Abstract
Objective
To examine the effect of arginine, β-hydroxy-β-methylbutyrate (HMB), and glutamine supplementation on wound collagen accumulation in a double-blind, randomized study.
Summary Background Data
Control of wound collagen synthesis has been an elusive goal for clinicians and scientists alike. In many clinical instances, it is desired to increase collagen deposition as a means of enhancing wound strength and integrity. Arginine, a semiessential amino acid, has been shown to increase wound collagen accumulation in rodents and humans. HMB, a metabolite of leucine, regulates muscle proteolysis in animals and humans and increases collagen deposition in rodents.
Methods
Thirty-five healthy, nonsmoking human volunteers 70 years or older were enrolled and underwent subcutaneous implantation of two small, sterile polytetrafluoroethylene (PTFE) tubes into the deltoid region under strict aseptic techniques. The tubes were 1 mm in diameter and 6 cm in length with pore size of 90 to 120 μm to allow optimal ingrowth of fibroblasts and the deposition of matrix. Eighteen volunteers (mean age 75.4 years; 2 men, 16 women) were randomized to receive daily supplementation of 14 g arginine, 3 g HMB, and 14 g glutamine (total nitrogen 3.59 g) in two divided doses. The control group (n = 17; mean age 75.3 years; 6 men, 11 women) received an isonitrogenous, isocaloric supplementation of nonessential amino acids. Catheters were removed at 7 and 14 days postimplantation and analyzed for hydroxyproline (OHP, nmol/cm catheter, an index of collagen accumulation) and α-amino nitrogen (α-AN, mmol/cm, an index of total protein deposition).
Results
Supplements were well tolerated, without any reported side effects. Supplementation with the specialized amino acid mixture led to a significant rise in plasma arginine and ornithine levels. The specialized amino acid supplement led to a significant increase in collagen deposition (as reflected by OHP content) in the PTFE tubes without an effect on total protein accumulation.
Conclusions
Collagen synthesis is significantly enhanced in healthy elderly volunteers by the oral administration of a mixture of arginine, HMB, and glutamine. This provides a safe nutritional means for increasing wound repair in patients.
Successful wound healing remains the cornerstone of all surgical interventions. The majority of surgical wounds require collagen synthesis and scar formation for successful completion. Wound complications, such as infections and delayed healing, can undo the best-executed surgical procedure and significantly contribute to the financial burden of healthcare systems worldwide.
Numerous attempts have been made over the past 50 years to modulate wound collagen synthesis. A great deal of research has also demonstrated the intimate relationship between host nutritional state and wound collagen accumulation. 1 More recently it has been shown that certain nutrients can enhance collagen deposition in a specific and pharmacological manner. 2
Arginine is a dietary semi essential amino acid that becomes conditionally indispensable during critical illness and severe trauma. 3 Dietary arginine supplementation, above the amounts required for optimal growth, nitrogen balance, or health, increases wound collagen accumulation in healthy rodents and humans. 3–7
After trauma, arginine supplementation significantly reduces weight loss and nitrogen excretion. 8,9 Traumatized rats given intravenous hyperalimentation mixtures containing higher concentrations of arginine showed significant improvement in nitrogen retention and accelerated wound healing. 4 Injured rodents given a perioperative 1% dietary supplementation of arginine have a marked improvement in wound healing, as assessed by wound breaking strength and the hydroxyproline (OHP) content of subcutaneously implanted polyvinyl alcohol sponges. 3,5 Human volunteers, given either 17 or 24.8 g of arginine per day, demonstrate increased wound collagen deposition after 14 days of supplementation. 6,7
These studies suggest that supplementation of arginine at higher levels than currently available through standard nutritional formulations can have significant beneficial effects on the wound reparative processes and might reduce the extent of catabolic metabolic changes in states of biologic stress.
β-hydroxy-β-methylbutyrate (HMB) is a naturally occurring metabolite of the essential amino acid leucine. Several recent studies have hypothesized HMB to be the bioactive metabolite of leucine responsible for inhibiting muscle proteolysis and for modulating protein turnover, both in vitro and in vivo. 10 HMB supplementation in humans, when combined with exercise, results in increased muscle mass accretion. 11 This was associated with decreased urinary excretion of 3-methyl-histidine, indicating that most of the effect was due to inhibition of muscle proteolysis. More recently we have shown that dietary supplementation with 60 mg/kg/d of HMB results in enhanced wound collagen deposition in rats (unpublished data).
