Abstract
Objective
To analyze the effectiveness of new techniques of mitral valve reconstruction (MVR) that have evolved over the last decade, such as aggressive anterior leaflet repair and minimally invasive surgery using an endoaortic balloon occluder.
Summary Background Data
MVR via conventional sternotomy has been an established treatment for mitral insufficiency for over 20 years, primarily for the treatment of patients with posterior leaflet prolapse.
Methods
Between June 1980 and June 2001, 1,195 consecutive patients had MVR with ring annuloplasty. Conventional sternotomy was used in 843 patients, minimally invasive surgery in 352 (since June 1996). Anterior leaflet repair was performed in 374 patients, with increasing use over the last 10 years. Follow-up was 100% complete (mean 4.6 years, range 0.5–20.5).
Results
Hospital mortality was 4.7% overall and 1.4% for isolated MVR (1.1% for minimally invasive surgery vs. 1.6% for conventional sternotomy;P = .4). Multivariate analysis showed the factors predictive of increased operative risk to be age, NYHA functional class, concomitant procedures, and previous cardiac surgery. The 5-year results for freedom from cardiac death, reoperation, and valve-related complications among the 782 patients with degenerative etiology are, respectively, as follows (P > .05 for all end points): for anterior leaflet repair, 93%, 94%, 90%; for no anterior leaflet repair, 91%, 92%, 91%; for minimally invasive surgery, 97%, 89%, 93%; and for conventional sternotomy, 93%, 94%, 90%.
Conclusions
These findings indicate that late results of MVR after minimally invasive surgery and after anterior leaflet repair are equivalent to those achievable with conventional sternotomy and posterior leaflet repair. These options significantly expand the range of patients suitable for mitral valve repair surgery and give further evidence to support wider use of minimally invasive techniques.
Over the last two decades several large experiences with mitral valve reconstruction (MVR) have been reported, 1–4 and MVR has become the preferred treatment for patients with mitral insufficiency from degenerative disease. A 1989 report from our institution 2 demonstrated that patients undergoing MVR had fewer late valve-related complications than patients having mitral valve replacement, and this observation was subsequently confirmed in more than 10 large series, reviewed by Yun and Miller. 5 In the last 10 years two additional major breakthroughs have occurred: the increased ability to repair patients with pathology involving the anterior mitral leaflet, and the introduction of minimally invasive techniques for mitral valve repair surgery.
In the United States the vast majority of patients undergoing MVR have degenerative disease, and the earlier reports predominantly involved repair in patients with posterior leaflet pathology. Nevertheless, as more patients were evaluated for surgical repair of mitral valve prolapse, it became evident that anterior leaflet pathology was present in approximately one third of the patients. 6 Thus, to more effectively offer valve repair to this more complex group of patients with anterior leaflet disease, the traditional techniques of anterior leaflet repair of chordal shortening and chordal transfer described by Carpentier 7 were supplemented by several innovative approaches, such as the use of artificial chordal replacement 6,8,9 or anterior leaflet resection. 10,11 Using a variety of these methods, surgeons have become more aggressive and confident in offering valve repair to patients with anterior leaflet disease. 10,12,13 This report demonstrates a changing pattern of anterior leaflet mitral valve repair at our institution due to an increased use of anterior leaflet resection and compares the late results in these patients with the results in patients without anterior leaflet disease.
A more dramatic breakthrough in cardiac surgery, which has occurred over the last 5 years, was the introduction of minimally invasive techniques for the surgical treatment of valvular heart disease. A variety of techniques has been described, including the parasternal approach, 14,15 partial or hemisternotomy incisions, 16–18 minithoracotomy incisions (with or without balloon aortic occlusion for cardioplegia delivery), 19–22 video-assisted surgery, 23,24 and the use of robotics. 25–27 Reports by Cohn et al, 14 Cosgrove et al, 15 and Gillinov et al 28 demonstrated excellent early results using both the parasternal and partial sternotomy approaches.
Our initial results with minimally invasive mitral valve surgery, using a minithoracotomy technique with balloon aortic occlusion (termed “Port Access”), were reported at the American Heart Association in 1997. 20 These promising early results were confirmed in a multicenter trial 21 that demonstrated the safety and efficacy of the “Port Access” approach. Subsequently, using a case-controlled method, Grossi et al 29 reported less need for blood transfusions and fewer postoperative infections or wound complications in patients who underwent a minimally invasive technique for isolated valve surgery, while separate comparative studies by Glower et al 30 and Grossi et al 31 demonstrated that patients undergoing minimally invasive surgery had less postoperative pain and shorter recovery times than patients undergoing traditional surgery.
Nevertheless, despite these encouraging reports and the potential for lowering short-term morbidity with a minimally invasive approach, many surgeons have remained skeptical and hesitant to adopt a minimally invasive technique, possibly due to concerns over the learning curve or to the paucity of long-term data. The primary purpose of this study, therefore, was to provide late follow-up data in patients after minimally invasive valve repair. This prospective trial assessed late survival, repair durability, and late valve-related complications in all patients undergoing mitral valve repair at New York University over a 20-year interval and compares late outcomes in the minimally invasive versus the traditional cases.
