Abstract
Objective
To report the authors’ experience with pediatric lung transplantation (LTX) to provide an overview of patients selected for this procedure and their outcomes.
Summary Background Data
Pediatric LTX differs from adults in many ways, including recipient size, indications, posttransplant care, and rehabilitation.
Methods
Two hundred seven isolated lung transplants on 190 children under the age of 18 years were performed from 1990 to the present. This represents the single largest series of lung transplants in children in the world. Thirty-two patients were less than 1 year of age, 22 were 1 to 5 years of age, 32 were 5 to 10 years of age, and 121 were 10 to 18 years old. The groups by major diagnostic category were cystic fibrosis (n = 89), pulmonary vascular disease (n = 44), bronchiolitis obliterans (n = 21), pulmonary alveolar proteinosis (n = 12), pulmonary fibrosis (n = 15), and other (n = 26). The average age at the time of transplant was 9.5 ± 5.9 years (range 36 days to 18 years).
Results
Survival by Kaplan-Meier analysis was 77% at 1 year, 62% at 3 years, and 55% at 5 years. There was no significant difference in survival according to primary diagnosis leading to LTX or age at LTX. There were 25 early (<60 days) and 61 late deaths. The most common cause of early deaths was graft failure (13/25, 52%). The most common causes of late death were bronchiolitis obliterans (35/61, 57%), infection (13/61, 21%), and posttransplant malignancies (11/61, 18%). No patient died of acute rejection. In those surviving greater than 3 months (mean follow-up 3.5 years, range 3 months to 11 years), the overall rate of occurrence of bronchiolitis obliterans was 46% (80/175) and the overall incidence of posttransplant malignancies was 24/175 (14%). Major risk factors for the development of bronchiolitis obliterans were age older than 3 years, more than two episodes of acute rejection, and organ ischemic time longer than 180 minutes.
Conclusions
In children, LTX is a high-risk but viable treatment for end-stage pulmonary parenchymal and vascular disease. The major hurdle to overcome in long-term survival is bronchiolitis obliterans.
Beginning with the first successful operation in 1983, lung transplantation (LTX) has emerged as an accepted treatment for complications due to end-stage pulmonary parenchymal and vascular disease in adults. 1–7 Despite this, the viability of LTX in children has been much slower in gaining such widespread acceptance. Only 5% of all patients transplanted are under the age of 18 years. 8 This large discrepancy is due to a number of factors relating to a relative paucity of potential recipients, a relative paucity of donors, or a relative paucity of physicians enthusiastic about subjecting children to a procedure with unknown results beyond 5 years posttransplant. The most common indications for LTX in general are chronic obstructive pulmonary disease, α-1 antitrypsin disease, and cystic fibrosis. 8,9 Most patients with these diagnoses present with progressive respiratory disability after the age of 18 years; in fact, there are essentially no patients with obstructive lung disease who undergo transplantation before the age of 20 years. For the calendar year 2000, there were 179 lung donors and 43 recipients under the age of 18. 10 Thus, there would appear on the surface to be a reasonable supply of donors; however, the majority of these young donors are used in adults. The basis for much of the skepticism of physicians towards applying LTX to children undoubtedly lies in some of the important unknown issues that are peculiar to pediatric LTX recipients. These include concerns about growth (both somatic and of the lung itself), indications for transplantation, technical issues (surgical), posttransplant care, rehabilitation, infectious risks, and the complexity of care required by these individuals. Nonetheless, there are children dying of end-stage pulmonary parenchymal and vascular disease who are potential candidates for LTX and who might benefit long term from this treatment.
Despite these potential concerns LTX has, in the past 10 years or so, been adopted by a small number of institutions around the world as an acceptable option for children with lethal or progressively debilitating lung disease. According to the registry of the International Society of Heart and Lung Transplantation, 587 lung transplants have been performed on children under the age of 18 years in 30 centers throughout the world. 2 Since 1990, 207 lung transplants have been performed on 190 children at St. Louis Children’s Hospital. This represents approximately one third of all the pediatric lung transplants and thus represents the largest single-center series of lung transplants in children in the world. To further evaluate the viability and practicality of pediatric LTX, we reviewed our series of patients to provide a better understanding of the current applications of LTX in children.