With these findings in mind, we sought to examine the therapeutic efficacy of a specialized amino acid supplement consisting of arginine, HMB, and glutamine (Juven; MTI, Ames, IA) on collagen deposition using an experimental wound micromodel in healthy elderly volunteers.
METHODS
Thirty-five volunteers were recruited from the Baltimore area responding to advertisements placed within the hospital and in local newspapers. Volunteers of both sexes were enrolled, and the minimum age for entry was 70 years. Exclusion criteria included known immunosuppression (HIV, recent chemotherapy, organ transplantation), recent steroid use, smoking, uncontrolled diabetes mellitus (fasting blood glucose > 200 mg/dL or Hgb A1C > 10%), severe peripheral vascular disease, renal function impairment (BUN > 50 mg/dL and/or creatinine > 2 mg/dL, protein-losing nephropathy > 2 g/d), severe liver failure/cirrhosis (Child class B or C), known collagen vascular disease or autoimmune disease (i.e., Ehlers-Danlos, Marfan, rheumatoid arthritis, lupus, systemic vasculitis), known systemic malignancy, and significant malnutrition (body mass index > 85% or <15% of ideal body weight).
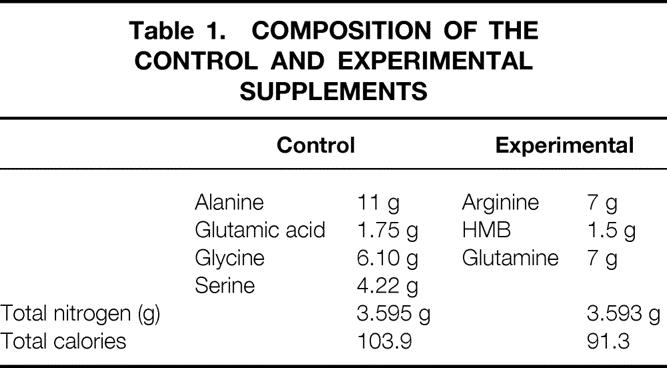
The Institutional Review Board of Sinai Hospital approved the study protocol. Informed consent was obtained from each subject before entry into the study. Subjects were blindly assigned to either the study group or the control group, using a randomization table. Eighteen volunteers (mean age 75.4 years, 2 men, 16 women) were randomized to receive the experimental amino acid mixture (Table 1). The control group (n = 17, mean age 75.3 years, 6 men, 11 women) received an isonitrogenous, isocaloric supplementation of nonessential amino acids. Supplements were administered twice daily at 8 am and 6 pm for 14 days. During this time no dietary restrictions were placed, but each subject was asked to keep a daily record of dietary activity to assess macronutrient intake. In addition, each subject was required to keep a log documenting the time each supplement dose was taken. The study was run in double-blind, randomized fashion and neither the researchers nor the subjects were aware of the treatment group assignments. Urine samples were collected on day 14 to evaluate dietary compliance by measuring HMB levels. Urine HMB was analyzed by a modified method of Nissen et al. 12 Absolute recoveries (quantitative amount of HMB) were ∼50% and relative recoveries (HMB relative to internal standard) were ∼100%. Intra-assay variation was ∼4%.
Table 1. COMPOSITION OF THE CONTROL AND EXPERIMENTAL SUPPLEMENTS
On enrollment, each patient underwent a complete medical history and physical examination. Plasma was obtained for baseline measurements of renal and hepatic profiles and for determination of amino acid levels. The wound healing model utilized involves the subcutaneous placement of polytetrafluoroethylene (PTFE) implants, modified from a technique originally described by Goodson and Hunt. 13 Under strict sterile technique and local anesthesia, two gas-sterilized PTFE tubes (Impra, Inc., Tempe, AZ) were implanted percutaneously into the subcutaneous region of the left deltoid region. Each PTFE tube measured 5 cm in length and 1 mm in diameter and had a pore size of 90 to 120 μm to allow optimal ingrowth of granulation tissue. An 11-gauge trocar and needle were inserted subcutaneously through a 3- to 4-mm stab wound in the left deltoid region. Care was taken to ensure that the needle was positioned in the subcutaneous layer. The trocar was then withdrawn, leaving the needle in place. The PTFE catheter was then inserted into the needle and the needle was withdrawn over the catheter, leaving the catheter in the subcutaneous tissue with a small portion of the catheter left exposed for retrieval. The two catheters were placed through the same stab wound at 45° to 60° from each other. After placement, the exit site was cleansed thoroughly and a sterile occlusive dressing was placed over the exposed ends of the catheters.
