Abstract
Objective
To evaluate the use of herpes simplex viral (HSV) amplicon vectors for production of tumor vaccines and to determine if such vaccines expressing combinations of immunostimulatory agents may be effective in the treatment of experimental liver cancer.
Methods
A hepatic metastatic tumor model using CT-26 colorectal cancer in syngeneic Balb/C mice was utilized. Tumor vaccines were produced by brief (20 minutes) exposure of irradiated tumor cells to herpes amplicon vectors carrying the transgene for RANTES, B7.1, or GM-CSF. The antitumor efficacy of vaccination using tumor cells secreting GM-CSF (single agent) or a combination of RANTES/B7.1/GM-CSF (multiagent) was tested. The effect of 60% hepatectomy or T-cell depletion was also tested in this model.
Results
In vitro assays confirmed high-level cytokine or costimulatory molecule production by cells transduced with amplicons. Antitumor efficacy was observed with single-agent or multiagent treatment. Without hepatectomy, immunization with single-agent or multiagent vaccine therapy appears equivalent. When administered in the setting of hepatectomy, multiagent regimens produced a higher cure rate than single-agent therapy (50% vs. 12.5%, P = .03). Animals treated with GM-CSF alone had an average nodule count of 40 ± 19 (P < .006 vs. Hep control 232 ± 30), while animals treated with multiagent therapy had an average nodule count of 11 ± 7 (P < .0004 vs. control). CD4+ and CD8+ lymphocyte blockade abrogated observed efficacy, confirming a lymphocyte-mediated response.
Conclusions
Tumor vaccines produced using HSV amplicon-mediated gene transfer may be useful in the treatment of liver malignancies. In the setting of hepatectomy, multiagent vaccine therapy offers an advantage over single-agent therapy. These data encourage consideration of such HSV-based neoadjuvant immunotherapy for treatment of liver malignancies.
Hepatic resection is the mainstay of therapy for primary and secondary neoplasms of the liver. 1–3 However, after complete gross resection of tumor, disease will recur in over two thirds of patients, 3,4 indicating that undetected residual microscopic tumors remain in the majority of patients undergoing a therapeutic hepatectomy. It has long been the clinical impression, supported by experimental data, that hepatic regeneration may indeed enhance growth of such residual tumors. 5–8 Recent data implicate local and systemic immune changes as partly responsible for such an enhancement of tumor growth 6,9 and suggest that immunostimulatory strategies may be useful in the treatment of residual hepatic tumor after hepatectomy.
Immunotherapy involving the transfer of genes coding for immunostimulatory proteins into tumor cells has been extensively investigated and shows promise in the treatment of cancer in many experimental models. 6,10–12 The premise of such tumor vaccine production is that expression of immunostimulatory molecules at the site of putative tumor antigens would enhance host response to cancer. In this regard chemokines such as RANTES (R egulated on A ctivation, N ormal T-cell E xpressed and S ecreted), adhesion/costimulatory molecules such as B7.1, and cytokines such as GM-CSF have each demonstrated success in preclinical models. 11–14 In the current study we investigated the possibility of combining these three classes of immunostimulatory proteins, postulating that using chemokines for recruitment of immunoreactive cells to tumor, along with costimulatory molecules and cytokines for binding and activation of these immune cells, may provide increased efficacy over single-agent therapy.
For gene transfer we used herpes simplex virus (HSV) type 1-based amplicon vectors. These replication-incompetent gene transfer vehicles can target a wide variety of tumor cells, regardless of cell cycle kinetics of the targeted cell, and are rapid and highly efficient in such gene transfer. These characteristics of the amplicon vector allow for a very simple protocol for tumor vaccine production requiring only small amounts of vector and a very short exposure time. Thus, such a protocol can easily be adopted as a clinical strategy. 9,15 This gene transfer protocol has also been shown to be feasible in the most common of human liver malignancies. 15 Evidence from the following preclinical experiments indicates that such HSV-mediated multiagent immunotherapy is effective against hepatic tumor growth, even in the setting of experimental hepatic resection.
METHODS
Herpes Simplex Virus Vectors
HSV-GMCSF, HSV-B7.1, HSV-RANTES, and HSV-lacZ were created by directionally cloning the murine GM-CSF, human B7-1, human RANTES and LacZ genes into HSVprPUC, which contains the HSV immediate early 4/5 promoter, a multiple cloning site, and an SV40 A sequence. 15,16 Packaging of amplicon vectors was performed in RR1 cells using helper mutant D30EBA virus, and amplicon titers were determined as described previously. 15 Amplicon titer in the different virus preparations ranged from 5 × 107 pfu/mL to 5 × 108 pfu/mL. HSVlac titers were between 1 and 2 × 108 blue-forming units/mL and titered by X-gal biochemistry on NIH3T3 cells.