METHODS
Patients
Since 1990, 207 isolated lung transplants were performed on 190 children under the age of 18 at St. Louis Children’s Hospital. Of these patients, 32 were less than 1 year of age, 22 were between 1 and 5 years old, 32 were between 5 and 10 years old, and 121 were between 10 and 18 years old (Fig. 1). The average age at the time of transplant for the children was 9.5 ± 5.9 years (range 36 days to 18 years). The average waiting time from listing to transplantation was 225.09 days (range 1–1,484 days). Seven patients underwent single lung transplant, while the remaining 199 were bilateral transplants. Thirty of our patients underwent living donor lobar transplantation and four others underwent cadaveric lobar transplantation.
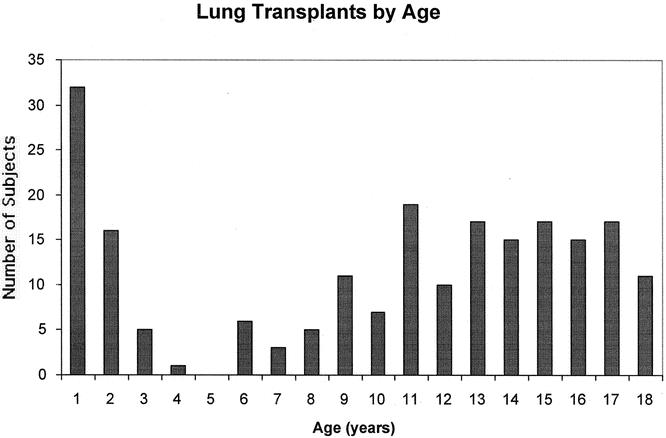
Figure 1. Number of lung transplants in each age group by year from birth to 18 years.
Pretransplant Diagnosis
The indication for LTX in general is irreversible pulmonary parenchymal or vascular disease. Based on the patient’s natural history, his/her life expectancy should be less than 2 years from diagnosis to qualify for this procedure. The patients were grouped into six major diagnostic categories: cystic fibrosis (n = 89, 42%), pulmonary vascular disease (n = 44, 21%), bronchiolitis obliterans (n = 21, 10%), pulmonary alveolar proteinosis (n = 12, 6%), pulmonary fibrosis (n = 15, 7%), and an “other” category of miscellaneous diagnoses (n = 26, 12%).
Pretransplant Evaluation
Before listing of patients, all other organ systems were evaluated. Evidence indicating severe, irreversible injury to any other organs precluded the child from listing, as did the presence of malignancy, colonization with pan-resistant bacteria, and HIV infection. In addition, before transfer to our institution, investigation of the social circumstances took place, including an open and frank discussion with the parents regarding the commitment and uncertainties that would be involved.
Transplant Technique
The transplant procedure was performed via a bilateral anterolateral transsternal (clamshell) thoracotomy incision. Whereas it is not always necessary in adults, cardiopulmonary bypass was used in all patients except one, because the airways in children are too small to safely accommodate the double-lumen endobronchial tubes that are necessary for single lung ventilation. Bilateral sequential lung transplant technique was employed in all but nine patients (those receiving single lung transplants), and bronchial anastomoses were wrapped with donor and recipient peribronchial tissue. The bronchial anastomosis was performed using monofilament absorbable sutures in a running fashion for the membranous portion and in an interrupted fashion for the cartilaginous portion. Thirty patients had bilateral living donor lung transplants. A Broviac catheter was placed in all patients to maintain chronic vascular access.