Each subject returned 7 and 14 days later for removal of one catheter. The removal process was performed using strict aseptic technique and was noninvasive and painless. Immediately after removal, the catheters were sectioned sterilely into 1-cm segments, which were stored in a −70°C refrigerator for subsequent analysis. The same 1-cm section of each implant was hydrolyzed at 130°C for 16 hours with 1 mL of 6 N hydrochloric acid. Hydrolyzed samples were mixed with internal standards and derivatized with polyisothionate (PITC) using Waters Picotag Vacuum Station (Millipore Corp., Milford, MA). PITC-derivatized amino acids were separated by reverse-phase chromatography (Waters HPLC System, Waters Chromatography Division, Millipore Corp.) and quantified by computerized analysis. Total alpha amino nitrogen (α-AN) levels were determined by colorimetric analysis, as an index of total protein content, and are expressed as μmol/cm of catheter length. Hydroxyproline is an amino acid unique to collagen and its level correlates well with the total amount of collagen; amino acid concentrations are expressed as nmol/cm catheter length.
At the 7- and 14-day return visits for catheter removal, additional venous blood was drawn. Plasma samples were stored at −70°C for subsequent analysis of hepatic and renal profiles. Additional blood was obtained for plasma amino acid determination, using the same reverse-phase chromatography technique described above.
The randomization code was not broken until all data were analyzed, entered, and submitted to an external study monitor. Data are reported as mean ± standard error of the mean (SEM). All data were entered into a G4 MacIntosh computer and analyzed using the StatView statistical package (Mountain View, CA). Statistical analysis was performed using the unpaired Student t test, and statistical significance was achieved at P < .05.
RESULTS
The dietary supplements were well tolerated, without any reported side effects. Laboratory evaluation of liver and renal function did not reveal any abnormalities in either group (data not shown). Compliance with the regimen was excellent, as judged by the volunteer-kept diaries as well as by monitoring urinary HMB excretion on day 14 of the trials. There was an almost 200-fold difference in the levels of urinary HMB excretion between the two groups. The SEM was high in the experimental group because in three volunteers the values were below 1,000 nmol/mL, suggesting inaccurate or incomplete compliance with the treatment regimen (Table 2).
Table 2. URINARY EXCRETION OF HMB
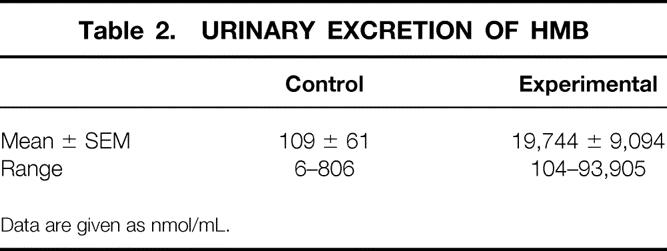
Data are given as nmol/mL.
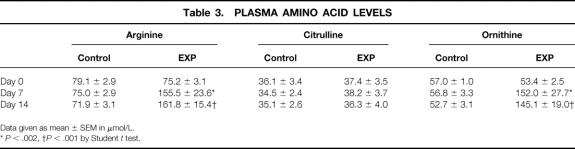
The administration of the experimental amino acid supplement resulted in significant increases in plasma arginine and ornithine levels when compared to baseline or to the control group (Table 3). Plasma citrulline, proline, aspartic acid, serine, glycine, or alanine levels were not affected by the treatment (data not shown).
Table 3. PLASMA AMINO ACID LEVELS
Data given as mean ± SEM in μmol/L.
*P < .002,
†P < .001 by Student t test.