METHODS
Between June 1980 and June 2001, 1,195 consecutive patients had MVR with ring annuloplasty. Patient demographics are listed in Table 1. The mean age was 60.7 ± 14.8 years (range 23–91); 743 patients (62%) were male and 452 (38%) were female. Isolated primary repair was done in 636 patients (53%), while 315 patients (26%) had concomitant coronary artery bypass grafting, 112 patients (9.4%) had concomitant valve surgery, and 73 patients (6.1%) had previous cardiac procedures.
Table 1. PATIENT DEMOGRAPHICS
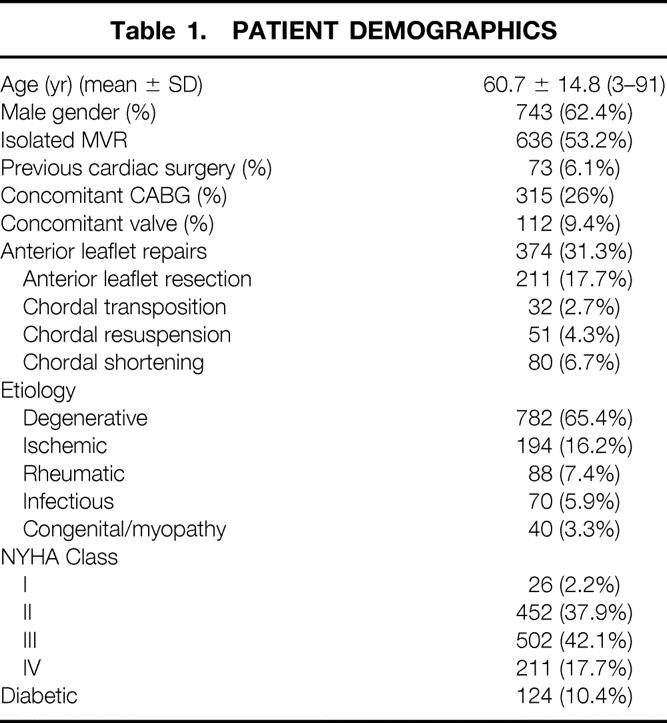
The etiology of the mitral insufficiency was degenerative in 782 patients (65.4%), ischemic in 194 (16.2%), rheumatic in 88 (7.4%), infectious in 70 (5.9%), and congenital or cardiomyopathy in 40 (3.3%). One hundred twenty-four patients (10.4%) had diabetes.
Preoperatively, 26 patients (2.2%) were New York Heart Association (NYHA) class I, 452 (37.9%) were class II, 502 (42.1%) were class III, and 211 (17.7%) were class IV.
Surgical Techniques
Overall, 374 patients (31.3%) had repair of anterior leaflet pathology. The techniques used for anterior leaflet repair included chordal transposition in 32 patients, chordal resuspension in 51, chordal shortening in 80, and anterior leaflet resection (Fig. 1) in 211. The increasing use of anterior leaflet resection by year is illustrated in Figure 2.
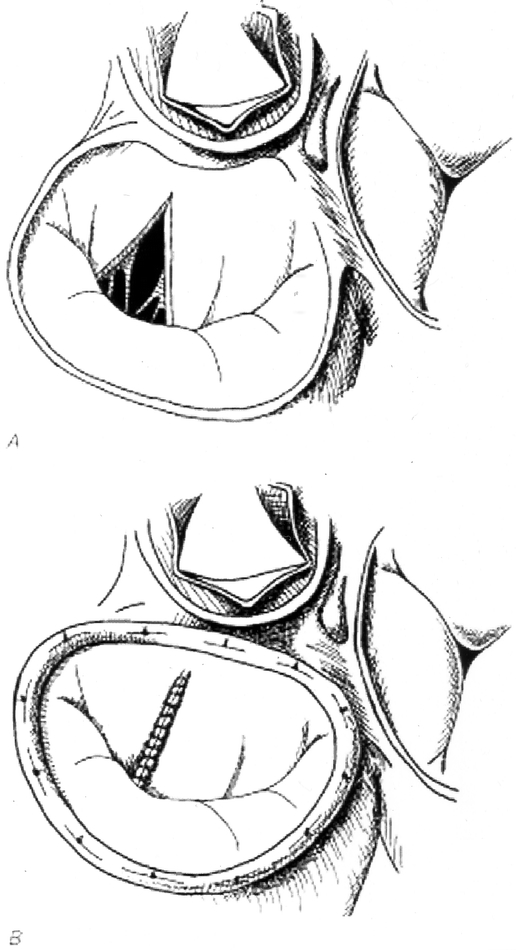
Figure 1. Anterior leaflet resection. (A) Triangular resection of the anterior leaflet of the mitral valve. (B) Sutured repair.