Immunosuppression
“Triple drug” (cyclosporine, azathioprine, steroids) immunosuppression is used. For the first year posttransplant, the target trough cyclosporine blood level is 300 to 400 ng/mL; subsequent levels are 200 to 300 ng/mL. The initial steroid dose is 0.5 mg/kg daily of prednisone. The steroid dose is progressively tapered over time, but we do not believe it is appropriate to stop this drug entirely. An azathioprine dose of 1.5 mg/kg daily is administered as long as the patient’s white blood cell (WBC) count exceeds 4,000 cells/mm3. All patients receive prophylaxis against Pneumocystis carinii pneumonia with either sulfamethoxazole-trimethoprim three times per week (orally), or monthly treatment with aerosolized pentamidine when sulfa allergy or intolerance is present. Prophylaxis against mucocutaneous candidal infections is also given.
Posttransplant Surveillance
Surveillance following transplantation is grounded on evaluation from two main observational methods: periodic spirometry and bronchoscopy with biopsies and bronchoalveolar lavage. Before the patients are discharged from the hospital, they are given a home spirometer and are instructed to perform spirometry at least once per day. If there is a decrease in FEV1 greater than 10% from baseline, an evaluation for infection and rejection is undertaken. Regardless of size or age at transplant, all patients undergo regularly scheduled surveillance bronchoscopy. The greatest challenge in doing this procedure comes in small infants, wherein a bronchoscope large enough to provide sufficient lumen for biopsy forceps may obstruct the airway. This problem has been somewhat rectified first by using a “blind” bronchial biopsy procedure that allows for nonbronchoscopic entry of the biopsy forceps, 3 and second by the development of a miniforceps that is compatible with the 3.5-mm-diameter pediatric bronchoscope. Bronchoscopy with biopsy is normally performed at 10 to 14 days, 6 weeks, and then 3, 6, 9, 12, 18, and 24 months posttransplant, and every 6 months thereafter. Formal pulmonary function tests are performed at those same time intervals. Children under the age of 5 years are generally not able to fully cooperate for standard pulmonary function tests. They are therefore evaluated with infant pulmonary function tests using standard techniques. 4,5 Outside these scheduled surveillance appointments, bronchoscopy with biopsy is also recommended for virtually all clinical changes. Bronchoalveolar lavage is performed at both scheduled and nonscheduled procedures to obtain quantitative bacterial, viral, and fungal cultures.
Rejection
All suspected episodes of rejection are confirmed with histology. Grade A2 rejection 6 or greater is treated with intravenous methylprednisolone (10 mg/kg) daily for 3 days. Refractory acute rejection is treated with a 10-day course of antithymocyte globulin followed by a switch of maintenance immunosuppression to tacrolimus and mycophenolate mofetil with prednisone.
Statistics
Values expressed are mean ± standard deviation. Differences between groups were evaluated using the Student t test. Survival was calculated using Kaplan-Meier life table analysis. Actuarial freedom from bronchiolitis obliterans for each risk factor was compared using life table analysis and evaluated using the generalized Wilcoxon test. P < .05 was deemed significant.
RESULTS
Donor Statistics and Organ Procurement
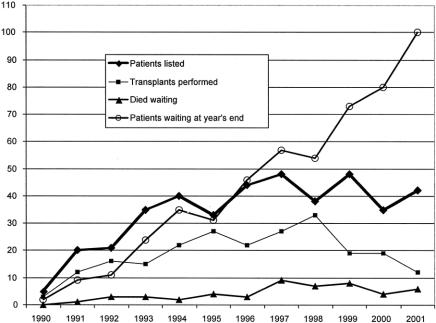
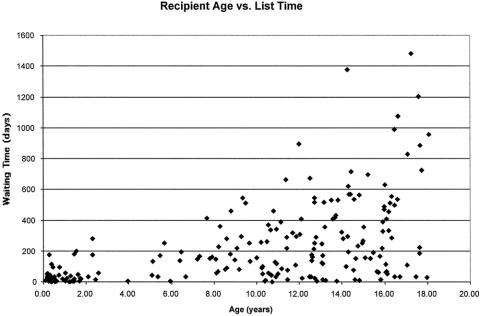
Donor availability remains a major limitation to the applicability of transplantation for end-stage lung disease. The average waiting time for donor organs for those patients transplanted was 225 ± 263 days. For the past 7 years, we have listed approximately 40 patients each year but have performed only 20 transplants per year; 5 to 10 patients have died each year while waiting for a donor. As a consequence of this pattern, our list has grown significantly and at the end of calendar year 2001 reached 100 patients waiting for LTX (Fig. 2). In general, it was found that the average waiting time per patient increased with age (Fig. 3). Fifty-one patients listed at our center died while awaiting donor organs. The average waiting time for those dying on the list was 184 ± 254 days, but this varied considerably (range 4–1,170 days). The only factor significantly associated with a shorter time between listing and death while waiting was age less than 2 years. For those listed at an age under 2 years the average time from listing to death was 36 ± 62 days (range 4–259 days). For those over 2 years of age at the time of listing who died before transplantation, the average time between listing and death was 267 ± 283 days (range 16–1,170 days). The waiting times for those surviving to LTX were 36 ± 46 days for those less than 2 years of age and 281 ± 274 days for those over 2 years of age. The average ischemic time to the left donor lung was 233 ± 78 minutes; the average ischemic time to the right donor lung was 271 ± 91 minutes.