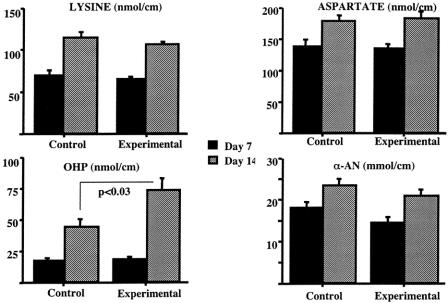
The PTFE catheters were removed at 1 and 2 weeks after implantation. Analysis of the catheters removed at 1 week revealed no significant differences in any of the parameters tested in the two groups. However, at 2 weeks posttreatment there was a significant enhancement in the amount of collagen produced, as reflected by the increase in OHP con-tent of the catheters removed from the supplemented group (72.2 ± 10.6 vs. 43.2 ± 7.2 nmol/cm implant, P < .03) (Fig. 1). These changes were not accompanied by increases in total protein deposition, as reflected by the lack of significant differences in the total amount of α-AN between the two groups. Consequently, the ratio of OHP between 1 week and 2 weeks was significantly higher in the sup-plemented group (4.64 ± 0.61 vs. 3.0 ± 0.48, P < .05) (Fig. 2). The ratios of the other amino acids or of α-AN were not affected by the treatment.
Figure 1. Selected amino acid and α-amino nitrogen (α-AN in μmol/cm tubing) assays in PTFE grafts removed at 7 and 14 days postimplantation. OHP, hydroxyproline. All values in nmol/cm tubing.
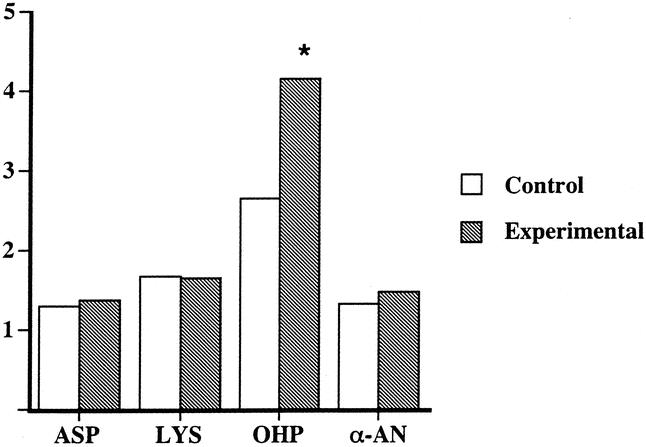
Figure 2. Ratios of 14-day to 7-day values for aspartate (ASP), lysine (LYS), hydroxyproline (OHP), and α-amino nitrogen (α-AN). *P < .05.
DISCUSSION
The present double-blind, randomized study demonstrates that healthy elderly volunteers accumulate 67% more collagen, as assessed by the OHP content, in an experimental wound model in response to supplementation with a specialized amino acid mixture containing arginine, HMB, and glutamine. The amount of wound total protein, as evaluated by the α-AN content of the implants, was not different between the two groups. The experimental supplement was well tolerated and did not affect the well-being of the subjects.
The three components of the experimental supplement have unique properties. Arginine has been shown to enhance wound healing in prior animal and human investigations. Rodents fed a diet supplemented with 1% arginine hydrochloride demonstrate enhanced wound healing as measured by collagen deposition and increased wound breaking strength. 3,5 The effect is noted whether the arginine supplementation is given for 3 or 10 days postwounding, suggesting an early effect on cells involved in the inflammatory and proliferative phases of wound healing. In young human volunteers, 2 weeks of arginine supplementation at doses of either 17 or 24.8 g/day enhanced collagen deposition by 74% and 137%, respectively. 6 Elderly subjects (>70 years of age) receiving a 17-g/day arginine supplementation for 2 weeks demonstrated a 52% increase in collagen deposition and a 98% enhancement in α-AN (protein) accretion. 7 In the latter human study, arginine supplementation did not affect the rate of wound epithelialization. Additionally, arginine did not increase DNA levels within the experimental wound, suggesting that arginine acts by enhancing fibroblastic synthetic processes, not by a proinflammatory mechanism. The 14 g of arginine contained in the experimental supplements administered in this study is an amount less than that used previously. This dose of arginine supplementation led to a significant elevation of plasma arginine and ornithine levels, elevations similar in magnitude to those observed in the prior studies with higher levels of arginine supplementation. 6,7
HMB is derived from the essential amino acid leucine. Nearly 80% of leucine is used for protein synthesis, while the remainder is converted to the α-ketoacid (α-ketoisocaproate, KIC). The major route of KIC metabolism (and indirectly leucine) is through oxidation to isovaleryl-CoA by the mitochondrial enzyme KIC-dehydrogenase, and a small proportion (∼5% of leucine) is oxidized to HMB by the cytosolic enzyme KIC-dioxygenase. 14 In the body, HMB appears to have two fates. After ingestion of HMB, nearly 20% to 50% of HMB is lost in the urine in humans, sheep, and pigs. A second pathway is the transformation of HMB to HMB-CoA. 15
Ingestion of HMB has been shown to increase lean body mass, improve skeletal muscle strength, and exert anticatabolic activity. Daily supplementation of HMB (3.0 g/day) to college-age men markedly decreased muscle damage during resistance exercise, as evidenced by less leakage of CPK from muscle and lower muscle proteolysis. There was also an associated increase in lean body weight gain. 11 In a prior unpublished study, we have shown that HMB supplementation can increase wound collagen deposition in an experimental model in rodents.