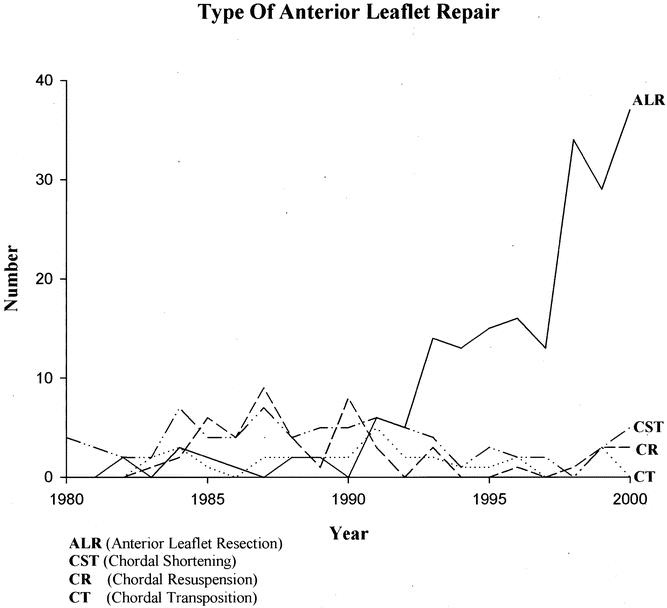
Figure 2. Increasing use of anterior leaflet resection for mitral valve repairs at New York University Medical Center.
A conventional sternotomy approach was used for valve repair in 843 patients, while a minimally invasive minithoracotomy technique was used in 352 patients. The minimally invasive approach was used increasingly since 1996 and was used in more than 90% of the isolated mitral valve repairs over the last 3 years. In the minimally invasive group a right anterior minithoracotomy incision was used in 97% of the patients, with 3% of patients receiving a left posterior minithoracotomy. Femoral perfusion was used in 79% of the minimally invasive cases; percutaneous or transthoracic direct aortic cannulation was used in 21%, with the latter technique used increasingly in recent years. More than 90% of the patients undergoing minimally invasive surgery had internal balloon catheter occlusion of the aorta rather than cross-clamping, with cardioplegia delivered either antegrade or retrograde through a percutaneous coronary sinus catheter. The typical operative setup (Fig. 3) and technique for minimally invasive mitral valve repair have been previously described. 20,32,33
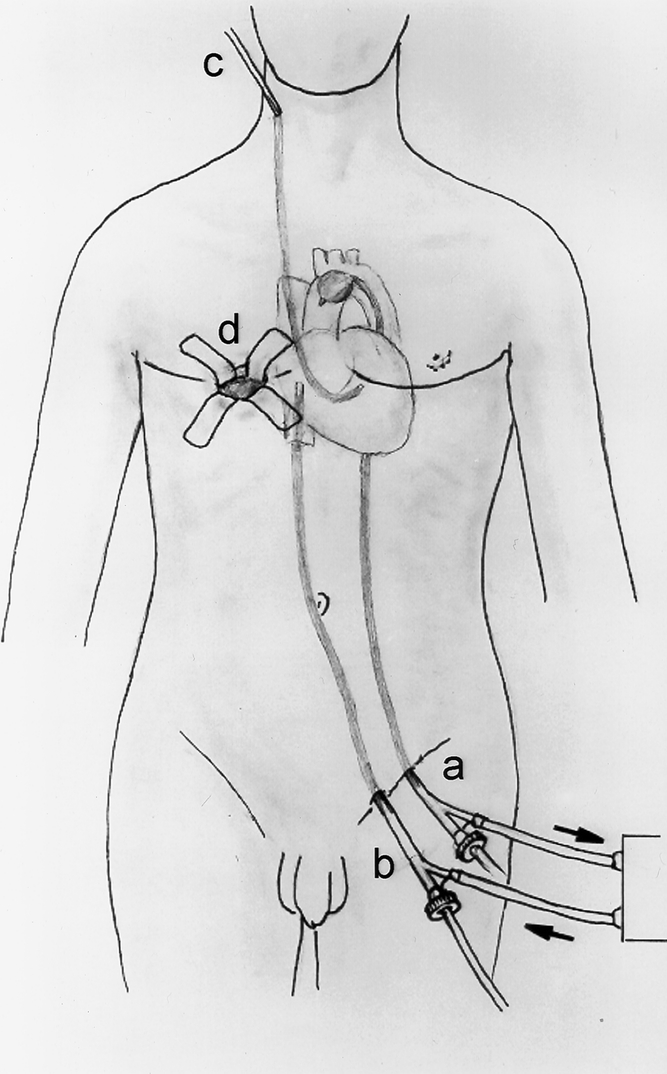
Figure 3. Typical operative setup for minimally invasive mitral valve repair at New York University Medical Center. (A) Femoral arterial cannulation with balloon catheter introduced for occlusion of the ascending aorta. (B) Femoral venous cannulation with tip of catheter positioned into the right atrium. (C) Percutaneous right internal jugular retrograde cardioplegia catheter positioned in the coronary sinus. (D) Right minithoracotomy incision performed via the inframammary crease through the fourth intercostal space.
