed venous flow remained patent during the postoperative courses. The other eight recipients with right liver grafts did not undergo reconstruction of MHV tributaries due to the existence of intrahepatic venous anastomoses (n = 3) or sufficient graft volume even after deduction of the venocongestive area (n = 5).
In summary, intraoperative Doppler ultrasonography revealed absent hepatic venous flow and regurgitated portal venous flow in the congested area. If the liver volume excluding the discolored area under occlusion of the artery is estimated to be insufficient for postoperative metabolic demand, reconstruction of the hepatic vein or its tributaries should be considered.
Footnotes
Keiji Sano, MD, executed clinical examinations, Masatoshi Makuuchi, MD, PhD, contributed to the planning, and Kenji Miki, MD, contributed to the analysis of the study. The other authors participated in the clinical execution of the study.
Correspondence: Masatoshi Makuuchi, MD, PhD, Hepato-Biliary-Pancreatic Surgery Division, Artificial Organ and Transplantation Surgery Division, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
E-mail: makuuchi-tky@umin.ac.jp
Accepted for publication November 1, 2001.
References
- 1.Bismuth H, Houssin D. Reduced-size orthotopic liver grafts for liver transplantation in children. Surgery 1984; 95: 367–370. [PubMed] [Google Scholar]
- 2.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation einer spenderbeber auf zwai empfanger (splitting-transplantation): eine neue methode in der weiterentwicklung der lebersegment transplantation. Langenbecks Arch Chir 1988; 373: 127–130. [PubMed] [Google Scholar]
- 3.Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990; 322: 1505–1507. [DOI] [PubMed] [Google Scholar]
- 4.Smith CME. Annual Report of the U.S. Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network-Transplant Data: 1988–1996. UNOS, Richmond, VA, and the Division of Transplantation, Bureau of Health Resources and Services Administration, U.S. Department of Health and Human Services, Rockville, MD, 1997.
- 5.Hashikura Y, Makuuchi M, Kawasaki S, et al. Successful living-related partial liver transplantation to an adult patient. Lancet 1995; 343: 1233–1234. [DOI] [PubMed] [Google Scholar]
- 6.Colledan M, Andorno E, Valente U, et al. A new splitting technique for liver grafts. Lancet 1999; 353: 1763. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka Y, Washida M, Honda K, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994; 57: 1127–1141. [PubMed] [Google Scholar]
- 8.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997; 226: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SG, Park KM, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation 2001; 71: 812–814. [DOI] [PubMed] [Google Scholar]
- 10.Sano K, Makuuchi M, Takayama T, et al. Technical dilemma in living donor or split-liver transplant. Hepato-Gastroenterology 2000; 47: 1208–1209. [PubMed] [Google Scholar]
- 11.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995; 21: 1317–1321. [PubMed] [Google Scholar]
- 12.Maguire R, Doppman JL. Angiographic abnormalities in partial Budd-Chiari syndrome. Radiology 1977; 122: 629–635. [DOI] [PubMed] [Google Scholar]
- 13.Couinaud C, Nogueira C. Les veines sus-hepatiques chez l’homme. Acta Anat 1958; 34: 84–110. [PubMed] [Google Scholar]
- 14.Lasinski W, Zientarski B. The anastomotic system of sub-hepatic veins in man. Bull Assoc Anat 1976; 60: 559–566. [PubMed] [Google Scholar]
- 15.Kakazu T, Makuuchi M, Kawasaki S, et al. Reconstruction of the middle hepatic vein tributary during right anterior segmentectomy. Surgery 1995; 117: 238–240. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko T, Kaneko K, Sugimoto H, et al. Intrahepatic anastomosis formation between the hepatic veins in the graft liver of the living related liver transplantation: observation by Doppler ultrasonography. Transplantation 2000; 70: 982–985. [DOI] [PubMed] [Google Scholar]
- 17.Rappaport AM. Hepatic venography. Acta Radiol 1951; 36: 165–171. [DOI] [PubMed] [Google Scholar]
- 18.Rousselot LM, Grossi CE, Slattery J, et al. Temporary hepatic outflow block with hepatic artery perfusion by anticancer agents. Surg Gynecol Obstet 1964; 118: 1295–1304. [PubMed] [Google Scholar]
- 19.Pollard JJ, Nebesar RA. Altered hemodynamics in the Budd-Chiari syndrome demonstrated by selective hepatic and selective splenic angiography. Radiology 1967; 89: 236–243. [Google Scholar]
- 20.Murata S, Itai Y, Asato M, et al. Effect of temporary occlusion of the hepatic vein on dual blood supply in the liver: evaluation with spiral CT. Radiology 1995; 197: 351–356. [DOI] [PubMed] [Google Scholar]
- 21.Jobsis FF. Non-invasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977; 198: 1264–1266. [DOI] [PubMed] [Google Scholar]
- 22.Delpy DT, Cope M, van der Zee P, et al. Estimation of optical path length through tissue from direct time of flight measurement. Phys Med Biol 1988; 33: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 23.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet 1973; 137: 179–199. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and inferior vena cava. Surg Gynecol Obstet 1981; 152: 43–50. [PubMed] [Google Scholar]
- 25.Hui AM, Makuuchi M, Takayama T, et al. Left hemihepatectomy in living donors with a thick middle hepatic vein draining the caudal half of the right liver. Transplantation 2000; 69: 1499–1501. [DOI] [PubMed] [Google Scholar]