Glutamine is essential for the metabolism of rapidly turning-over cells, such as lymphocytes and enterocytes. 16 In addition, it represents the largest nitrogen pool in the body, and, therefore, is intimately involved in the catabolic responses to starvation, trauma, and sepsis. 17,18 Past studies, however, have failed to demonstrate any specific benefit of glutamine on wound healing. 19
In the current study, the specialized amino acid supplement led to a statistically significant increase in hydroxyproline deposition within the catheters, without a concomitant effect on total α-AN accumulation (a measure of total wound protein synthesis). This result stands in contrast to previous studies where arginine supplementation led to increased α-AN accumulation. This may be a possible reflection of the fact that the current control group received nonspecific isonitrogenous amino acid supplementation. In the prior studies, the control group did not receive any nitrogen, and thus the effect on total protein may have represented a nonspecific nitrogen effect of the nitrogen load in the arginine supplement.
In the present study, the magnitude of the elevation in the amount of collagen deposited is in keeping with the prior studies cited above, further suggesting that the effect on collagen is specific and highly reproducible. The OHP levels were elevated at 14 days above all other amino acids evaluated; the increase in OHP levels between 1 and 2 weeks was fourfold, whereas other amino acids or the α-AN level only doubled. This suggests a specificity in the action of the experimental amino acid supplement on collagen deposition.
The model used herein has been useful in evaluating human collagen deposition. The model accurately reflects the effect of systemic factors on wound healing, such as sex, trauma, smoking, and nutritional state. 20–23 The model does not permit determinations of wound mechanical properties. In addition, it has not been validated against surgically relevant wounds, and the data obtained from the micromodel have not been correlated with the outcome of surgical wounds. This type of data acquisition has been hampered by lack of surgeon participation in such an endeavor (TK Hunt, personal communication). Despite this limitation, the model does offer relevant information on means to modulate wound collagen synthesis, as well as information on the physiology of this critical step to surgical healing.
In conclusion, a supplement of arginine, HMB, and glutamine significantly enhances wound collagen accumulation. The absence of side effects or complications within the study population indicates that the oral administration of a mixture of arginine, HMB, and glutamine is safe at this pharmacologic dosage. Furthermore, this formulation provides a safe and effective nutritional means for increasing wound repair in this age group. The effect of such supplementation in surgical patients or those patients with impaired healing is unknown, but further evaluation is warranted.
DISCUSSION
Dr. Stanley M. Levenson (Bronx, New York): I want to compliment Dr. Barbul on the concise, lucid description of his study in his written paper and his excellent presentation.
The basic finding of the investigation showing significantly more reparative collagen as assessed by hydroxyproline determination accumulated in subcutaneously implanted ePTFE tubes in the group of elderly volunteers ingesting the arginine, HMB, glutamine supplement as compared with the control group is clear-cut. It is probable, as Dr. Barbul and his colleagues have concluded, that this is due to the ingestion of the specialized dietary supplement, especially the arginine. HMB may have an effect, but the data regarding its effect in wound healing is, at present, limited to a yet unpublished study in rats by Dr. Barbul and associates. The only study I am aware of showing that glutamine has an effect on collagen is a paper reported in the 1950s which reported that administration of glutamine to rats ameliorated experimental osteolathyrism.
Getting back, though, to the conclusion of Dr. Barbul’s paper that the increased accumulation of reparative collagen was due to the specialized nutrient supplement, the design of the study and the very limited information in the paper and presentation about the individual volunteers in both groups does not, in my view, permit an unquestioned certainty to the conclusion. For example, there are no wound healing data in the volunteers prior to the administration of the two dietary supplements to show that the two groups were comparable to begin with. I can see why the authors did not obtain such important data: it would have added a minimum of 2 or more weeks to the study and would have made recruitment of volunteers more difficult. But because of the lack of such data, the comparability of the two groups of volunteers (a necessity for establishing the significance of the difference in wound healing data when the dietary supplements were given) depends on how successful the randomization of the subjects was. Not enough data about the individual volunteers are included in the paper or presentation for us to assess the comparability of the two groups prior to the initiation of the dietary supplements.