Cells
CT-26 is a well-characterized murine colorectal carcinoma cell line that is syngeneic with Balb/C mice. This cell line was obtained from the NCI tumor repository (Fredericksburg, MD) and was grown in RPMI supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 mmol/L nonessential amino acids (Gibco, Grand Island, NY).
Animals
All animal work was performed under guidelines approved by the Memorial Sloan-Kettering Institutional Animal Care and Use Committee. Male Balb/C mice were purchased from Charles River Labs (Wilmington, MA), submitted to a 12:12 hours light:dark cycle, housed five per cage, and allowed free access to food and water. Animals that underwent surgery were anesthetized with an intraperitoneal injection of ketamine/xylazine (70/10 mg/kg). For the animals that underwent hepatectomy, a 70% hepatectomy was performed through a midline incision using the method described by Higgins and Anderson. 17
CT-26 Production of RANTES or GM-CSF
CT-26 cells from culture were irradiated with 9,000 rads and rested for 1 hour. Cells were then exposed to HSV-GMCSF, HSV-RANTES, HSVlac, or medium at a multiplicity of infection of 1 for 20 minutes at 37°C with gentle agitation every 10 minutes. Cells were then washed with medium twice and maintained in culture for 7 days. One cubic centimeter of supernatant was harvested from individual wells at various time points after infection. Analysis for GM-CSF and for RANTES was performed by standardized ELISA (R&D Systems, Minneapolis, MN).
Cell Surface Expression of B7-1
As above, CT-26 cells were irradiated and rested for 1 hour and then exposed to HSV-B7.1, HSVlac, or medium. CT-26 (1 × 106) cells were then incubated (1 hour) with fluorescein-isothiocyanate (FITC)-labeled anti-CD80 (10 μL; rabbit-antihuman) antibodies (R&D Systems) and then rinsed three times in phosphate-buffered saline (PBS). After washing, cells were resuspended in PBS (1 × 106 cells/cc3) and then analyzed by FACS (Becton Dickinson, Franklin Lakes, NJ) for expression of CD-80. Human macrophages and lymphocytes were used for positive controls.
Immunization and Tumor Challenge
CT-26 cells were irradiated and infected with HSV (20 minutes) and then administered in 300 μL of serum-free medium via intrasplenic injection. Animals were immunized with either 106 total cells (high dose) or 105 total cells (low dose). In the high-dose groups, to maintain a standard number of cells and viral load, single-therapy groups were inoculated with 3.3 × 105 cells infected with HSV-GMCSF plus 6.7 × 105 HSVlac-infected cells, for a total inoculum of 1 × 106 infected cells. The combination group received 3.3 × 105 cells of each of HSV-GMCSF-, HSV-RANTES-, or HSV-B7.1-transduced cells. In the low-dose groups, single-agent immunizations consisted of 3.3 × 104 cells infected with the agent of interest and 6.7 × 104 cells infected with HSVlac. Animals receiving multiagent immunizations received 3.3 × 104 cells expressing each agent. Control animals were treated with HSVlac-treated cells. Treatment groups had six to eight animals per group, and the experiments were then repeated. Three weeks after immunization, animals were challenged with tumor via a second intrasplenic injection. Using a tumor model based on that described by LaFreniere and Rosenberg, 18 5 × 104 nonirradiated CT-26 cells from culture were injected in a total volume of 300 μL serum-free medium. Two weeks later, animals were killed and hepatic surface nodules were counted.
Effect of Hepatectomy on Vaccination
Data from our laboratory and others demonstrate that hepatectomy enhances the growth of microscopic tumors in the residual liver. 7–9,19 To determine if antitumor vaccine therapy may be useful in this clinical scenario, vaccination as above was investigated in hepatectomized animals. Animals underwent a similar immunization protocol as that described for prior experiments. At the completion of the 3-week incubation period, animals underwent 60% hepatectomy followed by tumor challenge. 9
CD4+/CD8+ Lymphocyte Blockade
To verify a role for the host immune cells in the effects of immunizations, animals treated as elaborated above were compared to animals depleted of CD4+ and CD8+ lymphocytes. Briefly, animals were treated with GK1.5 (anti-CD4) and 53 to 6.72 (anti-CD8) antibodies (0.2 mg intraperitoneal daily for 3 days) to deplete CD4+ and CD8+ T lymphocytes. 20,21 The efficacy of antibody treatment was verified by FACS analysis performed on splenocytes harvested 7 days after the first intraperitoneal injection of the antibodies. The blockade was maintained by an intraperitoneal injection of 0.2 mg of each antibody once per week thereafter for 2 weeks until animal sacrifice and liver harvest.
