Abstract
Objective
To review the long-term follow-up data from the authors’ institutional experience of 62 patients with locally advanced breast cancer (LABC) treated with a uniform multimodality regimen. The authors determined the rate of breast preservation, the disease-free and overall survival, and the factors associated with locoregional and distant recurrent disease.
Summary Background Data
It remains a challenge to achieve local and distant control of LABC. Over the last decade, preoperative or neoadjuvant chemotherapy has emerged as the standard of care for these patients. Successful tumor downstaging has been associated with increased rates of breast-conserving therapy (BCT), but the overall effect on long-term survival remains to be seen.
Methods
This study examines a cohort of 62 patients with LABC treated at the authors’ institution from 1992 to 1998. The uniform treatment regimen consisted of neoadjuvant doxorubicin (Adriamycin), followed by operation (BCT if sufficient clinical downstaging), followed by non-cross-resistant cyclophosphamide/methotrexate/5-fluorouracil, followed by radiation therapy. Treatment was both dose-intensive and time-intensive, with a total treatment time of 32 to 35 weeks.
Results
In this patient population, the median age was 44 years, with approximately two thirds white patients and one third African American. Eighty-two percent of patients were clinical stage III at presentation, 13 patients had T4d inflammatory cancers, and 3 patients were stage IV at diagnosis. Eighty-four percent of patients demonstrated a significant clinical response to doxorubicin. Twenty-eight patients had sufficient clinical downstaging to attempt BCT, and 22 (45%) of 49 noninflammatory patients underwent successful BCT. Pathologic complete response was seen in 15% of patients. Median follow-up for the cohort was 70 months. The local recurrence rate was 14%, including two ipsilateral breast tumor recurrences (10%) in the BCT patients. Seven (12%) patients developed a new primary cancer in the contralateral breast. Distant metastases occurred in 18 (31%) patients, and the 5-year overall survival rate for the cohort was 76%. Furthermore, in the patients who underwent an attempt at BCT, the survival rate was 96% at 5 years.
Conclusions
Dose-intensive and time-intensive multimodality neoadjuvant therapy was successfully administered to a mixed racial group over shortened times. Patients who had sufficient clinical downstaging to allow BCT have the best long-term outcome. Patients who required mastectomy are at a higher risk of relapse, as well as the development of new contralateral cancers, yet have 5-year survival rates of over 50%.
Despite efforts at early detection, locally advanced breast cancer (LABC) remains both a prevalent clinical problem and a challenge for achieving local and distant control of disease. In the past, many of these patients have been considered to be inoperable because of the sheer volume of their tumors and the belief that they would soon succumb to distant metastatic disease. Over the last decade, the use of neoadjuvant (preoperative) chemotherapy has emerged as the standard of care for patients with large primary tumors or matted axillary nodal metastases. 1 The rationale for neoadjuvant therapy is based on data from randomized and nonrandomized clinical trials that have demonstrated successful tumor downstaging and correlated the response to chemotherapy with patient outcome. 2 Two large randomized trials, the NSABP B18 and the EORTC 10902, compared preoperative versus postoperative chemotherapy in primary operative breast cancer. 2,3 While survival rates were equivalent in both arms of each trial, the tumor downstaging afforded by preoperative chemotherapy resulted in increased rates of breast-conserving therapy (BCT). Based on the data from smaller breast tumors, investigators have extended BCT to larger tumors that are successfully downstaged with neoadjuvant chemotherapy and report satisfactory rates of local control of disease. 4,5
In 1992, our group at the University of North Carolina-Lineberger Comprehensive Cancer Center (UNC-LCCC) began a trial for patients with LABC that used a particular regimen of aggressive neoadjuvant chemotherapy, surgery, and radiotherapy. Sixty-two patients were treated with dose-intensive and time-intensive chemotherapy. BCT was performed if indicated by successful tumor downstaging. In this report, we present the long-term outcomes of this study, having achieved a median follow-up of 70 months. We have examined the sites of recurrence and metastasis to determine the disease-free and overall survival rates, the factors associated with BCT, the factors associated with local and distant relapse, and the relationship of local downstaging to outcome.
METHODS
Breast cancer patients with primary tumors greater than 5 cm (T3), with skin or chest wall involvement (T4), or with matted axillary adenopathy (N2) were defined as having LABC and were eligible for the study. The diagnosis was obtained by either a fine-needle aspiration or a tissue biopsy. All of the patients were treated at UNC-LNCC with a uniform multimodality regimen that included dose- and time-intense neoadjuvant doxorubicin (90 mg/m2 given over 48 hours every 2.5 weeks for four cycles) followed by surgery. BCT was performed when there was sufficient downstaging to a tumor size of 4 cm or less and segmental mastectomy could be achieved with acceptable cosmetic result. All patients had a complete (levels I–III) axillary dissection. Within 14 to 21 days postoperatively, additional adjuvant dose- and time-intense chemotherapy was initiated with a combination of cyclophosphamide, methotrexate, and fluorouracil (CMF) at escalating doses up to 1,200 mg/m2 cyclophosphamide, 900 mg/m2 methotrexate, and 1,200 mg/m2 fluorouracil (termed the dose-dense modified Bonadonna regimen). G-CSF support was given to all patients. Adjuvant radiotherapy followed the CMF. Tamoxifen (10 mg twice daily for 5 years) was given to all patients greater than 50 years old at the time of diagnosis, or younger patients with estrogen receptor (ER)-positive or progesterone receptor (PR)-positive tumors by biochemical assay or immunohistochemistry. Our treatment protocol emphasized timely completion of all modalities of therapy within 32 weeks, with minimal interval between modalities.