For example, it is stated in the paper that potential volunteers with significant malnutrition were excluded. But the criteria regarding malnutrition listed were history and physical examination (details not given), body mass index greater than 85%, or weight loss not more than 15% of ideal body weight. Important nutritional abnormalities not readily detected by these criteria may exist in such individuals. One would like to know, for example, at a minimum, what were the serum albumin concentrations and peripheral blood lymphocyte counts? No information is given about the socioeconomic status of the volunteers. How many were single? How many married? How many were living alone? How many were recently widowed or had suffered other major emotional disturbances? All these may influence wound healing in important ways.
Also, the number of women included in the study was 11% in the control group and about 30% in the experimental group. There is very, very little information available whether gender affects wound healing. We do know that a recent paper reported that hydroxyproline accumulation in a wound model such as used in Dr. Barbul’s study is greater in premenopausal women than in men of similar ages. As far as I know, there are no published reports comparing wound healing in elderly men and women, the age group in the study of Dr. Barbul and his colleagues.
The authors also state that potential volunteers with severe liver disease were excluded. Does that mean that some volunteers with moderately severe liver disease were included? If so, how were they distributed in the two groups? A similar question can be raised in terms of renal function.
So, all in all, I hope that Dr. Barbul can present data regarding these and other critical points to convince us that the two groups in his study were indeed comparable at the start of the study. Finally, I suggest that he and his coauthors change the phrase “collagen synthesis” in the title of their paper. Synthesis of collagen was not measured, it is inferred by them. Reparative collagen accumulation (measured in this paper by measurement of hydroxyproline) is the net result of deposition of collagen and collagenolysis, which begins as soon as the collagen is deposited. The increased collagen accumulation in the experimental group (subject to the concerns raised earlier in this discussion) may represent increased collagen synthesis, but that was not demonstrated, it is an inference by the investigators.
Presenter Dr. Adrian Barbul (Baltimore, MD): Dr. Levenson, thank you for your comments. I want to publicly acknowledge your critical role in getting us interested and stimulated in the areas of wound healing and surgical metabolism.
To address your question about the groups. The assignment of groups occurred by a randomization table, we were not aware of their assignation to the groups, and the code was not broken until all data had been fully analyzed.
The question about serum albumin. No patient had serum albumins below 3.2.
The preponderance of women in the two groups really is a reflection of the difficulty in finding 70-year-olds and older who are healthy and meet the criteria of lack of other disease processes that would interfere with wound healing. I have to express my gratitude to Dr. Williams, who really canvassed a lot of retirement homes within the Baltimore area and spoke to multiple groups in order to get this number of patients to volunteer within the time frame of his 1 year in the laboratory.
There is no known effect of sex on wound healing in the elderly. It is known in the childbearing age. But again, your point is well taken that that is one variable that perhaps should be eliminated.
Lastly, the comment about collagen synthesis versus collagen accumulation. Again you are scientifically correct.
Dr. Thomas K. Hunt (San Francisco, CA): This is a very simple paper. It was crisply given and has one point. I want to make sure that the significance of that point is not lost. Dr. Barbul has found enhanced wound healing in elderly patients who were given a dietary supplement. This is prosaic, perhaps, but significant!
First, failed healing is an increasingly important public health problem in our aging population. It is clearly multifactorial, and many correctable issues contribute. For instance, the competence of wound healing is proportional to blood IGF levels. IGF levels decline with age and comorbidity. However, IGF-1 levels can be supported in elderly persons to the equivalent of early middle age by small doses of growth hormones, and healing improves as a result. How many other hidden problems are there in the elderly? We don’t know. In this case, Dr. Barbul appears to have found a nutritional deficiency that we didn’t even know existed, and has provided the cure “for pennies a day.”