Follow-up and Statistical Analysis
All patients were entered into the study prospectively and followed with yearly examinations by their surgeon or by phone interviews conducted by the research study coordinator. All data were entered into a computer and analyzed by use of the statistical software SPSS (SPSS, Inc., Chicago, IL). Follow-up was 100% complete, with a mean follow-up interval of 4.6 years (range 0.5–20.5). Continuous variables were analyzed by the Student t test and categorical variables by chi-square. Preoperative and intraoperative risk factors were analyzed by multivariate analysis for correlation with hospital mortality. Late follow-up data in the 782 patients with degenerative etiology were analyzed for differences between study groups by Cox logistic regression analysis.
RESULTS
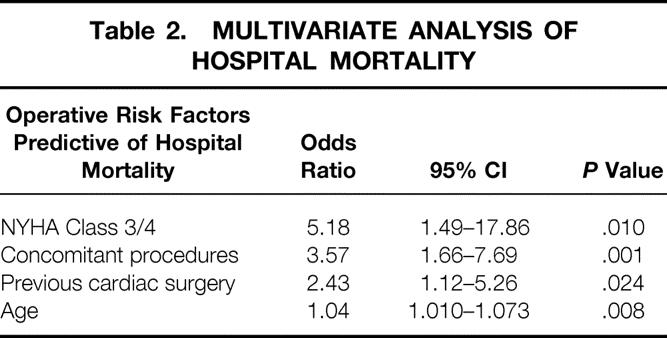
The overall hospital mortality was 4.7% and the mortality for isolated mitral valve repair was 1.4% (1.1% for minimally invasive surgery and 1.6% for conventional sternotomy, P = .4). The factors found be predictive of increased operative risk by multivariate analysis were NYHA functional class, concomitant procedures, previous cardiac surgery, and age (Table 2). Neither anterior leaflet repair nor minimally invasive surgery had a significant impact on operative risk.
Table 2. MULTIVARIATE ANALYSIS OF HOSPITAL MORTALITY
Among the patients with degenerative etiology, late results were compared between the 374 patients who had a repair of the anterior leaflet and the 821 patients who did not. In the patients with anterior leaflet repair the 5-year survival from late cardiac death was 93%, the 5-year freedom from reoperation was 94%, and the 5-year freedom from valve-related complications was 90%. In the patients without anterior leaflet procedures the 5-year survival from cardiac death was 91%, the 5-year freedom from reoperation was 92%, and the 5-year freedom from valve-related complications was 91% (Table 3;P > .05 for all end points). Also, the technique used for anterior leaflet repair had no significant impact on late repair durability. For example, and interestingly, the 5-year freedom from reoperation after chordal shortening was 91%, which was not significantly different than the other methods of anterior leaflet repair (P > .05).
Table 3. FIVE-YEAR FREEDOM FROM LATE CARDIAC DEATH, REOPERATION, AND VALVE-RELATED COMPLICATIONS
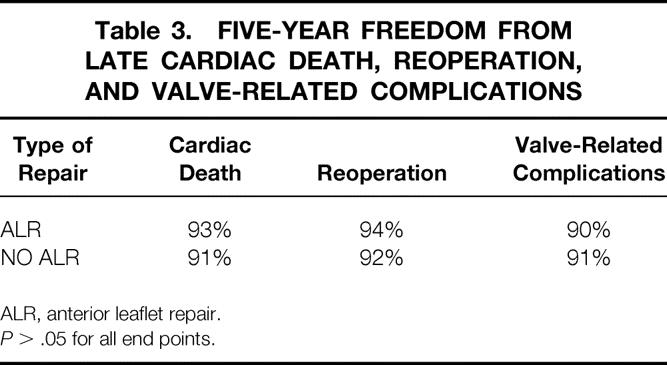
ALR, anterior leaflet repair.
P > .05 for all end points.
Similarly, in the patients with degenerative etiology, the late results were compared between the 352 patients in whom a minimally invasive approach was used and the 843 patients who had conventional surgery through a sternotomy incision. For the minimally invasive patients the 5-year survival from cardiac death was 97%, the 5-year freedom from reoperation was 89%, and the 5-year freedom from valve-related complications was 93%. In the conventional sternotomy patients the 5-year survival from cardiac death was 93%, the 5-year freedom from reoperation was 94%, and the 5-year freedom from valve-related complications was 90% (Table 4;P > .05 for all end points).