Data Analyses
Results are expressed as average nodule counts ± SEM. Comparison of tumor growth was by unpaired t test. Comparison of rates of cure was by the Fisher exact test. P < .05 was considered statistically significant.
RESULTS
In Vitro Production of RANTES and GM-CSF
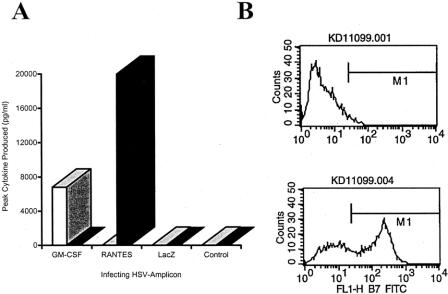
Expression of RANTES or GM-CSF peaked on day 2 after infection and persisted for 7 days. CT-26 cells infected with HSV-RANTES produced high levels of this chemokine, peaking on day 2 at a mean of 20,000 ± 9,200 pg/mL/106 cells, while in cells infected with HSV-GMCSF or HSVlac no expression of RANTES could be detected (limit of detection = 7 pg/mL) (Fig. 1A). Similarly, only cells infected with HSV-GMCSF produced GM-CSF. Peak production occurred on day 2 after infection (6,800 ± 2,100 pg/mL/106 cells). Cells infected with HSV-RANTES or HSVlac did not produce GM-CSF.
Figure 1. Expression of transgenes transferred by HSV amplicon-mediated gene transfer. (A) Peak amount of either GM-CSF (white/gray bars) or RANTES (black bars) secreted into the growth medium by cells infected with HSV amplicons (multiplicity of infection = 1) carrying the genes for GM-CSF, RANTES, or lacZ. GM-CSF or RANTES expression peaked at day 2 after infection. (B) Flow cytometry results using an FITC-conjugated anti-B7-1 antibody after infection with HSVB7.1. The upper graph represents noninfected cells; the bottom graph represents cells exposed 2 days before HSV-B7.1 (multiplicity of infection = 1). Fifty-nine percent of cells exposed to the amplicon were FITC positive, while only cells not exposed were FITC negative.
Expression of B7-1
Uninfected cells had no detectable surface expression of B7-1 by flow cytometry, nor was B7-1 detected on the surface of cells infected with HSVlac. In contrast, 59 ± 2% of the cell population infected with the amplicon containing the gene for B7-1 demonstrated peak surface expression by day 2 after infection (see Fig. 1B).
Treatment with HSV-Modified Tumor Vaccination Without Hepatectomy
Three weeks after vaccination with an inoculum of 1 × 105 (low dose) or 1 × 106 (high dose) irradiated CT-26 cells, animals were challenged with 5 × 104 nonirradiated CT-26 cells intrasplenically. After 2 weeks of incubation the animals were killed and liver nodules were counted. Control animals, treated with medium alone, were found to have 103 ± 32 (mean ± SEM) nodules. Treatment with cells infected with HSV-GMCSF alone or infected with the combination of HSV-RANTES, HSV-B7.1, and HSV-GMCSF produced significant suppression of tumor growth. Animals treated with high-dose single-agent therapy (GM-CSF alone) had a mean nodule count of 0.7 ± 0.4 (P < .01 vs. controls), while animals treated with multiagent therapy demonstrated a nodule count of 0.1 ± 0.1 (P < .01 vs. controls). Animals treated with low-dose HSV-GMCSF-treated cells had 12 ± 6 nodules, while low-dose multiagent therapy resulted in 24 ± 9 nodules. Both combination and single-dose therapy were statistically different from the control group (P < .02) but not from each other. Single-agent low-dose or high-dose therapy produced cure rates of 0% and 67% respectively, while multiagent low-dose and high-dose therapy showed cure rates of 25% and 88%. In this model of liver metastases without additional hepatectomy, single-agent and multiagent vaccine therapy were equally effective in preventing growth of tumors.