However, many of you probably wonder about the model. Is it really a wound? Is it predictive in practical terms? The answers are “yes” and “yes.” As Dr. Barbul pointed out, my colleagues and I developed this assay, and we have kept track of its results. It is the most commonly used measure of human healing. It has measured the healing defects in hypoxia, hypovolemia, radiation, uncorrected diabetes, smoking, distant trauma, renal failure, and steroid use. In several of these it has been used to determine mechanisms and outline effective therapy. It has shown that uncomplicated anemia to a hematocrit as low as 20 is not a factor in wound healing, thus avoiding many unnecessary transfusions. It has shown that added oxygen and perfusion significantly reduce wound infections by half; and it predicted that intra- and postoperative warmth and more aggressive perioperative fluids will enhance repair and reduce infection.
Dr. Barbul’s observation is not yet complete. He must show us which of the additives is/are active; what is the best dose, etc. He has already shown an effect of arginine. The method can meet this challenge.
Now, as a result of Dr. Barbul’s research, we are faced with the prediction that elderly persons will heal better by a simple enhancement of diet. Given the track record of the method, we should believe it. If we don’t believe it, we are faced by the only other alternative; that is, to discover it years from now by counting failed wounds and probably wound infections.
Dr. Douglas W. Wilmore (Boston, MA): Dr. Barbul, a very nice paper. Three questions.
You gave three substances. Do you have dose-response data on each substance? Is there any information that tells us whether we are looking at synergy or additivity of these substances? Could we just give arginine alone, for example, and expect to see the same effect?
Secondly, in your abstract you conclude that this may be useful in patients. Do you have any patient data? Moving from the tube technology to open wound assessment is quite a jump. Do you have any clinical data that would help us feel that this could be utilized in patients?
Thirdly, I would like to just comment on the safety of arginine. There are now several studies that suggest that the use of diets containing high doses of arginine in seriously ill patients—not in elective surgical patients, but in seriously ill patients—actually increases mortality. There were two papers in the British literature in the past year or so that really emphasize this point. I wonder if you would comment about the dose of arginine used, its safety, and the types of patients or the populations which you would target for this approach.
Dr. Josef K. Fischer (Boston, MA): I would like to add on something to Dr. Wilmore’s first question. Would you have seen the same result if you just gave HMB alone?
Dr. Adrian Barbul (Baltimore, MD): In terms of prior studies that we have done with arginine alone, we have used a dose of 17 grams per day and 24.8 grams per day, both of which were shown to be effective in elevating the amount of collagen accumulated in this micromodel. The dose of arginine used in this study is less than the prior studies, but here it is supplemented with the HMB and glutamine. We do not have data on the single amino acids in humans. We only have it on arginine alone or the combination supplement that you see.
In terms of safety, I think the point that you raise, Dr. Wilmore, is a very good point. Clearly, arginine in particular when given intravenously very rapidly can cause fatal cardiac arrhythmias. Large oral doses of arginine have been shown also to be detrimental in the very, very sick patients.
My vision, if you will, for this product or this combination, or even arginine alone, would be that if for example I were to undergo a hernia repair tomorrow, I would probably take this for a week before and then a few weeks subsequent to my operative intervention, in spite of taking great care to choose a very fine surgeon.
Lastly, the prior experiments on arginine alone, although the studies were blinded and so forth, the control group did not receive any nitrogen. Historical comparisons are dangerous, but in looking at the amount of collagen deposited in the present study control group compared to prior control groups, it is about twice as high, indicating perhaps the nonspecific effect of nitrogen on collagen accumulation.
On top of that accumulation, then we can see the specific effect of the amino acid utilized in this study on collagen deposition specifically. That is one of the unexpected and yet very intriguing findings of the current study.
Dr. Basil A. Pruitt, Jr. (San Antonio, TX): The supplements were comparable in terms of nitrogen and calorie content, but what about the basic diet? Did you obtain calorie counts throughout the study? Secondly, did either of the supplements affect appetite, either increase it or decrease it? Lastly, did the difference you observed correlate with levels of growth hormone, insulin-like growth factor-1, or insulin-like growth factor binding protein-3?
Dr. Adrian Barbul (Baltimore, MD): We asked the patients to keep dietary diaries, which we examined. We did calorie counts. They did not seem to be affected by the treatment regimen. We did not measure any peripheral hormone levels. The data that we have in animals, the growth hormone response is much more immediate and pulsatile. We don’t have data on peripheral levels of islet growth hormone IGF-1 or IGF-binding protein.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Supported by a grant from the Metabolic Technologies Inc., Ames, Iowa.
Correspondence: Adrian Barbul, MD, FACS, Department of Surgery, Sinai Hospital of Baltimore, 2435 West Belvedere Ave., Baltimore, MD 21215.