Table 4. FIVE-YEAR FREEDOM FROM LATE CARDIAC DEATH, REOPERATION, AND VALVE-RELATED COMPLICATIONS
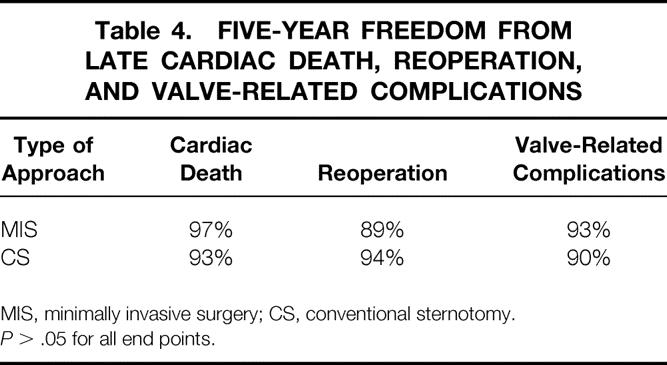
MIS, minimally invasive surgery; CS, conventional sternotomy.
P > .05 for all end points.
Thus, late clinical outcomes were not affected by the use of anterior leaflet repair or by the use of the minimally invasive operative approach.
DISCUSSION
In recent years several trends have emerged in the treatment of patients with valvular heart disease, and in particular in the treatment of patients with mitral valve insufficiency. One significant change has been an increased echocardiographic scrutiny of patients with valvular heart disease by cardiologists, with more aggressive recommendations for earlier surgery based on the echocardiographic findings of progressive cardiac dilation or left ventricular systolic dysfunction, before the onset of significant symptoms or left ventricular injury. In patients with mitral insufficiency, changes in management algorithms were fueled, at least in part, by the excellent surgical results achieved with mitral valve repair and by the low operative risk and decreased late morbidity seen in these patients compared to valve replacement patients. 2,5,34,35 Cardiologists’ confidence in surgeons’ ability to perform a durable valve repair consequently led to a shift in the treatment paradigm toward earlier surgery.
Repair of Anterior Leaflet Prolapse
Since patients with mitral insufficiency from anterior leaflet prolapse were initially less likely to have successful repair, these patients were often referred for surgery later in the course of their disease. Thus, the observation in this report that patients undergoing repair of the anterior leaflet had late outcomes that were equivalent to patients without anterior leaflet repair is quite significant. In this series, the operative techniques used to repair the anterior leaflet varied, although triangular resection of the anterior leaflet was used most frequently and increasingly in the later part of the study. The technique was used primarily in patients with severe myxomatous degeneration of the mitral valve, where redundant, excessive anterior leaflet tissue was present.
Interestingly, the late durability after anterior leaflet repair was not influenced by the technique used (chordal shortening, chordal reimplantation, chordal transposition, or anterior leaflet resection). This result differs from the findings of Phillips et al 13 and Smedira et al, 12 where patients receiving a chordal shortening procedure for anterior leaflet repair had an increased late failure rate. One possible explanation for the difference in findings may be in patient selection. In the current series chordal shortening was used selectively and was not used if the chordae were extremely thin. Another possible explanation may be in the technique used for chordal shortening. Our preferred method involves imbricating the elongated primary or secondary chordae onto the free edge of the prolapsed leaflet and not into the papillary muscle head, as this may produce necrosis. Since the late failures reported by Phillips et al 13 after using chordal shortening were primarily due to late rupture at the papillary muscle head, this difference in technique might explain the different results. While no patients in our series received artificial chordae for anterior leaflet repair, the results using artificial chordae reported by David et al, 9 Smedira et al, 12 and Phillips et al 13 were excellent, and the use of artificial chordae seems to be a viable option for repair of anterior leaflet pathology.
The most significant observation from this study concerning anterior leaflet repair was the durability after anterior leaflet resection. Importantly, the late results were completely equivalent to those achieved in patients with only posterior leaflet pathology. While in our experience anterior leaflet resection has been an extremely reliable technical option, it is ultimately up to the surgeon to use each technique selectively for optimal results.
Minimally Invasive Valve Repair
Probably the most dramatic change in surgical technique over the last 5 years was the introduction of minimally invasive surgery for the treatment of valvular disease. Although this approach remains controversial, at our institution minimally invasive surgery has made a significant impact on the care given to patients with mitral insufficiency. For example, over the last 3 years less than 10% of the patients undergoing isolated mitral valve repair had a traditional median sternotomy approach. As summarized in the introduction of this paper, the early results with minimally invasive valve repair have been exceedingly good, 22 with measurable patient benefits. 29,30,36 It has been imperative, however, for late results to be carefully documented and analyzed after minimally invasive valve surgery if the surgical community was to accept this approach as a viable option for the vast majority of patients. The current study begins to provide these data. Significantly, the 5-year results after minimally invasive valve repair were indistinguishable from the results obtained after conventional sternotomy.
In summary, the minimally invasive technique for mitral valve repair produced durable and effective results with no increased risk. These data offer further support for wider use of minimally invasive techniques for the treatment of patients with mitral valve disease.
DISCUSSION
Dr. Irving L. Kron (Charlottesville, VA): I very much appreciate the opportunity to comment on this excellent paper by Dr. Galloway and colleagues from New York University. They present a very large series of patients undergoing mitral valve repair. And for those in the audience who don’t know, mitral valve repair is far superior than mitral valve replacement, and they have basically shown that they can amplify this technique. I have several questions for the authors.