The case report forms and medical charts provided clinical and outcomes data for this study. Tumor measurements and response assessments were performed by several physicians, and when there was more than one measurement, the medical oncologist’s measurements and response assessments were used. Clinical responses were defined by the primary tumor response and were categorized as follows: complete response (CR) = complete clinical and radiographic resolution of tumor; partial response (PR) = 50% or greater diminution of bidimensional tumor; minimal response (MR) = 25% to 50% diminution of tumor; stable disease (SD) = no more than 25% increase or decrease in tumor size; progressive disease (PD) = more than 25% increase in tumor. Since patients with a poor response to chemotherapy could receive a second neoadjuvant regimen, response was assessed uniformly after doxorubicin and did not include the response to a second regimen. A pathologic complete response was defined as no evidence of invasive cancer in the breast or axilla. Axillary nodal status was grouped as follows: 0 involved lymph nodes after chemotherapy, 1 to 3 lymph nodes, 4 to 9 lymph nodes, and 10+ lymph nodes. ER and PR were routinely assayed using either a biochemical assay or immunohistochemistry. For the purposes of this analysis, patients with borderline ER or PR were grouped with “positive.” Most patients had ER, PR, and tumor grade evaluated pretreatment, although some had these analyses performed on the final surgical specimen. When receptors and tumor grade were assessed both before and after therapy, the pretreatment measure was used. Outcomes data were obtained from protocol follow-up forms, medical records, and the tumor registry. Relapse or disease-free survival was calculated as the time from primary tumor diagnosis to time of last follow-up, development of either in-breast or chest wall local recurrences (for locoregional), development of systemic metastases (for distant disease), or death.
Statistical Analyses
We used logistic regression to examine factors associated with the attempt of BCT in noninflammatory cancers. We used Cox regression to evaluate possible predictors in the time-to-event outcomes of locoregional relapse-free survival, distant disease-free survival, and overall survival. Survival curves were calculated using the Kaplan-Meier (or product limit) method. We used the log-rank test for differences between survival curves. All analyses were performed using JMP 3.2.1 and SAS version 8.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Demographics, Tumor Staging, and Treatment
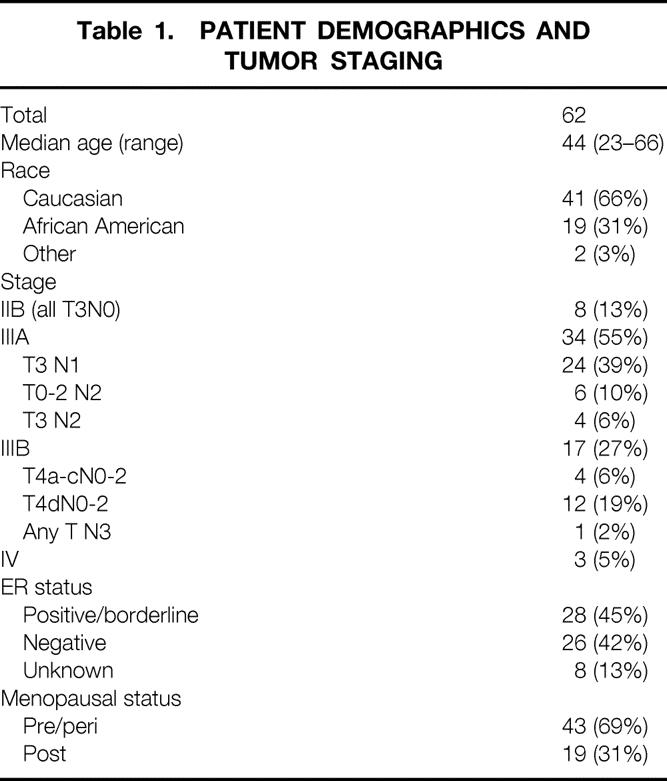
Between 1992 and 1998, 62 patients with LABC were treated with this uniform multimodality treatment. Median age was 44 years (Table 1). Approximately two thirds of the patients were white and one third African American. Fifty-one (82%) of the patients were clinical stage III at diagnosis, with 34 stage IIIA and 17 stage IIIB. Three patients with LABC were considered to be stage IV at diagnosis. In addition, our cohort included 13 (21%) patients with inflammatory cancer, defined as tumor stage T4d (12 stage IIIB, 1 stage IV). Forty-five percent of patients were ER positive and 69% were premenopausal or perimenopausal. Median follow-up for the cohort was 70 months (range 35–117).
Table 1. PATIENT DEMOGRAPHICS AND TUMOR STAGING
Fifty-eight (94%) of patients received neoadjuvant doxorubicin, while four patients received both the single agent doxorubicin and the CMF neoadjuvantly. After neoadjuvant chemotherapy, all patients underwent surgical resection of the primary tumor and a full axillary dissection, although one declined surgery until undergoing a mastectomy 2 years after diagnosis. Two patients were converted to conventional-dose AC for their neoadjuvant therapy due to toxicity to dose-intense doxorubicin. One patient declined adjuvant CMF. Fifty-nine (95%) of the 62 patients received radiation therapy. The radiation was administered postlumpectomy in 22 patients, postmastectomy in 33, and before surgery in 4 patients in whom operability was uncertain after all chemotherapy. Thirty-four received tamoxifen, including, in that era, 11 of the 34 who had ER-negative tumors. Eight of the 36 patients with ER-positive tumors declined or did not tolerate tamoxifen.
Clinical Response to Neoadjuvant Chemotherapy
Overall, 84% of patients demonstrated a significant clinical response to chemotherapy. There was a complete clinical and mammographic response to doxorubicin in 13 (22%) of 58 measured responses. Thirty-six patients (62%) had a partial response while 8 (14%) had a minimal response and one patient had stable disease. No patient progressed on dose-intense doxorubicin.