E-mail: abarbul@jhmi.edu
Accepted for publication April 24, 2002.
References
- 1.Levenson SM, Demetriou AA. Metabolic factors. In: Cohen IK, Diegelmann RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders, 1992: 248–273.
- 2.Schäffer MR, Barbul A. Use of exogenous amino acids in wound healing. In: Ziegler TR, Pierce GF, Herndon DN, eds. Growth Factors and Wound Healing. New York: Springer-Verlag, 1997: 79–91.
- 3.Seifter E, Rettura G, Barbul A, Levenson SM. Arginine: An essential amino acid for injured rats. Surgery 1978; 84: 224. [PubMed] [Google Scholar]
- 4.Barbul A, Sisto DA, Wasserkrug HL, et al. Metabolic and immune effects of arginine in post-injury hyperalimentation. J Trauma 1981; 21: 970–974. [DOI] [PubMed] [Google Scholar]
- 5.Barbul A, Rettura G, Levenson SM, et al. Arginine: A thymotropic and wound healing-promoting agent. Surg Forum 1977; 28: 101–103. [PubMed] [Google Scholar]
- 6.Barbul A, Lazarou S, Efron DT, et al. Arginine enhances wound healing in humans. Surgery 1990; 108: 331–337. [PubMed] [Google Scholar]
- 7.Kirk SJ, Hurson M, Regan MC, et al. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993; 114: 155–160. [PubMed] [Google Scholar]
- 8.Pui YML, Fisher H. Factorial supplementation with arginine and glycine on nitrogen retention and body weight gain in the traumatized rat. J Nutr 1979; 109: 240–246. [DOI] [PubMed] [Google Scholar]
- 9.Elsair J, Poey J, Issad H, et al. Effect of arginine chlorhydrate on nitrogen balance during the three days following routine surgery in man. Biomedicine 1978; 29: 312–317. [Google Scholar]
- 10.Nissen S, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB). J Nutr Biochem 1997; 8: 300–311. [Google Scholar]
- 11.Nissen S, Sharp R, Ray M, et al. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol 1996; 81: 2095–2104. [DOI] [PubMed] [Google Scholar]
- 12.Nissen S, Van Koevering M, Webb D. Analysis of β-hydroxy-β-methylbutyrate in plasma by gas chromatography and mass spectrometry. Anal Biochem 1990; 188: 17–19. [DOI] [PubMed] [Google Scholar]
- 13.Goodson WH, Hunt TK. Development of a new miniature method for the study of wound healing in human subjects. J Surg Res 1982; 33: 394. [DOI] [PubMed] [Google Scholar]
- 14.Coon MJ. Enzymatic synthesis of branched chain acids from amino acids. Fed Proc 1955; 14: 762–764. [PubMed] [Google Scholar]
- 15.Rudney H. The synthesis of β-hydroxy-β-methylglutaric acid in rat liver homogenates. J Am Chem Soc 1954; 76: 2595. [Google Scholar]
- 16.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep 1985; 5: 393–400. [DOI] [PubMed] [Google Scholar]
- 17.Bulus N, Cersosimo E, Ghishan F, et al. Physiologic importance of glutamine. Metabolism 1989; 38: 1–5. [DOI] [PubMed] [Google Scholar]
- 18.Cersosimo E, Williams PE, Radosevich PM, et al. Role of glutamine in adaptations in nitrogen metabolism during fasting. Am J Physiol 1986; 250: E622–E628. [DOI] [PubMed] [Google Scholar]
- 19.McCauley R, Platell C, Hall J, et al. Effects of glutamine on colonic strength anastomosis in the rat. J Parent Ent Nutr 1991; 15: 437. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen LN, Sorensen LT, Kallehave F, et al. Premenopausal women deposit more collagen than men during healing of an experimental wound. Surgery 2002; 131: 338–343. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson K, Jensen JA, Goodson WH III, et al. Tissue oxygenation, anemia and perfusion in relation to wound healing in surgical patients. Ann Surg 1991; 214: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen LN, Kallehave F, Christensen E, et al. Less collagen production in smokers. Surgery 1998; 123: 450–455. [PubMed] [Google Scholar]
- 23.Haydock D, Hill G. Impaired wound healing in surgical patients with varying degrees of malnutrition. J Parent Ent Nutr 1986; 10: 550–554. [DOI] [PubMed] [Google Scholar]