Abstract
Objective
To assess the evolution of treatment and outcome for resected esophageal cancer at a single institution.
Summary Background Data
Strategies for optimizing the treatment of resected esophageal cancer continue to evolve over time. The outcomes of these evolving treatments in the context of improved diagnostic staging and changing epidemiology have not been carefully analyzed in a single institution.
Methods
One thousand ninety-seven consecutive patients with primary esophageal cancer underwent surgery during the period 1970 to 2001. Nine hundred ninety-four patients underwent curative esophagectomy and were analyzed for changing demographics. Eight hundred seventy-nine patients who did not have systemic metastases and survived the perioperative period were assessed by multivariate analysis for factors associated with long-term survival.
Results
During the study period the overall median survival increased from 17 to 34 months, and combined hospital and 30-day mortality decreased from 12% to 6%. The R0 resection rate increased from 78 to 94%, and adenocarcinoma replaced squamous cell carcinoma as the predominant histology (83% vs. 17%). No change in survival with time was noted for patients treated with surgery alone having the same postoperative pathologic stage (pTNM). An increased proportion of patients had preoperative chemoradiation in the last 4 years of the study (59% vs. 2%). Preoperative chemoradiation was associated with a longer survival and increased likelihood of achieving a complete resection. Multivariate analysis showed that long-term survival was associated with a complete resection and the preoperative staging strategy used, while the use of preoperative chemoradiation was the most significant factor associated with ability to achieve an R0 esophageal resection.
Conclusions
This study shows favorable trends in the survival of patients with resected esophageal cancer over time. The increased use of preoperative chemoradiation, better preoperative staging, and other time-dependent factors may have contributed to the observed increase in survival.
The treatment and epidemiology of patients with resectable esophageal carcinoma has changed dramatically over time. In the Western Hemisphere, there has been an increasing prevalence of adenocarcinoma and a shift to a predominantly lower or gastroesophageal junction location. 1–3 Preoperative staging techniques have improved with time, allowing better selection of patients, while operative techniques and perioperative management have evolved, leading to decreased morbidity and mortality. 1 Because of poor long-term survival with surgery alone in locoregionally advanced esophageal cancer, treatment strategies in recent years have evaluated more frequent use of preoperative concurrent chemoradiation. 4–8 Because of the simultaneous changes in treatment and epidemiology, the interpretation of the effect of these factors on long-term survival has been difficult. Therefore, we reviewed our experience at the University of Texas M.D. Anderson Cancer Center from 1970 to 2001 in 1,097 consecutive patients with resectable esophageal carcinoma in an attempt to identify the changing epidemiologic and treatment factors and to analyze the impact of these changes on long-term outcome using multivariate statistical analysis.
METHODS
Patient Population
We retrospectively reviewed 1,097 consecutive patients with primary esophageal cancer who underwent surgery at the M.D. Anderson Cancer Center between 1970 and 2001. Nine hundred ninety-four of these patients were resected for cure and had squamous cell carcinoma or adenocarcinoma and were analyzed for changes in epidemiology and factors related to treatment outcome. Multivariate analysis of factors associated with long-term survival was performed on the 879 patients with adenocarcinoma or squamous cell carcinoma who were resected for cure, showed no evidence of distant metastatic disease at surgery, were discharged from the hospital, and survived greater than 30 days after resection. Patients with primarily gastric adenocarcinomas (i.e., cardia tumors) and patients with histology limited to high-grade dysplasia were also eliminated from this study group (n = 879). For purposes of analyzing differences in outcome, patients were analyzed by eras that reflected major temporal shifts in preoperative workup or management (1970–1985, 1986–1996, 1997–2001). In the multivariate survival analysis (i.e., 879 patients), an adjustment was made for stage migration due to inaccurate preoperative staging by eliminating those patients who were found to have unsuspected M1b disease at surgery. This compensated in part for the increasing accuracy of preoperative staging that may have eliminated these advanced-stage patients before surgery in later years.