Surgical Treatment and Final Pathologic Staging
A total of 28 (45%) patients had sufficient clinical downstaging to permit an attempt at BCT by performing a segmental mastectomy. Of these patients, 22 (79%) had successful BCT, while 6 (21%) required completion mastectomy for positive pathologic margins after segmental mastectomy.
Thus, in this cohort, 35% of all patients had breast preservation. As previously noted, 13 patients had inflammatory (T4d) disease and were never considered candidates for BCT, regardless of clinical response. If this subgroup is discounted, BCT was attempted in 28 (57%) of 49 noninflammatory patients, with successful conservation in 22 (45%).
Pathologic complete response was seen in nine (15%) patients. Six were among the patients with BCT, representing 27% of the patients with conserved breasts, and 3 were among the modified radical mastectomy (MRM) group, representing 8% of the mastectomy group. There was no axillary lymph node involvement after chemotherapy in 21 (34%), 1 to 3 involved lymph nodes in 16 (26%), 4 to 9 lymph nodes in 16 (26%), and 10+ involved lymph nodes in 9 (14%).
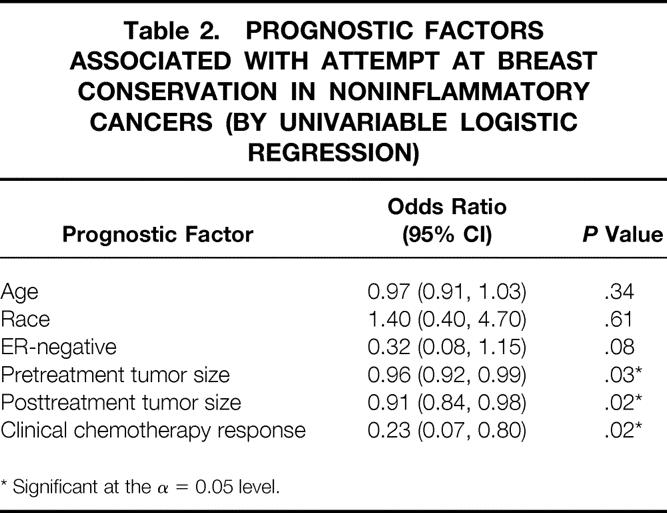
We analyzed the data to determine what clinical factors predicted for the ability to attempt BCT in these patients. We excluded the 13 patients with T4d inflammatory tumors, as they were never candidates for BCT, and analyzed the factors associated with attempting BCT. Factors significantly associated with the ability to attempt BCT included initial tumor size and posttreatment tumor size and the clinical response to chemotherapy (Table 2). Age, race, and ER status were not significantly associated. When this analysis was repeated only for patients with successful BCT, similar results were obtained, although ER negativity was also significantly (P = .02) associated with successful BCT.
Table 2. PROGNOSTIC FACTORS ASSOCIATED WITH ATTEMPT AT BREAST CONSERVATION IN NONINFLAMMATORY CANCERS (BY UNIVARIABLE LOGISTIC REGRESSION)
* Significant at the α = 0.05 level.
Outcome Analyses
Three patients in this cohort had clinical stage IV disease with distant metastases at diagnosis and were excluded from outcome analysis; thus, our analysis was based on a total of 59 patients.
Locoregional Recurrence
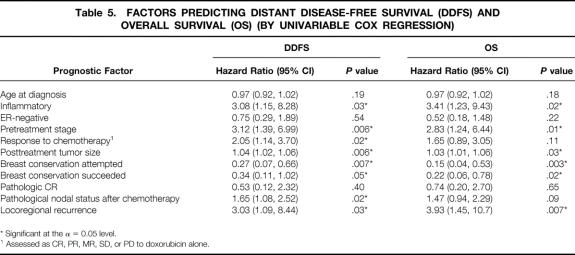
Eight (14%) patients suffered 11 locoregional recurrences, including in-breast, chest wall, axillary, or supraclavicular lymph node (Table 3). Overall, a low percentage of locoregional recurrences occurred in both the BCT group (2/21, 10%) and in the MRM group (6/38, 16%). Two (10%) ipsilateral breast tumor recurrences occurred in the BCT group, and one of these recurrences was also associated with a supraclavicular nodal recurrence. There were five chest wall recurrences in the MRM group, including one patient who declined mastectomy for 2 years. One of the five patients with chest wall recurrences had declined radiotherapy, and two of the five patients with chest wall recurrences had inflammatory cancers. Table 4 depicts factors associated with locoregional recurrence. In Cox regression analysis, patients with inflammatory disease, higher pretreatment stage, or poor response to chemotherapy, and those who were not candidates for BCT were more likely to suffer locoregional recurrences.
Table 3. LOCAL AND REGIONAL RECURRENCES
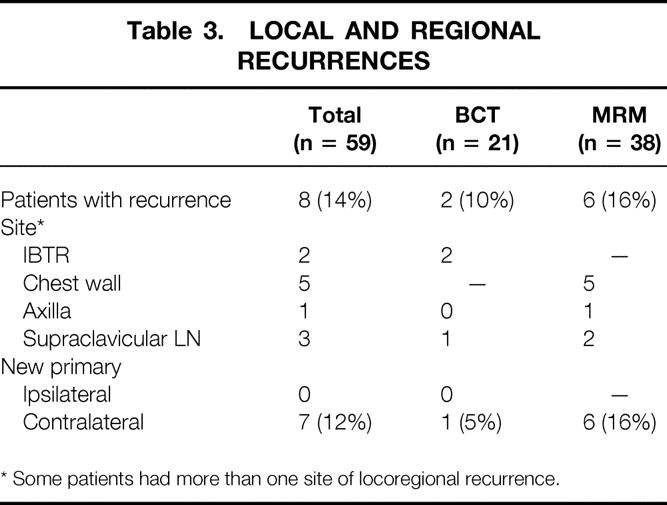
* Some patients had more than one site of locoregional recurrence.