Figure 2. Relationship of the patients listed for transplantation, the number transplanted, those dying while on the list, and the number of patients waiting for lung transplantation at our center for each year of the program. This illustrates the discrepancy between donor supply and patients listed.
Figure 3. This scattergram shows a trend toward longer waiting time for transplantation according to age at listing.
Rejection
For patients for whom there was greater than 6 months of follow-up (n = 166), there were an average of 1.95 episodes of rejection. When analyzed by age, the incidence of rejection was less for younger patients. All patients less than 2 years of age had an average of 0.6 episodes of rejection, and those less than 1 year of age at the time of transplant had an average of 0.2 episodes of rejection. The follow-up period for all age groups was approximately 3.5 years. This difference in rejection was statistically significant comparing older children to both those less than 2 and those less than 1 year of age (P < .01).
Bronchiolitis Obliterans
Because bronchiolitis obliterans occurs only after a significant period has elapsed since transplantation, only those with more than 6 months of follow-up were analyzed (n = 166). Eighty-four patients were diagnosed with bronchiolitis obliterans. 7 Among these patients, the average onset time for the bronchiolitis obliterans following transplantation was 679 ± 653 days. Risk factors that we identified for this complication were greater than two episodes of acute rejection, prolonged ischemic time (>120 minutes), age greater than 3 years, and length of follow-up. Seventeen percent of patients (15/88) with two or fewer episodes of rejection developed bronchiolitis obliterans compared with 48% (38/79) of those with more than two episodes of acute rejection. When the ischemic time was less than 2 hours, the incidence of bronchiolitis obliterans was 20% (5/25) compared with a 52% incidence when the ischemic time was greater than 2 hours. All of the patients transplanted using living donors had an ischemic time of less than 2 hours. The incidence of bronchiolitis obliterans in this group was 15% (4/26 long-term survivors). This is obviously significantly less than those undergoing cadaveric transplantation, although it relates more to the shorter ischemic times. For patients less than 3 years of age at the time of LTX, 28% (11/40) developed bronchiolitis obliterans; 58% of patients over 3 years of age at transplant did so. Unfortunately, the risk of bronchiolitis obliterans clearly increases with the length of follow-up, regardless of the ischemic time and probably other issues (Fig. 4). Other factors that did not increase the risk were pretransplant diagnosis, early graft dysfunction, and the presence of cytomegalovirus infection.
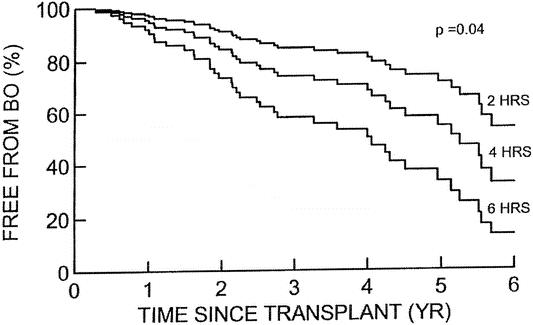
Figure 4. Kaplan-Meier life table analysis of freedom from bronchiolitis obliterans (BO) when the patients were segregated according to the organ ischemic time. There was a statistically significant difference in the incidence of bronchiolitis by 5 years posttransplant at each 2-hour interval of organ ischemic time. However, it would appear that even those with short ischemic times would likely develop this complication.