Effects of CD4+/CD8+ T-cell Depletion on Antitumor Actions of Vaccine Therapy
To verify that the response obtained in these experiments was a result of T-cell activity, the experiments were repeated in animals depleted of CD4+ and CD8+ cells. After vaccination, animals were depleted of CD4+ and CD8+ cells via a standard protocol and then challenged with tumor. Animals were killed 2 weeks later and liver nodules counted. Control animals had a tumor count of 160 ± 50 nodules; CD4/CD8-depleted controls had a tumor count of 199 ± 50. Undepleted low-dose and high-dose treatment with the multiagent vaccine therapy resulted in tumor counts of 24 ± 9 and 0.1 ± 0.5 respectively (P < .01 vs. control;P < .05 high vs. low dose). Animals depleted of their CD4+ and CD8+ cells had a mean nodule count of 234.4 (± 28.7) despite being vaccinated against the tumor (P < .002 vs. unblocked animals) (Fig. 2). There appeared to be a dose-related response to the multiagent vaccine therapy that is T cell-mediated.
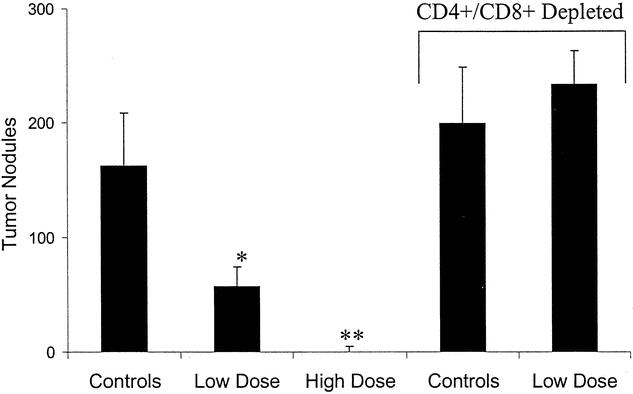
Figure 2. Hepatic tumor nodule count 2 weeks after tumor challenge with 5 × 104 CT-26 cells. Animals were vaccinated 3 weeks prior to tumor challenge with 1 × 105 (low dose) or 1 × 106 (high dose) irradiated cells infected with HSV amplicons carrying genes for GM-CSF, RANTES, and B7-1. Results are also compared to animals receiving vaccination after CD4+ and CD8+ T-cell depletion using specific antibodies directed at these T-cell subsets. Results are mean ± SEM. Depletion of T cells resulted in abrogation of the protective effect of vaccination. *P < .05; **P < .01 vs. undepleted controls.
Hepatectomy after Low-Dose Vaccination
Finally, the vaccination protocols were tested in a model of hepatectomy with residual microscopic tumors within the remnant liver. Animals were vaccinated by the previously described low-dose protocol and subsequently underwent hepatectomy immediately before tumor challenge in a model previously used in our laboratory. 6,9 The results demonstrated that hepatectomy enhanced tumor growth: nodule counts in nonhepatectomized control animals were 106 ± 30, while in hepatectomized control animals the mean nodule count after 2 weeks was 232 ± 30. Treatment was efficacious in this model despite the effects of hepatectomy. Animals treated with GM-CSF alone had an average nodule count of 40 ± 19 (P = .006 vs. control), while animals treated with multiagent therapy had an average nodule count of 11 ± 7 (P = .0004 vs. control) (Fig. 3). In this experiment, multiagent therapy again showed an enhanced cure rate when compared to single-agent therapy (50% vs. 12.5%, P = .03). There was no nonsurgical mortality in this study, an indication of the safety of this therapeutic approach.
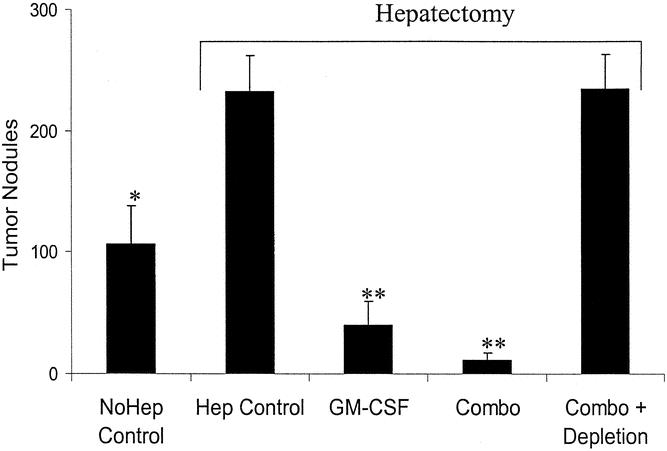
Figure 3. Hepatic nodule counts after hepatectomy and vaccination with 1 × 105 irradiated cells infected with either HSV-GMCSF or a combination of HSV-RANTES, HSV-B7.1, and HSV-GMCSF. Animals were vaccinated and after 3 weeks underwent tumor challenge with 5 × 104 CT-26 colorectal carcinoma cells and simultaneous hepatectomy to simulate microscopic residual disease at the time of resection. Two weeks after tumor challenge animals were killed and hepatic nodules counted. Nodule counts are expressed as mean ±SEM. *P < .05; **P < .01 versus controls.