Table 4. PREDICTORS OF LOCOREGIONAL RELAPSE-FREE SURVIVAL (BY UNIVARIABLE COX REGRESSION)
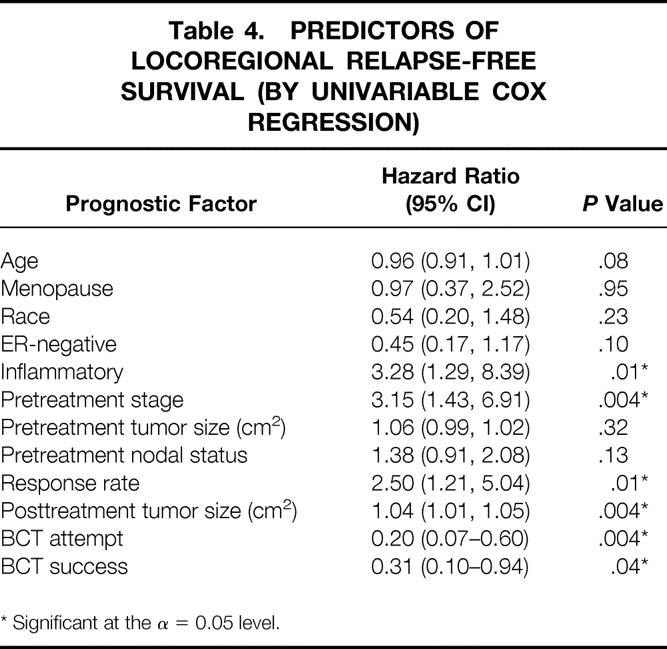
* Significant at the α = 0.05 level.
Among those with locoregional recurrence, the median time to recur was 23 months (range 9–55). Four (50%) of the eight patients with locoregional recurrence also developed distant metastases at −1 month and +1, 27, and 61 months later.
Contralateral New Primary Breast Cancers
In this group of high-risk patients, there was an elevated rate of new primary cancers developing in the contralateral breast. Overall, seven (12%) patients developed new contralateral breast primaries during the follow-up period (see Table 3). Six of these cancers occurred in the MRM group, representing a rate of 16%, indicating an unusually high incidence of second primaries in this group. In contrast, only one new contralateral primary was detected in patients who underwent BCT. No BCT patient developed a new primary cancer in the conserved breast.
Distant Relapse and Death
The Kaplan-Meier curves for these 59 patients reveal outstanding distant disease-free survival and overall survival (Fig. 1). At a median follow-up of 70 months, only 19 (32%) developed systemic metastases, and 18 (30%) died. Seventeen of 18 deaths occurred in patients who had relapsed, 16 systemically and 1 locoregionally (chest wall). Among those patients with distant disease, median overall survival from diagnosis was 54 months (range 12–112). Only two patients died without evidence of distant metastases. The median time to developing distant metastases was 33 months (range 11–96). The 5-year overall survival for the entire patient cohort was 76% (95% confidence interval 65–88%).
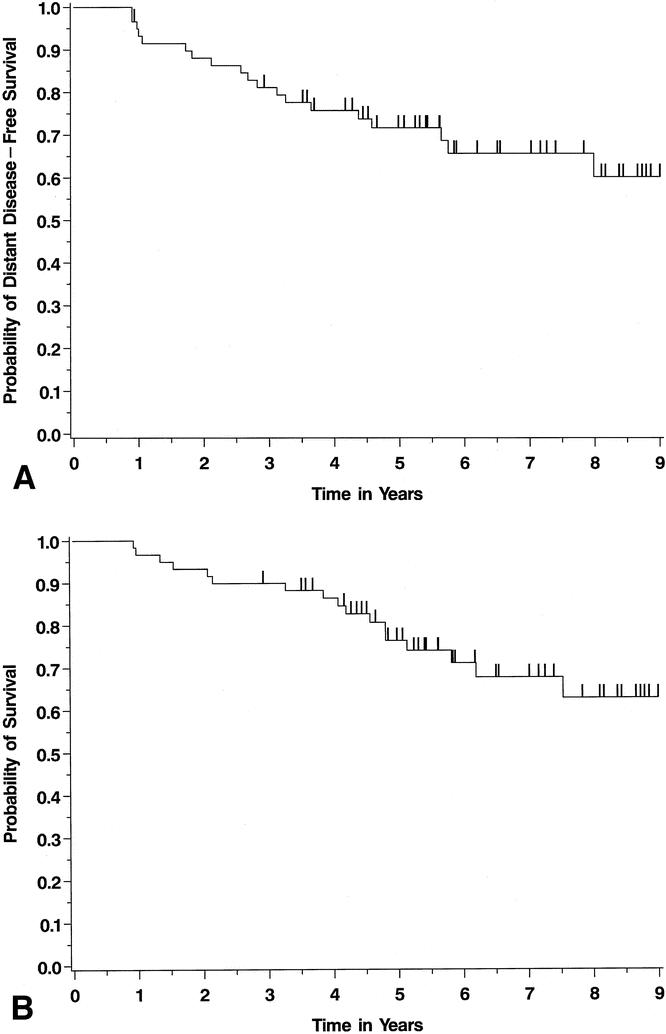
Figure 1. Kaplan-Meier survival curves for distant disease-free (A) and overall (B) survival in the entire patient cohort. This figure analyzes the survival of 59 patients, excluding 3 patients who presented with LABC and stage IV disease.