Preoperative Staging and Treatment
Patients were preoperatively staged according to the available technology of the era and physician preference. Between 1970 to 1985, barium swallow and chest x-ray were the predominant methods used, with 86% of the patients having only these two studies. Clinical staging criteria (barium swallow) were as follows: T1, no mass or ulcer; T2, less than 5 cm ulcer/mass; T3, more than 5 cm ulcer/mass; and T4, tracheoesophageal fistula. Within the second time period, 1986 to 1996, 73% of the patients who were operated on were staged with computed tomography scanning as well as barium swallow, chest x-ray, and endoscopy. Clinical staging criteria (CT scan) were as follows: T1: no mass; T2, esophageal thickening; T3, esophageal mass; T4, invasion of adjacent organ; N0, no enlarged nodes; N1, enlarged nodes; M1a, enlarged nodes in AJCC (1997 9)-designated nodes; and M1b, systemic metastases. This was followed by the period from 1997 to 2001, in which evaluation consisted primarily of high-resolution CT scanning and esophageal ultrasound, with fine-needle aspiration in some cases of suspicious lymph nodes. Clinical staging criteria (endoscopic ultrasound/CT scan) were as follows: T status as determined by endoscopic ultrasound; N status and M1a status as determined by endoscopic ultrasound, fine-needle aspiration, and CT scan criteria; and M1b status determined by CT scan. Since late 2001, PET scanning has been added in a few patients. Routine medical workup for respiratory, cardiac, and metabolic deficiencies was performed in all patients.
Preoperative staging and medical assessment allowed a decision to be made regarding the feasibility of achieving a complete resection (R0). Patients whose tumors were considered to be resectable but at high risk of recurrence (IIB or higher) because of local extension or possible lymph node metastases were eligible to receive neoadjuvant therapies according to institutional protocols. In all, 53% of the resected patients underwent some form of neoadjuvant therapy. The neoadjuvant regimen did not vary based on histology and was not given to patients with an early clinical stage (i.e., T1N0).
Surgical Technique
Surgical technique was determined by tumor location and surgeon’s preference. In the 1970s some patients underwent two-stage operations (“other”) with bypass of the tumor from the abdomen to neck in the first operation and resection of the esophageal tumor at a second operation. From the late 1970s to the present, most patients underwent a single operation. Fifty-two percent of the patients underwent a transthoracic resection with a separate laparotomy and right thoracotomy. The anastomoses in these cases were placed at the level of the azygous vein. A transhiatal approach was used in 25% of the patients and was performed as described in previous publications by Orringer 8 with a cervical hand-sewn anastomosis. A three-field esophagectomy with a transthoracic resection and cervical anastomosis was done in 9% of the patients. Esophageal reconstruction was performed with a gastric conduit (90%), colon interposition (9%), or free jejunal graft (1%) according to the clinical situation. Lymph node dissections included systematic removal of the celiac axis, lesser curvature, paracardial, periesophageal, and subcarinal lymph nodes.
Resected specimens underwent routine histopathology. All patients were retrospectively assigned a TNM classification and categorized according to 1997 AJCC guidelines. 9 Ninety-four patients, however, were unclassifiable (stage 0-Rx) after neoadjuvant therapy secondary to a complete pathologic response at the primary tumor (T0N0, T0N1, or TcisN1 classifications). Patients were followed by direct evaluation or phone interview until death or up to the current study period. Follow-up is complete with a median potential follow-up as follows: 1970 to 1985, 264 months; 1986 to 1996, 121 months; and 1997 to 2001, 29 months.
Statistical Analysis
Survival probability analyses were performed using the Kaplan-Meier method and were calculated from the date of surgery to the date of death or most recent follow-up. Statistical significance was assessed by the log-rank test. Independent predictive factors for long-term survival were determined using Cox regression analysis. Factors independently influencing complete (R0) resection were determined by logistic regression analysis. Univariate analyses were performed by chi-square analysis. Two-tailed probability values (P values) of .05 or less were considered significant. All data were analyzed by SPSS (Chicago, IL) and Graph Pad Prism (Graph Pad Software, San Diego, CA) software analysis packages.
RESULTS
Demographics
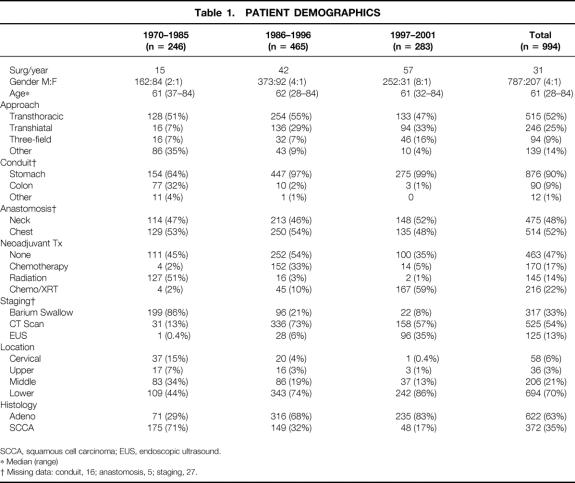
Patients showed a similar age range throughout the study period. There was an increase with time in the proportion of men versus women undergoing surgical resection (Table 1). Adenocarcinoma became the predominant histology in the 1986–1996 study period, with a simultaneous increase in the number of tumors located in the lower esophagus. Over time there was an improvement in preoperative staging techniques: most patients were staged only with barium swallow and chest x-ray from 1970 to 1985, while most patients from 1986 to 1996 also underwent CT scan evaluation. From 1997 to 2001 many patients underwent endoscopic ultrasound, and beginning in 2001 some patients were evaluated by PET scan.