DISCUSSION
Hepatic tumors are most effectively treated by surgical resection. 22 Unfortunately, nearly two thirds of patients fail this treatment modality due to residual microscopic disease undetected at the time of resection. 3,10,22 It is also clear by previous and the current study that liver regeneration potentiates the growth of local tumors. 5 Investigations aimed at identifying the mechanisms underlying this enhancement of tumor growth have implicated immunosuppression as a major contributor. Kupffer cell 6 as well as T-cell 9 dysfunction have both been found after liver resection. Studies have targeted each cell population in attempts to improve tumor surveillance in preclinical models. Administration of Kupffer cell stimulatory agents such as gamma-interferon or muramyl-tripeptide have been partly successful at eradicating tumors, 6 as has the use of single-agent tumor vaccines expressing cytokines such as GM-CSF 9 or IL-12. 10,23,24 Other investigators have also sought to treat other tumor types by genetically engineering tumor cells to secrete various cytokines. 13,16,23,25 The current study attempts to extend these previous observations by examining the difference in efficacy of multiagent therapy compared to single-agent therapy in the treatment of a model of metastatic colorectal carcinoma to the liver, focusing on their use as adjuvants to hepatic resection. Without hepatectomy, both single-agent and multiagent vaccine therapies were highly effective in retarding hepatic tumor growth, and no statistical difference was discernible. When tumor growth was exaggerated by hepatectomy, the multiagent therapy was more effective at securing curative results.
In this investigation, an HSV amplicon-based gene delivery system was used to manufacture the tumor vaccines. This was chosen because it is a highly efficient gene transfer vehicle that produces high-level production of transgenes in a wide variety of cell types. 16,26,27 Using these vectors, Tung et al 15 demonstrated that tumor vaccine secreting high levels of the cytokine IL-2 can be produced freshly harvested human liver tumors in a matter of minutes. HSV amplicon-mediated gene transfer and expression is also independent of cell replication. Thus, HSV-mediated gene transfer is compatible with the irradiation that is necessary in ex vivo tumor vaccine production to render the tumors cells incapable of replication. 15 Furthermore, the transgene remains largely episomal, resulting in only transient expression. This characteristic is the major reason that these vectors have not found widespread use in gene replacement therapy for diseases involving congenital or acquired genetic defects. This transient gene expression, however, is another desirable characteristic of these replication-incompetent vectors in tumor vaccine production, in that a potentially toxic gene product will express only in a self-limited fashion. The successful results from the current study confirm these desired characteristics of HSV-mediated tumor vaccine production. Rapid gene transfer was accomplished in irradiated tumor cells that led to high-level production of secretory as well as cell surface molecules. The resultant transient expression of these immunostimulatory molecules resulted in effective suppression of hepatic tumor growth. Additionally, no mortality was noted as a result of either single-agent or multiagent vaccinations, confirming other studies that amplicons offer a safe approach to cytokine delivery even in the setting of hepatectomy. 9,10
A number of prior studies had focused on using genetically engineered cytokine-secreting tumor cells in the treatment of tumor types outside the liver. 6,9,9,10,12,13,28 Others have shown that a wide variety of agents are capable of producing an experimental antitumor response, including interferon-gamma, 9 ICAM-1, 29 IL-12, 10,23 B7-1, 14 and IL-2. 28,29 More recently, studies have addressed the benefit of combining agents and evaluating their benefit compared to that of single-agent immunotherapy. 9,11 In the current studies, we chose GM-CSF as the single agent of comparison because in an analysis of 10 different cytokines, Dranoff et al 13 demonstrated that GM-CSF was most efficient at producing potent, tumor-specific immunity. For the purpose of this investigation, the three agents in the multiagent therapeutic strategy were selected because they potentially stimulate the immune system by different mechanisms. RANTESis a secreted protein that attracts a wide variety of im-munoactive cells to the area in which it is being produced. B7.1 is a surface costimulatory molecule that allows cells expressing it to present antigen directly to T cells. GM-CSF is thought to play a role in the maturation of antigen-presenting cells. It was hoped that the broadest response to a multiagent approach would be generated by combining agents that act via different mechanisms.