The most common sites of distant relapse included bone, central nervous system, lung, lymph node, and liver, with soft tissue, skin, and ovary involved less frequently. Table 5 illustrates factors associated with distant disease-free and overall survival. Using Cox regression modeling, becoming a BCT candidate after neoadjuvant chemotherapy was the strongest predictor of long-term survival. Intriguingly, this was a stronger predictor than actual successful BCT, since none of the six patients who went on to completion mastectomy after initial efforts at conservation relapsed systemically. Other significant factors for overall and distant disease-free survival included pretreatment clinical stage, posttreatment tumor size, inflammatory disease, successful completion of BCT, and locoregional recurrence. Pathologic complete response was uncommon (nine patients, 15%) and was not significantly associated with outcome, although response to chemotherapy and pathologic nodal status after chemotherapy were associated with increased distant disease-free survival.
Table 5. FACTORS PREDICTING DISTANT DISEASE-FREE SURVIVAL (DDFS) AND OVERALL SURVIVAL (OS) (BY UNIVARIABLE COX REGRESSION)
* Significant at the α = 0.05 level.
1 Assessed as CR, PR, MR, SD, or PD to doxorubicin alone.
Finally, we analyzed the outcomes of three subgroups of this cohort of 59 patients: patients with locally advanced, noninflammatory breast cancers who were BCT candidates after neoadjuvant chemotherapy (n = 27), patients with noninflammatory cancers who underwent MRM (N = 20), and patients with inflammatory breast cancers treated with mastectomy after chemotherapy (n = 12). As expected, the LABC patients without inflammatory disease who were BCT candidates after neoadjuvant chemotherapy had an excellent long-term distant disease-free and overall prognosis, with a 5-year overall survival rate of 96% (Fig. 2; log-rank test among groups P = .007 for distant disease-free survival, P = .002 for overall survival). The BCT candidates had a significantly better outcome than patients who did not achieve tumor downstaging sufficient to consider BCT, who had a prognosis similar to the inflammatory cohort. In the noninflammatory patients who underwent MRM, the 5-year overall survival rate was 51%. Furthermore, although inflammatory breast cancer carries a high risk of relapse, there was a 67% 5-year overall survival among the 12 inflammatory breast cancer patients treated with aggressive multimodality therapy. Thus, this dose- and time-intensive multimodality treatment regimen offered significant rates of long-term survival even in patients who had the extremes of LABC.
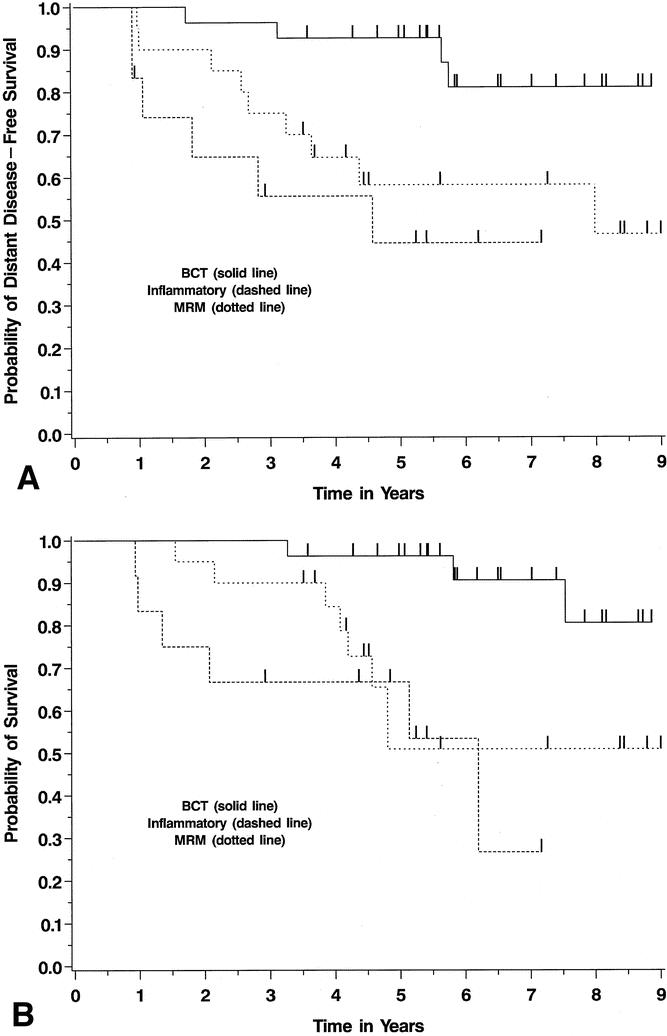
Figure 2. Kaplan-Meier survival curves for distant disease-free (A) and overall (B) survival in three subgroups of the LABC patients. These Kaplan-Meier curves illustrate outcomes among patients with locally advanced noninflammatory breast cancers who were breast-conservation candidates after neoadjuvant chemotherapy (BCT) versus those who were not conservation candidates (MRM). These are compared with survival curves for inflammatory breast cancer patients, all of whom were treated with mastectomy after chemotherapy.
DISCUSSION
This study reports mature data from a cohort of patients with LABC treated with a uniform regimen and followed for a median interval of over 5 years. Our results demonstrate that patients with LABC can achieve outstanding control of disease and long-term survival by undergoing a dose- and time-intensive regimen of neoadjuvant chemotherapy. These data provide evidence that BCT can reasonably be accomplished in up to 45% of patients with noninflammatory LABC. Furthermore, the tumor response to chemotherapy can help identify the subgroup of LABC patients with a more favorable long-term prognosis.