Table 1. PATIENT DEMOGRAPHICS
SCCA, squamous cell carcinoma; EUS, endoscopic ultrasound.
* Median (range)
† Missing data: conduit, 16; anastomosis, 5; staging, 27.
Over time surgical techniques changed. Many patients underwent two separate staged procedures in the 1970s (labeled as “other” in Table 1), in which esophageal bypass from the abdomen to the neck was performed at the first operation, followed by resection of the tumor and esophagus at another operation. An increasing number of transhiatal esophagectomies were performed after 1985, with fewer colon conduits. The proportion of neck and chest anastomoses was similar with time.
The type of preoperative neoadjuvant therapy also shifted with time. From 1970 to 1985, the most common treatment modality was preoperative radiation therapy alone. This shifted to preoperative chemotherapy alone in 1986 to 1996 and finally to preoperative concurrent chemotherapy and radiation therapy from 1997 to 2001. The changes in preoperative treatment reflected the evolving therapeutic strategies and protocols of the different eras.
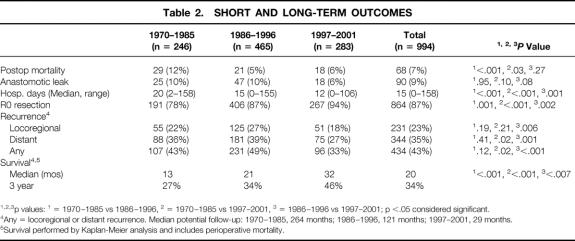
Short-Term Outcomes
The length of stay decreased with time, and overall hospital and 30-day mortality also decreased after 1985 (Table 2). Anastomotic leaks occurred in 9% of patients overall; this was not related to time, although there was an increased tendency for leaks in patients with cervical anastomoses (anastomotic leak: cervical location 12%, thoracic location 6%, P = .005) and colon replacements (anastomotic leak: gastric conduit 8%, small bowel 11%, colon 19%, P < .01).
Table 2. SHORT AND LONG-TERM OUTCOMES
*, †, ‡p values:
* = 1970–1985 vs 1986–1996,
† = 1970–1985 vs 1997–2001,
‡ = 1986–1996 vs 1997–2001; p <.05 considered significant.
††Any = locoregional or distant recurrence. Median potential follow-up: 1970–1985, 264 months; 1986–1996, 121 months; 1997–2001, 29 months.
**Survival performed by Kaplan-Meier analysis and includes perioperative mortality.
Long-Term Outcomes
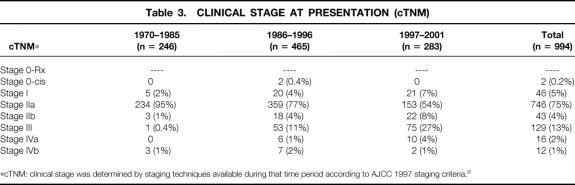
No change in survival with time was noted for patients treated with surgery alone having the same postoperative pathologic stage (pTNM) over time (Table 3). Three-year survival rates for surgery with a pathologic stage IIA patient from 1970 to 1985, 1986 to 1996, and 1997 to 2001 were 63%, 52%, and 44% respectively (P = .88). Three-year survival rates for surgery alone with a pathologic stage III patient from the same time periods were 10%, 18%, and 6%, respectively (P = .06). This suggests that the natural history of surgically treated esophageal cancer has not changed significantly over this time period.