Our own results for mitral valve repair confirm the authors’ low mortality. We found, as the authors did, that there is essentially minimal risk for patients with myxomatous disease, but we have some risks from ischemic mitrals. Since their isolated cases indeed had only minimal mortality, were the ischemics the bad actors in this crowd?
We have changed our approach for the ischemic mitral, particularly as it relates to the patient with a large ventricle, and we have added ventricular remodeling operations for these patients and stabilization of the posterior papillary muscle. I am wondering if Dr. Galloway and his colleagues have changed their approach to the ischemic mitral.
While his focus on the anterior mitral repair is unique and truly has transformed this difficult area, basically these patients in the past have not been referred for mitral valve repair surgery since it was thought not to be repairable.
We, too, very much like the NYU approach of shortening of chordae of the anterior leaflet, and we are pleased to see that the authors had good results with this technique. However, I wonder if Dr. Galloway can tell me and the audience when they would use chordal shortening as opposed to anterior leaflet resection.
Finally, the authors have demonstrated excellent results for the minimally invasive approach, but I wonder if they could tell us the relative costs of the minimally invasive approach to standard sternotomy, and do they think the robotic approach might add something to this?
This is a great series, Dr. Galloway, and you ought to be proud.
Presenter Dr. Aubrey C. Galloway, jr. (New York, NY): Thank you, Dr. Kron, for your questions. Regarding the first question, on ischemic disease. I think that most reports have observed an increased mortality in these patients. In our multivariate analysis, concomitant procedures, which represented primarily concomitant bypass grafting for ischemic disease, increased the odds ratio for hospital death by 3.57. The most important factor increasing the operative risk, however, was the NYHA functional classification, which is a surrogate of left ventricular function. Patients with NYHA class III or IV status preoperatively had an increased odds ratio for hospital mortality of 5.18. Thus, ischemic etiology increases the operative risk because concomitant bypass grafting is necessary and because these patients generally have worse left ventricular function and a poor NYHA functional class, often with symptoms of congestive heart failure.
Certainly mitral repair in ischemic patients can often be tricky. The major problem in most patients with chronic ischemic mitral insufficiency is a combination of a dilated annulus and restricted valve motion due to the decreased ejection fraction. The latter problem may prevent the mitral valve leaflet from reaching the proper closing plane, since the leaflet may be tethered or displaced towards the apex of the heart by the dilated ventricular wall. In general, our approach to repair of these patients has been to overcorrect the annular dilation with a small annuloplasty device, and we have been able to achieve extremely durable results as long as the valve leaflets are not overly displaced towards the apex of the heart. Simply elevating the posterior annulus and correcting annular dilation with the annuloplasty will provide a good coaptation point for the anterior leaflet in most cases. We have not performed chordal elongation in any patients, but your idea of removing tension from the displaced posterior papillary muscle is appealing, as this addresses the pathology present in the extremely dilated heart. The patients that fail late after ischemic valve repair generally do so from excessive apical displacement of the valve leaflets, resulting in restrictive leaflet motion that is not corrected by annuloplasty alone.
Regarding the questions on the durability of the various types of anterior leaflet repairs and the choice of anterior leaflet procedures, there are no differences in the durability after chordal shortening, chordal transfer, or anterior leaflet resection in our experience. These findings are different from other reports, and our results may be explained both by our technique of choral shortening and by our selection criteria for the particular type of anterior repair technique used.
Our preferred method for chordal shortening for approximately 10 years has been to shorten or imbricate the elongated chord onto the undersurface of the free edge of the anterior leaflet. This is different than the technique of chordal shortening described by Carpentier, which advocated implantation of the chord into the tip of the papillary muscle, potentially producing necrosis, with resultant rupture and recurrent insufficiency. The technique of shortening elongated chordae onto the free edge of the leaflet has been quite successful, as long as the elongated chordae are not extremely thin.
In making the choice between triangular anterior leaflet resection and either chordal transfer or chordal shortening, the amount of redundant anterior leaflet tissue is the main determining factor. In patients who have myxomatous disease with excessive anterior leaflet tissue, triangular resection of the prolapsing segment is our preferred method. As this report suggests, the late results have been exceedingly good. Care must be taken not to remove too much leaflet tissue, but if excessive tissue is present and the patient can be left with a normal-sized anterior leaflet after repair, this technique results in a more normal anterior leaflet, and also lowers the risk of postrepair systolic anterior motion. In patients with nonmyxomatous prolapsing valves, where no excessive tissue is present, triangular resection would result in too small of an anterior leaflet. In these patients, either chordal transfer or chordal shortening is preferred, depending on the thickness and strength of the prolapsing anterior chordae, and the suitability of the corresponding posterior leaflet and chordae for transfer. Thus, the pathology present dictates the repair technique used.