It is well established that neoadjuvant chemotherapy can effectively downstage the primary breast cancer in patients with LABC. 1,4–9 However, in these patients as a whole, the extent of the survival advantage provided by neoadjuvant therapy remains less clear. In most larger studies of patients with LABC, the 5-year survival rates range from approximately 20% to 55%. 10–12 The patients in this series represent the entire clinical spectrum of LABC, including a group of T4d inflammatory cancers. Despite this diversity, the entire group had 5-year disease-free and overall survival rates of over 70%. This suggests that the treatment regimen changed the natural history of the disease in the majority of patients.
The subgroup of patients who had the most favorable outcomes represented those who had the best clinical response to the doxorubicin. This finding is similar to other reports that correlated response in the breast with long-term outcomes. 7,13–15 In fact, some investigators have found that patients who had a pathologic complete response (pCR) had the best outcomes. 7,13 In our series, only nine patients had a pathologic complete response, and this did not correlate with long-term outcome. However, in our series, the strongest predictor of long-term survival was whether these patients had sufficient clinical downstaging such that BCT could be attempted. This likely reflects a surrogate marker for the response rate of the tumors to the doxorubicin. In these 28 patients offered BCT, there have been only three deaths during the long follow-up period of this study and the 5-year overall survival rate is 96%. These data strongly argue not only that BCT is appropriate in suitably downstaged patients, but also that this subgroup of patients has been selected for an excellent overall outcome.
The BCT rate of 45% in this series is higher than other studies that have examined neoadjuvant therapy in T3 and T4 tumors, suggesting that multi-modality therapy, combined with an aggressive BCT attempt, can save more breasts in this group. The NSABP B-18 study compared preoperative versus postoperative doxorubicin/cyclophosphamide chemotherapy in 1,523 women with operable, early-stage (T1–3) breast cancer. Preoperative chemotherapy was associated with increased lumpectomy rates compared to postoperative chemotherapy (67% vs. 60%, P = .002) when examining the entire cohort of patients. 16 However, the patients with T3 tumors who underwent preoperative chemotherapy had a 22% rate of breast conservation. In the subsequent (B-27) neoadjuvant study, there was a slightly lower rate of BCT than the B-18, but this trial consisted of larger tumors. 17 In a series of 89 patients with stage III breast cancer from the University of Michigan, 28% were treated with BCT. 14 Finally, a French group reported a breast conservation rate of 62% in 97 stage II and III patients treated with neoadjuvant chemotherapy. 15
This group of patients had a 14% long-term local recurrence rate, even with a significant number of inflammatory cancers. Notably, despite the large and locally extensive nature of the primary tumors in this study, in-breast tumor recurrence occurred in only 10% of the 22 patients with conserved breasts. We have included supraclavicular lymph node metastases as locoregional disease because this site was included within our radiation ports and was treated with curative intent. Other groups consider supraclavicular lymph node metastases to be locoregional disease and not distant metastatic disease because of a more favorable long-term outcome. 18 Not surprisingly, half of the patients with local recurrence also developed distant metastatic disease, suggesting the local recurrence was a manifestation of aggressive biologic behavior of the tumor. Intriguingly, the strongest factor associated with reduced rates of local recurrence was an attempt at BCT, again illustrating the favorable outcomes in this subgroup of patients. In contrast, the patients who required mastectomy had an unusually high rate of developing contralateral new primary cancers, suggesting that their other breast also has a persistent high risk of cancer.
The chemotherapy regimen in this study focused both on dose intensity and time intensity. In particular, the time interval between surgery and the beginning of the next course of non-cross-resistant chemotherapy was kept as short as possible, often beginning 2 weeks after operation. There is experimental evidence that the actual excision of the primary tumor may augment a systemic response that enhances residual tumor growth, and this has implications in the timing of adjuvant chemotherapy. 19,20 Patients tolerated this quick return to chemotherapy well, with no evidence of major wound healing problems. Furthermore, the entire time of treatment was 32 to 35 weeks, including all three treatment modalities. This contrasts to other neoadjuvant studies where the chemotherapy treatments alone totaled up to 52 weeks. 14
Finally, the chemotherapeutic regimen used in this study predated the era of the taxanes that are now being used in neoadjuvant regimens with good efficacy. 17,21 We have evolved from our high-dose CMF regimen to one that incorporates a taxane. Our current neoadjuvant approach to LABC uses doxorubicin/cyclophosphamide followed by paclitaxel and Herceptin for HER-2-positive tumors. Before treatment, the tumors are marked by the mammographers so that their sites can be identified even with complete clinical responses. 22 Based on these data from our initial trial, we hypothesize that treating the patients to the best clinical response will provide the best outcome.
In conclusion, neoadjuvant chemotherapy provides a significant survival advantage to patients with LABC. Those patients who have clinical downstaging are candidates for BCT and have the best long-term outcome. Patients who require mastectomy are at a higher risk of relapse as well as development of new, contralateral cancers, yet have 5-year survival rates of over 50%. Thus, dose-intense and time-intense neoadjuvant chemotherapy should be standard of care for patients with LABC.
DISCUSSION
Dr. Timothy J. Eberlein (St. Louis, MO): The past 10 years has seen an increase in neoadjuvant chemotherapy protocols in breast cancer. This study has been beautifully presented by Dr. Cance and is unique in two regards. First is the neoadjuvant regimen dose-intensive as well as time-intensive; that is, repeating the Adriamycin every 2.5 weeks. In fact, completion of all modalities occurred within 32 weeks in most of the patients, in contrast to the usual 52-week regimen in most of our neoadjuvant trials. The second unique feature of the study is the long-term follow-up, with the median of almost 70 months.