Malignancies
Posttransplant lymphoproliferative disease (PTLD) occurs frequently in association with a primary Epstein-Barr virus infection. In total, 26 of 207 patients (13%) acquired PTLD. All were treated initially with reduction in immunosuppression therapy. Those not responding to that were treated with chemotherapy. Seven of these patients died directly due to PTLD. Other malignancies observed in our series included gastric leiomyosarcoma and hepatic sarcoma; both patients with sarcomas died secondary to these lesions.
Survival
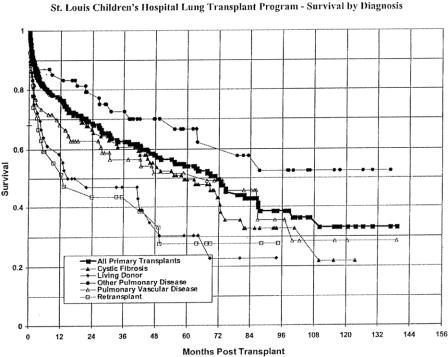
The overall survival rate for the first year posttransplantation was 77%. The 3-year and 5-year survival for these children declined to approximately 63% and 54%, respectively. Overall survival by diagnosis for those transplanted can be seen in Figure 5. Although some pretransplant diagnoses appear to offer some slight advantage, there was no statistically significant difference between any of these groups in terms of survival. Graft failure accounted for the majority of deaths in the first 60 days posttransplant (14/25, 56%). Infection was a relatively infrequent cause of early death (8%). Bronchiolitis obliterans was the leading cause of late deaths (35/61, 62%). Infection accounted for 22% of late deaths and malignancies 14%. No patient in our series died of acute rejection. Although the incidence of bronchiolitis obliterans was lower in those patients undergoing living donor lung transplantation, the overall survival was not different in this group because of early deaths. This group represented patients too ill to wait for cadaveric lung donors, and their acuity of illness was generally higher pretransplant than other diagnostic groups.
Figure 5. Survival curve for the entire series as well as for each pretransplant diagnosis.
DISCUSSION
Survival following LTX remains the lowest of all solid organ transplants, with 5-year survival figures of just under 50% in all patients reported to the registry of the International Society for Heart and Lung Transplantation. 8 By comparison, 5-year survival for heart transplantation is approximately 70%8 and for liver transplantation approximately 80%. 9 Factors that limit the success of LTX in children are similar to adults: donor shortage, early graft failure, and bronchiolitis obliterans. Our survival statistics exceed those seen in adult LTX—55% versus 48% at 5 years. This difference is probably not statistically significant but reflects the notion that it is feasible to achieve results in children that have been deemed acceptable for adult LTX. Although no specific analysis of relative risk has been performed, pediatric lung transplant recipients as a whole are probably of higher risk based on their pretransplant diagnosis. The lowest-risk groups for transplantation in adult LTX are those with emphysema and α1-antitrypsin disease. This also represents the single largest diagnostic group. 8 There were no patients in our series with this diagnosis. In addition, pulmonary vascular disease is a high-risk diagnostic group and represented 21% of our transplant patients, as opposed to 5% to 10% in adult LTX. 8
As the waiting list grows, the waiting time for transplantation grows. Unfortunately, this is accompanied by more deaths in patients on the waiting list for LTX. From 1991 through 2000, 294 children under the age of 18 years have died while awaiting LTX; for the past 3 years an average of 40 patients have died annually. 10 Certainly our patients have not been immune to this problem. In spite of intense efforts at public education and other measures, the number of potential organ donors annually is unchanged. This has led to the development of techniques that might expand the donor pool such as cadaveric lobar transplantation using larger donors and living donor lobar transplantation as an alternative to waiting for a protracted period on the list, risking death. 11 Approximately 15% of our patients were transplanted using one of these techniques. Although the use of lobes from a larger cadaveric donor sounds like a reasonable approach, in reality those donors will not be available because there are more adult or large teenagers on the list waiting for longer periods of time than small children. Living donor lung transplantation should be reserved for situations in which the recipient clearly will not survive to receive a cadaveric donor offer. In addition, there must be two satisfactory donors willing to make such a sacrifice. This is a more technically challenging operation than cadaveric LTX because the donor bronchial, arterial, and venous cuffs are purposely short so that the vascular and airway structures for the donor can be safely handled. Although to date there have been no reported deaths among the donors, a thoracotomy is not a trivial operation and has been associated with a moderate degree of morbidity in our experience. 12 We endorse living donor transplantation but recognize that it is associated with risk for three individuals, not just one.