This study offers further support that immunosuppression occurring after hepatectomy may be partly responsible for the enhanced tumor growth in this setting. 9,10 The data are also a demonstration that HSV-mediated tumor vaccine therapy may result in sufficient immunostimulatory activity to overcome these detrimental effects after hepatectomy. Furthermore, the CD4/CD8 depletion studies demonstrate that the mechanism by which such vaccine therapy produces the antitumor response is via an immunologic, T cell-mediated pathway. 10 In this experimental system, multiagent use of HSV amplicons is a safe and effective method of abrogating tumor development after hepatectomy. Residual microscopic tumors are present in the majority of patients undergoing liver resection for malignancies. Current adjuvant therapy in this setting has had only limited success. These encouraging preclinical data, combined with the ease with which amplicon-mediated tumor vaccine production can be accomplished, are highly encouraging of clinical studies of this strategy in humans.
DISCUSSION
Dr. Adrian Barbul (Baltimore, MD): This study addresses two long-studied yet elusive biological phenomena. The first is the harnessing of the host immune response in the fight against cancer; and the second is the delivery of gene product to cells and tissues in order to achieve a desired and specific biological effect. Dr. Fong and his group show clearly that hepatic tumor regrowth, a process that all too often undoes superb surgical resectional therapy, can be manipulated by T-cell lymphocytes. They also demonstrate that the delivery of T-cell cytokines can enhance host antitumor responses.
I have several questions. One, what is the relationship of this immune-enhancing therapy to tumor burden in this particular model? Does the immunotherapy lose efficacy the higher the tumor burden? Secondly, once the tumor is established, can you induce immunotherapy and still be effective? And lastly, as you mentioned in your summary, what are the hurdles that you see in translating this research into the clinical arena?
Presenter Dr. Yuman Fong (New York, NY): In terms of the tumor burden and immunotherapy, I have not been very optimistic about using immunotherapy for big, bulky disease. I think that surgery to try to reduce tumor burden should be considered as part of any immuno strategy. That is why in this model we tried to look at minimal microscopic tumor. That is how it is in most of our models as we move forward to try to get this therapy as clinical reality in liver cancer patients.
For established tumors, the difficulty for most of the tumor models available is that liver tumors grow quite fast and do not completely resemble human disease. That is why this study is done more as a proof of principle rather than as a test of a specific strategy for use in man.
To examine viral effects on established tumors, we have looked at models that were easier to execute. We have examined established tumors such as in the flank, where tumors are much more obvious. And by implanting flank tumors and injecting these flank tumors with the various amplicon vectors, we are able to reduce tumor both at the site of injection and at remote sites from injection. So we believe, at least in the preclinical models, we can impact upon established tumors by such strategy. Whether that will happen in man is unclear.
But in terms of moving forward to clinical reality, where do we envision that all of this may head? Right now, the main target we are trying to get to is to use this kind of strategy as a neoadjuvant therapy before resection, to try to put in immunostimulatory genes while the immune system is relatively intact, not hampered by any of the surgical issues such as recovery from surgery. For example, to inject tumors directly with the amplicon vectors 3 or 4 weeks before a liver resection may generate an immune response that hopefully will carry over after liver resection to produce an immunoadjuvant result.
In terms of the hurdles to get there, obviously gene therapy is greatly regulated at the current time. Just getting a class of vectors to human testing is difficult. Right now in the world there are only three herpes trials that are currently in progress. We have one of them. We are the first group to put the herpes virus into the bloodstream for treatment of any human disease. Nobody has taken amplicons yet to that level. That is where we are hoping to go. We are hoping to be able to raise the required funding to generate clinical-level vector and to bring it to human testing.
Dr. Douglas L. Fraker (Philadelphia, PA): I have one observation and several questions.