Concerning costs: new technology is costly and must be evaluated both in terms of effectiveness and cost-effectiveness. The Port Access technology currently costs approximately $4,000 to $5,000 per case. We studied this, and interestingly, the upfront technology costs were recouped by shorter hospital and ICU stays and less of a need for blood transfusions, so the technology ended up being cost-neutral.
Finally, regarding the use of the surgical robot for mitral valve repair. We have used robotic assistance experimentally for mitral surgery at our institution and so far have found no added value. Certainly, surgical robotics is in its infancy, and these techniques deserve further scrutiny as the technology continues to develop.
Again, I appreciate your comments, and I would like to thank the Association for the privilege of the floor.
Dr. Richard J. Shemin (Boston, MA): I want to compliment the NYU group for bringing this data to our attention. It is a very excellent and large series of mitral valve reconstructions.
I personally have found that the most reliable technique to repair the anterior leaflet, particularly if you are not worried about excess redundant tissue, is to use Gore-Tex suture and produce artificial chords. I am interested in whether or not you have used that technique.
Also, have you analyzed your failures of anterior leaflet repair? Since you used a variety of techniques, is there one that you feel at this time is superior to the others?
Dr. Aubrey C. Galloway, jr. (New York, NY): I will answer the last part of that question first. When we looked at subgroup analysis of anterior leaflet repair comparing triangle resection, chordal shortening, and choral transfer, there was no difference in the 5-year durability of the valve repair, which is different than what others have reported. We think, particularly with chordal shortening, it may have to do with shortening the chordae on to the free edge of the leaflet rather than to the papillary muscle head, which could produce necrosis.
We have not used artificial chordal replacement, which is another one of the new techniques that have been described in the last 10 years. We comment on this in the manuscript. The reports have been good with chordal shortening and this is certainly another viable option, but we don’t have any experience with this approach.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Aubrey C. Galloway, MD, Professor of Surgery and Director of Cardiac Surgical Research, NYU School of Medicine, 530 First Avenue, Suite 9-V, New York, NY 10708.
E-mail: galloway@cv.med.nyu.edu
Accepted for publication April 24, 2002.
References
- 1.Galloway AC, Colvin SB, Baumann FG, et al. Long-term results of mitral valve reconstruction with Carpentier techniques in 148 patients with mitral insufficiency. Circulation 1988; 78 (3 Pt 2):I97–105. [PubMed] [Google Scholar]
- 2.Galloway AC, Colvin SB, Baumann FG, et al. A comparison of mitral valve reconstruction with mitral valve replacement: intermediate-term results. Ann Thorac Surg 1989; 47: 655–662. [DOI] [PubMed] [Google Scholar]
- 3.Deloche A, Jebara VA, Relland JY, et al. Valve repair with Carpentier techniques. The second decade. J Thorac Cardiovasc Surg 1990; 99: 990–1002. [PubMed] [Google Scholar]
- 4.Cosgrove DM, Chavez AM, Lytle BW, et al. Results of mitral valve reconstruction. Circulation 1986; 74 (3 Pt 2):I82–87. [PubMed] [Google Scholar]
- 5.Yun KL, Miller DC. Mitral valve repair versus replacement. Cardiol Clin 1991; 9: 315–327. [PubMed] [Google Scholar]
- 6.David TE. Invited letter concerning: correction of prolapse of the anterior leaflet of the mitral valve. J Thorac Cardiovasc Surg 1992; 104: 1489. [PubMed] [Google Scholar]
- 7.Carpentier A. Cardiac valve surgery—the “French correction.” J Thorac Cardiovasc Surg 1983; 86: 323–337. [PubMed] [Google Scholar]
- 8.David TE. Replacement of chordae tendineae with expanded polytetrafluoroethylene sutures. J Card Surg 1989; 4: 286–290. [DOI] [PubMed] [Google Scholar]
- 9.David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1991; 101: 495–501. [PubMed] [Google Scholar]
- 10.Grossi EA, Galloway AC, LeBoutillier M 3rd, et al. Anterior leaflet procedures during mitral valve repair do not adversely influence long-term outcome. J Am Coll Cardiol 1995; 25: 134–136. [DOI] [PubMed] [Google Scholar]
- 11.Grossi A, LaPietra A, Galloway AC, et al. History of mitral valve anterior leaflet repair with triangular resection. Ann Thorac Surg 2001; 72: 1794–1795. [DOI] [PubMed] [Google Scholar]
- 12.Smedira NG, Selman R, Cosgrove DM, et al. Repair of anterior leaflet prolapse: chordal transfer is superior to chordal shortening. J Thorac Cardiovasc Surg 1996; 112: 287–292. [DOI] [PubMed] [Google Scholar]
- 13.Phillips MR, Daly RC, Schaff HV, et al. Repair of anterior leaflet mitral valve prolapse: chordal replacement versus chordal shortening. Ann Thorac Surg 2000; 69: 25–29. [DOI] [PubMed] [Google Scholar]
- 14.Aklog L, Adams DH, Couper GS, et al. Techniques and results of direct-access minimally invasive mitral valve surgery: a paradigm for the future. J Thorac Cardiovasc Surg 1998; 116: 705–715. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove DM 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998; 65: 1535–1539. [DOI] [PubMed] [Google Scholar]
- 16.Gundry SR, Shattuck OH, Razzouk AJ, et al. Facile minimally invasive cardiac surgery via ministernotomy. Ann Thorac Surg 1998; 65: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 17.Nair RU, Sharpe DA. Limited lower sternotomy for minimally invasive mitral valve replacement. Ann Thorac Surg 1998; 65: 273–274. [DOI] [PubMed] [Google Scholar]
- 18.Gillinov AM, Cosgrove DM. Minimally invasive mitral valve surgery: mini-sternotomy with extended transseptal approach. Semin Thorac Cardiovasc Surg 1999; 11: 206–211. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz DS, Ribakove GH, Grossi EA, et al. Minimally invasive mitral valve replacement: port-access technique, feasibility, and myocardial functional preservation. J Thorac Cardiovasc Surg 1997; 113: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 20.Galloway AC, Ribakove GH, Grossi EA, et al. Minimally invasive port access valvular surgery: initial clinical experience in 151 patients. Seventieth Scientific Session of the American Heart Association. Orlando, FL: 1997.