Single versus bilateral LTX is somewhat controversial. Early results in adults have shown equivalent survival. However, with the passage of time, it is apparent that there is a survival advantage for bilateral versus single LTX. 8 We have generally preferred bilateral LTX for children because of the unclear growth potential in transplanted lungs. There is mounting evidence, however, that the transplanted lungs grow in very young patients 13 and immature animals. 14 Our approach may change in response to this animal and clinical research. Patients with cystic fibrosis (our largest diagnostic group) require bilateral lung transplantation because of the need to remove both chronically infected lungs.
The prevention of early graft failure has been the subject of a good deal of research. 15 The “ideal” preservation solution to extend potential ischemic times and avoid reperfusion injury has not yet been found. The relation we found between donor ischemic time and the development of bronchiolitis obliterans is also a subject that deserves further study. One must surmise that the preservation solution could have an impact on this issue.
Bronchiolitis obliterans is viewed by most to be a manifestation of chronic rejection, although it can present itself as a primary disorder that is a legitimate indication for LTX. The precise etiology is unknown, although the donor ischemic time and episodes of early acute rejection have been identified in our series as possible risk factors. Because bronchiolitis obliterans is one of the leading causes of late death in lung transplants, 8 clinical and basic research aimed at understanding the vectors of injury and disease progression are of supreme importance to the field of LTX. Furthermore, because the airway as the site of injury is accessible for assessment and therapy, bronchiolitis obliterans may provide a model system whereby chronic rejection—which also affects long-term success in heart, kidney, and liver transplants—can be understood and overcome.
In summary, LTX in children is a high-risk but viable treatment for end-stage pulmonary parenchymal and vascular disease. In general, this treatment modality is indicated for increasing the duration of life, not solely for improvement of the quality of life. The current survival results are somewhat encouraging, considering many of the unknown issues concerning LTX in children. Bronchiolitis obliterans remains the primary obstacle for lasting survival following LTX in children.
DISCUSSION
Dr. Larry R. Kaiser (Philadelphia, PA): I would just like to say that when we started this program back in, I guess it was 1990 or 1991, Dr. Cooper initially was against starting the program at the Children’s Hospital. And I think some of that was because of the technical limitations. I think that what Dr. Huddleston and his colleagues have shown today is that not only is it feasible to transplant children, but it is also feasible to transplant very young children with results as good as we see in the adult population.
I think that the same problems that occur in the adults, that is the problem of obliterative bronchiolitis, will occur in the children. We noticed early in the going that sometimes the complications occur even earlier. Dr. Huddleston, are you still seeing obliterative bronchiolitis occurring earlier, especially in the adolescent population that you are transplanting for cystic fibrosis? Also, tell us a little bit about who is not a candidate for transplant in the cystic fibrosis population.
I enjoyed the paper very much. It is a tremendous series and as you point out represents a significant number of the transplants performed in children worldwide.
Presenter Dr. Charles B. Huddleston (St. Louis, MO): Thank you, Dr. Kaiser. As with a lot of the transplant programs, and with our very early experience in particularly the young patients, we didn’t see any, but we thought that the infants would be completely immune to this process. Subsequently we found that that is not the case. It appears that at least it is delayed for a number of years in the particularly young patients. Apart from that, I would say that it is identical to the adult experience in terms of how soon this comes on and how quickly it progresses.