Yesterday we heard Dr. Jarnagin from Memorial present a paper of over 1,800 hepatic resections over a 10-year period. And one of the discussants said that this set the standard of excellence for this very complicated technique. Today, Dr. Fong has presented an elegant preclinical model directly related to this clinical practice, and, as this describes, plans to translate this into clinical experimental trials. I view Dr. Fong as a role model for young investigators who are surgeon-scientists who have contributed excellent work both in the clinical realm as well as an experimental realm, and I use his work as examples to my residents, fellows, and young faculty, as what one can accomplish if you are very focused in a specific area, particularly if you are Yuman Fong. And I have several questions.
The first relates to the immunogenicity of your colon cancer. I read the manuscript, Dr. Fong, and I couldn’t understand. In your control experiments, are you injecting just media or do you inject lacZ-infected cells, which would be the appropriate control, because you might have an immunogenic effect just from the cells alone, with irradiated cells given then a rechallenge? If that is a correct interpretation, it seemed that the number of tumors you had in the control group was 100. When you immunosuppressed either by T-cell depletion or by hepatectomy, you increased this to 200 to 250. Although you showed an experiment, you said it wasn’t significantly different. So the question is: Is this another example of a unique way to cure cancer in mice in the immunogenic cell line? Or does at apply to other cell lines in mice? That is the first question.
A related question involves the immunogenicity of colon cancer in humans, which, as we recognize, is either not immunogenic or very weakly immunogenic. Have you conducted in vitro studies? You mentioned plans to inject amplicons into liver. But clearly you have a large volume of tumor being resected from patients at Memorial. You have a very nice in vitro technique, a very nice technique to deliver genes, and there will be a great opportunity to do in vitro studies with in vitro end points, either interferon production or cytotoxicity. Have you done these studies to demonstrate that your vectors can produce an immune effect in human choloricocancers for initiating clinical trials?
The third question I believe you may have answered. You have a fairly complex model where you are doing a intrasplenic delivery to the liver. The question was: Does this create a specific vaccination in the liver only? Or could you challenge with pulmonary mass? I believe you answered or at least discussed in the last set of answers that it does apply.
The other question would be: Can you immunize sub-QIP and get the same production against the liver model?
And the final question relates to the mechanism of enhanced tumor growth when you do hepatectomies. Clearly, your vaccine works through T cell-mediated effects, the T cell depletes and you have enormous numbers of tumors, yet it can overcome the immunosuppressive effects in hepatectomy. Is that because the hepatectomy effects are not T cell-related? Or is it a difference in magnitude of the immunosuppression compared to antibody deletion?
Dr. Yuman Fong (New York, NY): In terms of the immunogenicity, the T-26 cells are widely used and are known to be mildly immunogenic. That is why the best controls are cells that are exposed to HSV-lacZ, a herpes amplicon carrying a marker gene.
In terms of mechanisms of enhanced tumor growth within the liver after hepatectomy, we know it is more than just T cells. We and others have performed studies demonstrating that local Kupffer cell-mediated tumor surveillance is tremendously deranged after liver resection. We also demonstrated that this derangement can be reversed by Kupffer cell enhancement processes. So we don’t think that it is just T cells, but we think T cells are one of the targets.
In terms of the immunization strategy, why did we go with the splenic injection, and can other routes still produce the same effects? The reason we went with the intrasplenic injection is because we had published experiments in the past where we compared single injection in the spleen versus multiple versus intraperitoneal or subcutaneous injections of vaccines. We discovered that you could get the same effect as immunizing an animal three or four times SQ or intraperitoneal with a single injection into the portal system. For whatever reason, the immune system is stimulated much quicker and much more efficiently. That is why we chose to use this model. However, we can also vaccinate by SQ or intraperitoneal injection.
In terms of the immunogenicity of tumorous colon cells in man, I totally agree with you. Those studies should be performed before we move forward to human clinical testing. They are in the plans, but not yet performed.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Supported in part by grants RO1CA76416, RO1CA72632, and RO1CA80982 (Y.F.) and grant MBC-99366 (Y.F.) from the American Cancer Society.
Correspondence: Yuman Fong, MD, Hepatobiliary Division, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: fongy@mskcc.org
Accepted for publication April 24, 2002.