- 21.Galloway AC, Shemin RJ, Glower DD, et al. First report of the Port Access International Registry. Ann Thorac Surg 1999; 67: 51–58. [DOI] [PubMed] [Google Scholar]
- 22.Grossi EA, Galloway AC, LaPietra A, et al. Minimally invasive mitral valve surgery. Ann Thorac Surg (In Press). [DOI] [PubMed]
- 23.Chitwood WR Jr. Video-assisted and robotic mitral valve surgery: toward an endoscopic surgery. Semin Thorac Cardiovasc Surg 1999; 11: 194–205. [DOI] [PubMed] [Google Scholar]
- 24.Grossi EA, La Pietra A, Galloway AC, et al. Videoscopic mitral valve repair and replacement using the port-access technique. Adv Card Surg 2001; 13: 77–88. [PubMed] [Google Scholar]
- 25.Chitwood WR Jr, Nifong LW. Minimally invasive videoscopic mitral valve surgery: the current role of surgical robotics. J Card Surg 2000; 15: 61–75. [DOI] [PubMed] [Google Scholar]
- 26.Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced “robotic” cardiac surgery: experience in 148 patients. J Thorac Cardiovasc Surg 2001; 121: 842–853. [DOI] [PubMed] [Google Scholar]
- 27.Grossi EA, Lapietra A, Applebaum RM, et al. Case report of robotic instrument-enhanced mitral valve surgery. J Thorac Cardiovasc Surg 2000; 120: 1169–1171. [DOI] [PubMed] [Google Scholar]
- 28.Gillinov AM, Banbury MK, Cosgrove DM. Hemisternotomy approach for aortic and mitral valve surgery. J Card Surg 2000; 15: 15–20. [DOI] [PubMed] [Google Scholar]
- 29.Grossi EA, Galloway AC, Ribakove GH, et al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001; 71: 807–810. [DOI] [PubMed] [Google Scholar]
- 30.Glower DD, Siegel LC, Frischmeyer KJ, et al. Predictors of outcome in a multicenter port-access valve registry. Ann Thorac Surg 2000; 70: 1054–1059. [DOI] [PubMed] [Google Scholar]
- 31.Grossi EA, LaPietra A, Ribakove GH, et al. Minimally invasive versus sternotomy approaches for mitral reconstruction: comparison of intermediate-term results. J Thorac Cardiovasc Surg 2001; 121: 708–713. [DOI] [PubMed] [Google Scholar]
- 32.Galloway AC, Grossi EA, Ribakove GH, et al. Minimally invasive cardiac surgery. In: Topol EJ, ed. Textbook of Cardiovascular Medicine: Updates, Vol 4. New Jersey: Lippincott Williams & Wilkins Healthcare, 1998.
- 33.Grossi EA, Ribakove GH, Schwartz DS, et al. Port-access approach for minimally invasive mitral valve surgery. Operative Techniques in Cardiac and Thoracic Surgery: A Comparative Atlas, Vol. 1. 1998.
- 34.Cosgrove DM. Surgery for degenerative mitral valve disease. Semin Thorac Cardiovasc Surg 1989; 1: 183–193. [PubMed] [Google Scholar]
- 35.Perier P, Deloche A, Chauvaud S, et al. Comparative evaluation of mitral valve repair and replacement with Starr, Bjork, and porcine valve prostheses. Circulation 1984; 70 (3 Pt 2):I187–192. [PubMed] [Google Scholar]
- 36.Grossi EA, Zakow PK, Ribakove G, et al. Comparison of post-operative pain, stress response, and quality of life in port access vs. standard sternotomy coronary bypass patients. Eur J Cardiothorac Surg 1999; 16 (suppl 2): S39–42. [PubMed] [Google Scholar]