Table 3. CLINICAL STAGE AT PRESENTATION (cTNM)
*cTNM: clinical stage was determined by staging techniques available during that time period according to AJCC 1997 staging criteria.9
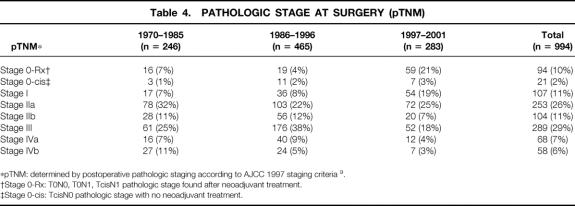
In an effort to assess the effect of various factors on long-term cancer survival, patients who died perioperatively from nontumor causes were excluded from multivariate analysis. Additionally, an attempt was made to correct for stage migration with time due to less accurate staging techniques by excluding patients with unsuspected metastatic disease found at surgery. In the 1,094 patients taken to surgery, the number of patients with “occult” unsuspected metastatic disease decreased over time from 11% in 1970 to 1985 to 7% in 1997 to 2001 and from 11% to 3% in the patients undergoing esophageal resection (Table 4, n = 994). This decrease in unsuspected metastatic disease may reflect improved preoperative staging with time, leading to better selection of operative candidates and decreased operative interventions for patients with unsuspected metastatic disease.
Table 4. PATHOLOGIC STAGE AT SURGERY (pTNM)
*pTNM: determined by postoperative pathologic staging according to AJCC 1997 staging criteria 9.
†Stage 0-Rx: T0N0, T0N1, TcisN1 pathologic stage found after neoadjuvant treatment.
‡Stage 0-cis: TcisN0 pathologic stage with no neoadjuvant treatment.
By univariate analysis, improved survival was associated with the surgery time period (Fig. 1), ability to achieve a complete resection (Fig. 2), use of preoperative concurrent chemoradiation (Fig. 3), and type of preoperative staging technique used (data not shown). Histology, location of tumor, age, gender, and type of surgical procedure did not affect survival (data not shown).
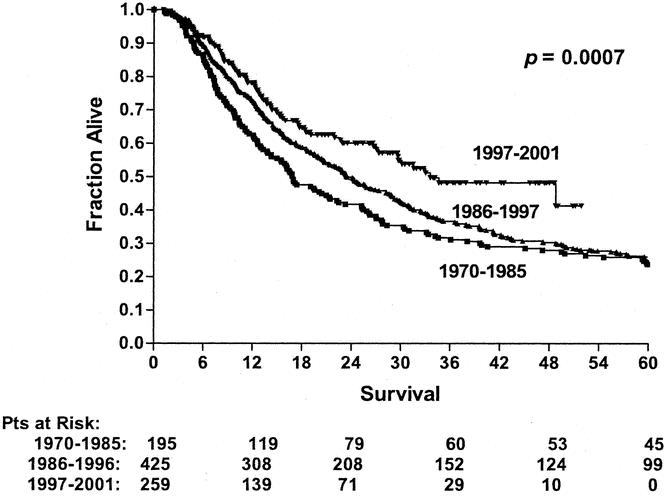
Figure 1. Long-term survival of patients undergoing esophagectomy over three different time periods (1970–1985, n = 195; 1986–1996, n = 425; 1997–2001, n = 259). Patients who died perioperatively or were found to have M1b disease were excluded from this analysis.
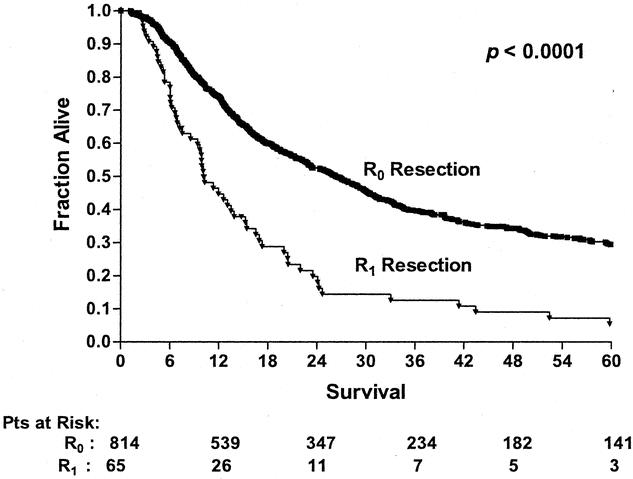
Figure 2. Long-term survival of patients undergoing esophagectomy according to R0 or Ri resection status (R0 resection, n = 814; R1 resection, n = 65). Patients who died perioperatively or were found to have M1b disease were excluded from this analysis.