References
- 1.Mackiewicz A, Rose John S, Schooltink H, et al. Soluble human interleukin-6-receptor modulates interleukin-6- dependent N-glycosylation of alpha 1-protease inhibitor secreted by HepG2 cells. FEBS Lett 1992; 306: 257–261. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picardo A, Karpoff HM, Ng B, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery 1998; 124: 57–64. [PubMed] [Google Scholar]
- 6.Karpoff HM, Jarnagin W, Delman K, et al. Regional muramyl tripeptide phosphatidylethanolamine administration enhances hepatic immune function and tumor surveillance. Surgery 2000; 128: 213–218. [DOI] [PubMed] [Google Scholar]
- 7.Paschkis KE, Cantarow A, Stasney J, et al. Tumor growth in partially hepatectomized rats. Cancer Res, 1955; 579–582. [PubMed] [Google Scholar]
- 8.Fisher ER, Fisher B. Experimental studies of factors influencing hepatic metastases. Cancer 1959; 12: 929–932. [DOI] [PubMed] [Google Scholar]
- 9.Karpoff HM, D’Angelica M, Blair S, et al. Prevention of hepatic tumor metastases in rats with herpes viral vaccines and gamma-interferon. J Clin Invest 1997; 99: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarnagin WR, Delman K, Kooby D, et al. Neoadjuvant interleukin-12 immunogene therapy protects against cancer recurrence after liver resection in an animal model. Ann Surg 2000; 231: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuurman B, Heuff G, Beelan RHJ, et al. Enhanced killing capacity of human Kupffer cells after activation with human granulocyte/macrophage-colony-stimulating factor and interferon gamma. Cancer Immunol Immunother 1994; 39: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aruga E, Aruga A, Arca MJ, et al. Immune responsiveness to a murine mammary carcinoma modified to express B7–1, interleukin-12, or GM-CSF. Cancer Gene Ther 1997; 4: 157–166. [PubMed] [Google Scholar]
- 13.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting immunity. Proc Natl Acad Sci USA 1993; 90: 3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993; 259: 368–370. [DOI] [PubMed] [Google Scholar]
- 15.Tung C, Federoff HJ, Brownlee M, et al. Rapid production of interleukin-2-secreting tumor cells by herpes simplex virus-mediated gene transfer: implications for autologous vaccine production. Hum Gene Ther 1996; 7: 2217–2224. [DOI] [PubMed] [Google Scholar]
- 16.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli B-galactosidase in cultured peripheral neurons. Science 1988; 241: 1667–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931; 12: 186–202. [Google Scholar]
- 18.LaFreniere R, Rosenberg SA. A novel approach to the generation and identification of experimental hepatic metastases in a murine model. J Natl Cancer Inst 1986; 76: 309–322. [PubMed] [Google Scholar]
- 19.Panis Y, Ribeiro J, Chretien Y, et al. Dormant liver metastases: an experimental study. Br J Surg 1992; 79: 221–223. [DOI] [PubMed] [Google Scholar]
- 20.Wong RJ, Patel SG, Kim SH, et al. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum Gene Ther 2001; 12: 253–265. [DOI] [PubMed] [Google Scholar]
- 21.Delman KA, Bennett JJ, Zager JS, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther 2000; 11: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 22.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999; 341: 2039–2048. [DOI] [PubMed] [Google Scholar]
- 23.Tahara H, Zeh HJ III, Storkus WJ, et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res 1994; 54: 182–189. [PubMed] [Google Scholar]
- 24.Karpoff HM, Kooby D, D’Angelica M, et al. Efficient cotransduction of tumors by multiple herpes simplex vectors: implications for tumor vaccine production. Cancer Gene Ther 2000; 7: 581–588. [DOI] [PubMed] [Google Scholar]
- 25.Wei MX, Tamiya T, Hurford RKJ, et al. Enhancement of interleukin-4-mediated tumor regression in athymic mice by in situ retroviral gene transfer. Hum Gene Ther 1995; 6: 437–443. [DOI] [PubMed] [Google Scholar]
- 26.Fong Y, Federoff HJ, Brownlee M, et al. Rapid and efficient gene transfer in human hepatocytes by herpes viral vectors. Hepatology 1995; 22: 723–729. [PubMed] [Google Scholar]
- 27.Federoff HJ, Gerschwind MD, Geller AI, et al. Expression of nerve growth factor in vivo from a defective herpes simplex virus 1 vector prevents effects of axotomy on sympathetic ganglia. Proc Natl Acad Sci USA 1992; 89: 1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearon ER, Pardoll DM, Itaya T, et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell 1990; 60: 397–403. [DOI] [PubMed] [Google Scholar]
- 29.D’Angelica M, Tung C, Allen P, et al. Herpes simplex virus (HSV)-mediated ICAM-1 gene transfer abrogates tumorigenicity and induces anti-tumor immunity. Mol Med 1999; 5: 606–616. [PMC free article] [PubMed] [Google Scholar]