Dr. Cance and his colleagues have shown superb results. In patients with locally advanced breast cancer without inflammatory disease who were breast-conserving therapy candidates after neoadjuvant therapy, the overall 5-year survival is an astounding 96%. In fact, even in the patients with inflammatory breast cancer there is an impressive 67% survival.
This brings me to my first question. What is the toxicity of this regimen? It appeared that there were no treatment dropouts. Is this true? Other than the G-CSF, that you mentioned in your presentation and in the manuscript, to support the postoperative CMF regimen, were there other difficulties treating these patients?
I have two questions with respect to survival. In the noninflammatory patients who underwent modified radical mastectomy, the 5-year overall survival rate was 51%, even less than the 67% 5-year overall survival for the inflammatory breast cancer patients. Was this due to chemoresistance? Were you able to identify any molecular markers that predicted response or nonresponse to the neoadjuvant therapy, such as herz-neu?
Even more impressive than the overall survival is the fact that no patient with breast-conserving therapy developed a new primary in the conserved breast. However, you had seven patients in the entire cohort who had new contralateral breast primaries, only one of these in patients who underwent breast-conserving therapy. Again, do you have an explanation for this observation? And is it related to the responsiveness of patients to the chemotherapy?
Finally, as I mentioned, these are impressive results. However, since you instituted this treatment regimen, new drugs such as taxanes and new molecular markers such as herz-neu and others have been identified. So would you recommend for us the best-case current regimen that we all might care to use in our own institutions?
Once again I would like to congratulate you and your group for an outstanding study and for contributing to breast-conserving therapy in women who present with advanced breast cancer.
Presenter Dr. William G. Cance (Chapel Hill, NC): To answer Dr. Eberlein’s first question, there was toxicity in this group. It tended to be relatively mild grade III toxicity. There were rare patients that did develop severe toxicities, and three patients did not get the complete course of the chemotherapy. The toxicity was largely related to bone marrow suppression, even though they were supplemented with G-CSF. It was a tolerable regimen, but this high-dose chemotherapy did have side effects for some of these patients.
You asked about the difference between the noninflammatory patients who underwent mastectomy and the patients with inflammatory disease. I agree that this likely reflected chemoresistance. Again, we selected for those patients as being nonresponders, and that would seem to indicate chemoresistance, although it is hard to establish definitive proof.
Dr. Eberlein asked about molecular markers, which is the next part of our study. When these patients were being enrolled, we weren’t routinely measuring the molecular markers that are standard today. The trial was begun in the pre-HER-2 era. Current efforts are focusing on the determination of molecular markers in these patients at various times during their treatment.
The contralateral cancer rate likely relates to chemoresistance and probably also to some as-yet-undefined genetic defects. One additional issue in the neoadjuvant patients is that some of them have strong family histories of breast cancer. During the first phase of their neoadjuvant treatment, many are getting genetically tested, which will help them determine what operation they ultimately elect.
The current regimen that we recommend involves the use of a taxane. I think that the high-dose CMF was standard back in 1992, but has been replaced by the combination of Adriamycin and Cytoxan along with a taxane. In our current regimen, we are focused on the molecular markers. So, we are doing pretreatment biopsies and have moved to surgery after the second non-cross-resistant chemotherapy so that we can get two biopsies during treatment plus the final surgical specimen, to look at chemoresistance. In addition, we have added Herceptin in the HER-2-positive patients.
Finally, one issue to watch is that the estrogen receptor-positive patients do not seem to be responding as well to a taxane as estrogen receptor-negative patients. In the ER-positive, HER-2-negative patients, we are considering going back to the CMF regimen as the second chemotherapy treatment.
Dr. William C. Wood (Atlanta, GA): Two pet peeves. Small breast cancer trials and discussants who use the podium to present their own studies. So please indulge my reference to our intergroup study of 111 stage III patients with 9.4 years of follow-up, neoadjuvant chemotherapy of the same agents at the same dose that you gave, although less time-intense, manuscript in preparation by Dave Duggin. I mention it only to praise the study that we have just heard.
Fifty-nine stage III tumors may sound like a small study. But compared to 111 study patients that took three cooperative groups the same period of time to accrue, and you realize what an accomplishment this is from a single institution. Seventy-four percent major response rate in the intergroup series compares with Dr. Cance’s 84% major response rate. A median survival of just over 50% in our group trial compares with the 76% that you achieved in this trial. Not only that, but a 45% breast-conserving rate in stage III breast cancer has to be compared with the usual 20% to 30% breast-conserving therapy rate even with modern induction regimens. And you didn’t achieve it by pushing indications, as demonstrated by only a 10% in-breast failure rate after this.
So I appreciated receiving this manuscript in advance. It has an excellent discussion. I concur with your conclusions as outlined in the subtitle of your paper. I really loved everything until you showed your conclusions here, and then I have a quibble. I am not convinced that you have demonstrated that multimodality neoadjuvant therapy provides a survival advantage within this population, or the time intensity of treatment is clearly the factor that is responsible for your suburb results.
Three questions:
The 12% incidence of contralateral breast cancer you report in 6 years is more than twice the rate that you would expect in this age group. Could some of these have been metastases? Were all clearly arising in a background of in situ disease?
Secondly, there are 62 patients. You appropriately excluded the three who were stage IV, but you included eight as locally advanced disease who were stage II rather than stage III breast cancer. I suspect you may have already looked at the effect this may have had on your outcomes. And if you have, I wonder if you could share that with us.
Thirdly, as you demonstrated again, response to induction therapy predicts for prognosis. In larger multi-institutional breast cancer trials, a pathologic complete response predicts for the very best prognosis. You conclude that the best predictor is “a candidate for breast-conserving therapy.” This looks a bit data-derived to me. Is this your new hypothesis? Or is this the play of chance, Dr. Cance?