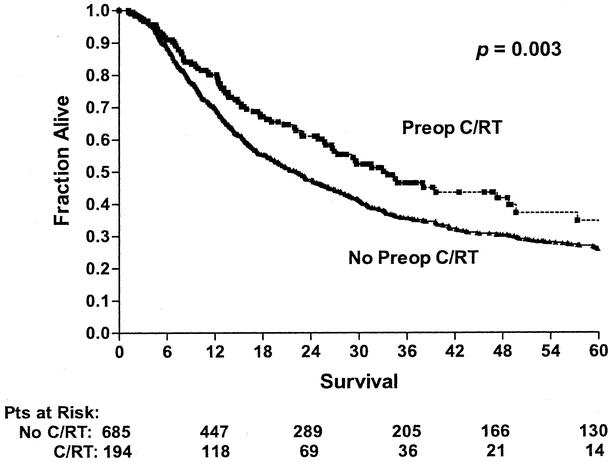
Figure 3. Long-term survival of patients undergoing esophagectomy according to whether they received preoperative concurrent chemoradiation (Preop C/RT, n = 194) or no preoperative chemoradiation (No Preop C/RT, n = 685; includes preoperative radiation alone, preoperative chemotherapy alone, and no preoperative treatment). Patients who died perioperatively or were found to have M1b disease were excluded from this analysis.
Multivariate analysis using Cox regression analysis revealed that significant prognostic factors for long-term survival were the type of preoperative staging technique used and the ability to achieve R0 resection (Table 5). In an attempt to identify factors leading to R0 resection, multivariate analysis was performed using logistic regression. This revealed that the use of preoperative concurrent chemoradiation was the factor most closely associated with an ability to achieve R0 resection (Table 6).
Table 5. COX REGRESSION ANALYSIS OF LONG-TERM SURVIVAL
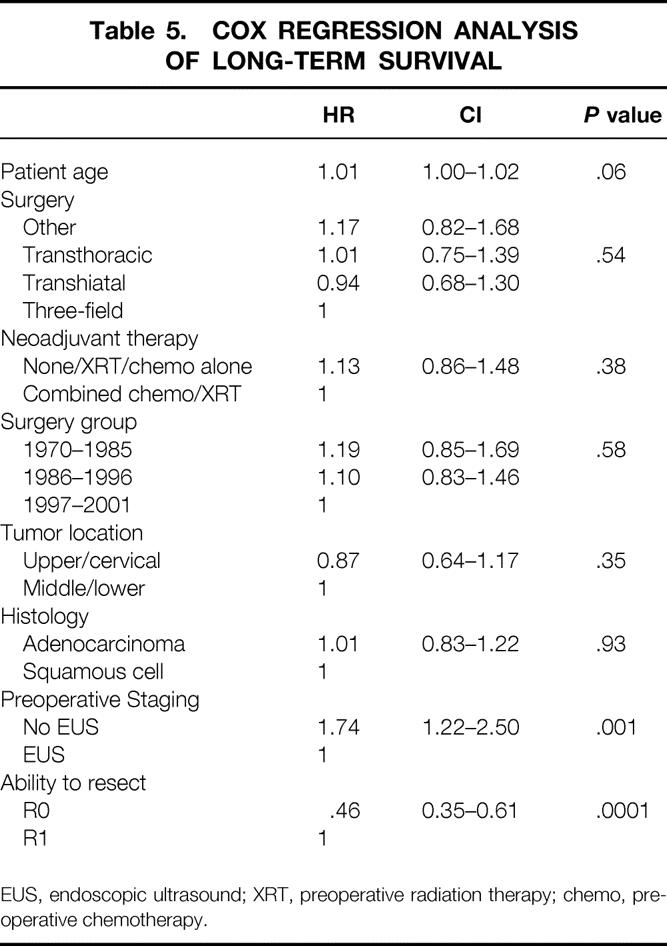
EUS, endoscopic ultrasound; XRT, preoperative radiation therapy; chemo, preoperative chemotherapy.