We have undertaken a number of retransplants for bronchiolitis obliterans. Unfortunately, many of those have developed the recurrence of bronchiolitis obliterans and the retransplanted lung failed early on following that. It is hoped that perhaps with the different immunosuppressants that are coming on the market that this might be put off a bit longer.
The contraindication for lung transplant in cystic fibrosis patients is the presence of multiresistant bacterial organisms, particularly Burkholderia cepacia (it used to be called Pseudomonas cepacia). Those patients have had uniformly a very poor outcome following lung transplantation. Apart from that, there haven’t been any specific contraindications to ascribe to the cystic fibrosis group as a whole.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Charles B. Huddleston, MD, #1 Children’s Place, Suite 5S 50, Children’s Hospital, St. Louis, MO 63110.
E-mail: huddlestonc@msnotes.wustl.edu
Accepted for publication April 24, 2002.
References
- 1.Cooper JD, Ginsberg RJ, Goldberg M, the Toronto Lung Transplant Group. Unilateral transplantation for pulmonary fibrosis. N Engl J Med 1986; 314: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 2.Boucek MM, Faro A, Novick RJ, et al. The registry of the International Society for Heart and Lung Transplantation: fourth official pediatric report—2000. J Heart Lung Transplant 2001; 20: 39–52. [DOI] [PubMed] [Google Scholar]
- 3.Mullins D, Livne M, Mallory GB, Kemp JS. A new technique for transbronchial biopsy in infants and small children. Ped Pulmon 1995; 20: 253–257. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt T, Hehre D, Bancalari E, Watson H. A simple method for measuring functional residual capacity by N2 washout in small animals and newborn infants. Pediatr Res 1985; 19: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 5.Taussig LM, Landau LI, Godfrey S, Arad I. Determinants of forced expiratory flows in newborn infants. J Appl Physiol 1982; 53: 1220–1227. [DOI] [PubMed] [Google Scholar]
- 6.Berry GJ, Brunt EM, Chamberlain D, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. J Heart Transplant 1990; 9: 596–601. [PubMed] [Google Scholar]
- 7.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant 1993; 12: 713–716. [PubMed] [Google Scholar]
- 8.Hosenpud JD, Bennett LE, Keck BM, et al. The registry of the International Society for Heart and Lung Transplantation: eighteenth official report—2001. J Heart Lung Transplant 2001; 20: 805–815. [DOI] [PubMed] [Google Scholar]
- 9.Smith CM, Davies DB, McBride MA. Liver transplantation in the United States: a report from the UNOS Liver Transplant Registry. Clinical Transplants, 1999;: 23–34. [PubMed] [Google Scholar]
- 10.2000 Annual Report of the U.S. Scientific Registry for Transplant Recipients and the Organ Procurement and Transplantation Network, Transplant Data: 1990–1999. U.S. Department of Health and Human Services, Health Resources and Services,
- 11.Barr ML, Baker CJ, Schenkel FA, et al. Living donor lung transplantation: selection, technique, and outcome. Transplant Proc 2001; 33: 3527–3532. [DOI] [PubMed] [Google Scholar]
- 12.Battafarano RJ, Anderson RC, Meyers BF, et al. Perioperative complications after living donor lobectomy. J Thorac Cardiovasc Surg 2000; 120: 909–915. [DOI] [PubMed] [Google Scholar]
- 13.Hislop AA, Odom NJ, McGregor CGA, Haworth SG. Growth potential of the immature transplanted lung: an experimental study. J Thorac Cardiovasc Surg 1990; 100: 360–370. [PubMed] [Google Scholar]
- 14.Cohen AH, Mallory GB, Ross K, et al. Growth of lungs after transplantation in infants and in children younger than 3 years of age. Am J Respir Crit Care Med 1999; 159: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 15.Huddleston CB, Mendeloff EN. Heart and lung preservation for transplantation. J Cardiac Surg 2000; 15: 108–121. [DOI] [PubMed] [Google Scholar]