Dr. William G. Cance (Chapel Hill, NC): Dr. Wood, I agree it is very difficult to prove a survival advantage with a trial such as this, and I think your point is well taken. It is even more difficult to prove that time intensity matters. Some data from Dr. Bernard Fisher in the past has suggested that metastases can grow more quickly after surgery in experimental animals. That was one of the bases for our time-intense regimen of moving to the next phase of chemotherapy 2 weeks after surgery. Hopefully, the CALGB 9741 trial, looking at time intensity comparisons, should answer some of those questions.
Regarding the new contralateral cancers, these do not appear to be metastases, but appear to be new primaries. We excluded the cross-chest wall inflammatory cancers, so these should all be new primary cancers.
There were only eight T3N0 patients in the study, and they did not appear to influence the survival curves. When we took those patients out and ran the analyses, the distant disease-free survival was about 71%, essentially equivalent to the entire group.
The pathological complete response does appear to be the best prognostic factor from other studies, and I think that that would be the gold standard for neoadjuvant therapy. We did not have the same level of preoperative chemotherapy that the patients are currently getting as part of other trials, and we did have distant disease develop in our pathological complete responders. So, we suspect that the CMF had an impact when the patients went back to the next phase of chemotherapy. So, we were perhaps in an earlier end point where we couldn’t pick up those patients who were going to respond to the CMF. Since we couldn’t measure response to the CMF, our surrogate was a response to the doxorubicin chemotherapy by downstaging to allow breast conservation. As you said, it is more data-driven than hypothesis-driven. Nonetheless, I agree that the pathological complete response is the current gold standard for a more favorable long-term prognosis.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: William G. Cance, MD, CB 7210, 3010 Old Clinic Building, University of North Carolina, Chapel Hill, NC 27599.
E-mail: cance@med.unc.edu
The first two authors contributed equally to this manuscript.
Accepted for publication April 24, 2002.
References
- 1.Singletary SE. Neoadjuvant chemotherapy in the treatment of stage II and III breast cancer. Am J Surg 2001; 182: 341–346. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol 2001; 28: 389–399. [DOI] [PubMed] [Google Scholar]
- 3.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001; 19: 4224–4237. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, Singletary SE, Buzdar AU, et al. Surgical conservation planning after neoadjuvant chemotherapy for stage II and operable stage III breast carcinoma. Am J Surg 2001; 182: 601–608. [DOI] [PubMed] [Google Scholar]
- 5.Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 1999; 10: 47–52. [DOI] [PubMed] [Google Scholar]
- 6.Vlastos G, Mirza NQ, Lenert JT, et al. The feasibility of minimally invasive surgery for stage IIA, IIB, and IIIA breast carcinoma patients after tumor downstaging with induction chemotherapy. Cancer 2000; 88: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 7.Eltahir A, Heys SD, Hutcheon AW, et al. Treatment of large and locally advanced breast cancers using neoadjuvant chemotherapy. Am J Surg 1998; 175: 127–132. [DOI] [PubMed] [Google Scholar]
- 8.Touboul E, Lefranc JP, Blondon J, et al. Primary chemotherapy and preoperative irradiation for patients with stage II larger than 3 cm or locally advanced non-inflammatory breast cancer. Radiother Oncol 1997; 42: 219–229. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Bonadonna G, Zurrida S, et al. Conservation surgery after primary chemotherapy in large carcinomas of the breast. Ann Surg 1995; 222: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hortobagyi GN, Ames FC, Buzdar AU, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 1988; 62: 2507–2516. [DOI] [PubMed] [Google Scholar]
- 11.Jacquillat C, Baillet F, Weil M, et al. Results of a conservative treatment combining induction (neoadjuvant) and consolidation chemotherapy, hormonotherapy, and external and interstitial irradiation in 98 patients with locally advanced breast cancer (IIIA-IIIB). Cancer 1988; 61: 1977–1982. [DOI] [PubMed] [Google Scholar]
- 12.Valagussa P, Zambetti M, Bignami P, et al. T3b-T4 breast cancer: factors affecting results in combined modality treatments. Clin Exp Metastasis 1983; 1: 191–202. [DOI] [PubMed] [Google Scholar]
- 13.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999; 17: 460–469. [DOI] [PubMed] [Google Scholar]
- 14.Merajver SD, Weber BL, Cody R, et al. Breast conservation and prolonged chemotherapy for locally advanced breast cancer: the University of Michigan experience. J Clin Oncol 1997; 15: 2873–2881. [DOI] [PubMed] [Google Scholar]
- 15.Touboul E, Buffat L, Lefranc JP, et al. Possibility of conservative local treatment after combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer. Int J Radiat Oncol Biol Phys 1996; 34: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 17.Bear HD, for NSABP. The effect on primary tumor response of adding sequential Taxotere to Adriamycin and cyclophosphamide: preliminary results from NSABP Protocol B-27. Breast Cancer Res Treat 2001; 69: 210. [Google Scholar]
- 18.Brito RA, Valero V, Buzdar AU, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001; 19: 628–633. [DOI] [PubMed] [Google Scholar]
- 19.Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 1979; 39: 3861–3865. [PubMed] [Google Scholar]
- 20.Fisher B, Gunduz N, Saffer EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res 1983; 43: 1488–1492. [PubMed] [Google Scholar]
- 21.Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 2002; 20: 1456–1466. [DOI] [PubMed] [Google Scholar]
- 22.Braeuning MP, Burke ET, Pisano ED. Embolization coils as tumor markers for mammography in patients undergoing neoadjuvant chemotherapy for carcinoma of the breast. AJR Am J Roentgenol 2000; 174: 251–252. [DOI] [PubMed] [Google Scholar]