Table 6. LOGISTIC REGRESSION ANALYSIS OF R0 RESECTION
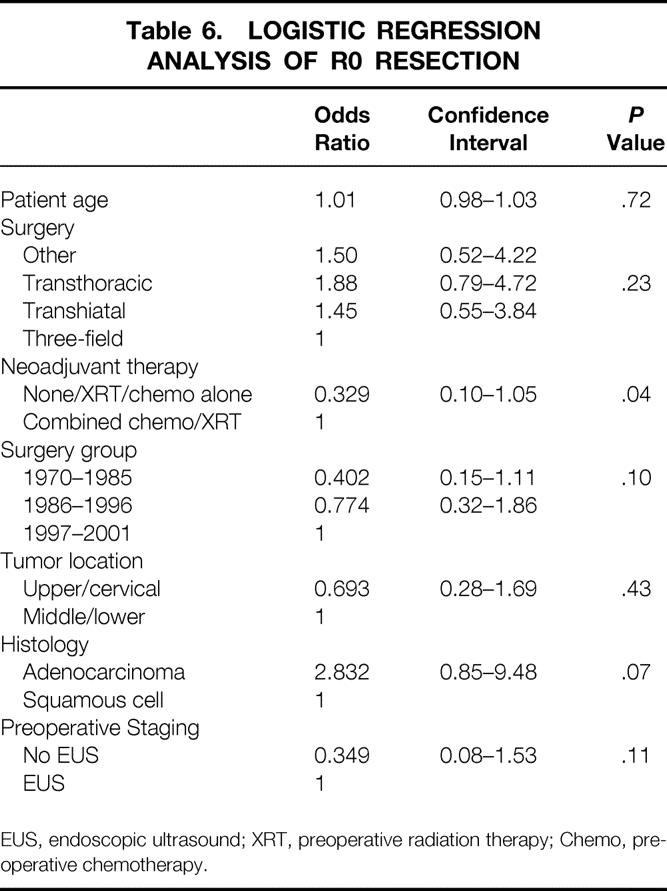
EUS, endoscopic ultrasound; XRT, preoperative radiation therapy; Chemo, preoperative chemotherapy.
Recurrence
For the patients undergoing esophageal resection (n = 994), recurrence was identified in 43% of patients (see Table 2). The number of locoregional and distant recurrences decreased in the last time period, but follow-up was also shorter in this group (median potential follow-up: 1970–1985, 264 months; 1986–1996, 121 months; 1997–2001, 29 months). In the first time period, with 264 months of median potential follow-up, 50% of the recurrences occurred within 9 months of surgery and 75% of the recurrences occurred within 21 months.
Patients who were treated with preoperative chemoradiation had a significantly decreased locoregional recurrence rate compared to those not receiving preoperative chemoradiation (17% vs. 25%, P = .01) and a tendency towards decreased distant recurrence (31% vs. 35%, P = .12). Additionally, Kaplan-Meier analysis of survival demonstrated an increase in 3-year survival in those patients who received preoperative chemoradiation (see Fig. 3) from 34% to 56% (P = .003).
DISCUSSION
Our study confirms the shift in esophageal cancer noted in the Western world from squamous cell carcinoma to adenocarcinoma. 1–3 In 1997 to 2001, 83% of the resected patients with esophageal cancer had adenocarcinoma, as opposed to 29% from 1970 to 1985. The cause for this shift is not clear but may be related to changing risk factors, such as decreased smoking and alcohol use and increased gastroesophageal reflux. 10 This change in histology was accompanied by a shift in location from the upper and mid-esophagus to the gastroesophageal junction and lower esophagus. We also noted a trend towards a higher proportion of males that has not previously been reported; this may reflect epidemiologic shifts or changing local factors such as regional referral patterns. Our study confirmed the observation of others that the short-term outcomes following esophageal resection have improved, with operative mortality dropping from 12% to 6%, despite the increasing use of neoadjuvant therapies such as preoperative chemoradiation. These improvements in short-term outcome may be due to modifications in surgical techniques or perioperative care or possibly improved patient selection. 1,11 Another factor accounting for the decreased perioperative mortality may be the increased number of resections performed per year at our institution (see Table 1). Several authors have noted that increasing esophagectomy volume is directly correlated with decreased operative mortality, morbidity, and hospital costs. 12–14
The dramatic increase in overall survival with time that we observed with resected esophageal cancer is of great interest. The cause for the improved survival, however, is difficult to determine because of the retrospective nature of this review. It is likely multifactorial, reflecting improved patient selection, changing epidemiology, increased use of preoperative chemoradiation, and improved surgical technique. Although patients presenting in the most recent years in our study showed an increased frequency of adenocarcinoma, univariate and multivariate analyses showed no significant survival differences attributable to histology, a finding differing from a recent review by Siewert et al. 3 In addition, when comparing patients who presented with adenocarcinoma versus squamous cell carcinoma, there were no differences in clinical or pathologic stage stratification (data not shown); although they may represent a diverse population of patients, combined analysis did not appear to change the outcome. It is possible that the reason certain other authors 3 reported a survival difference with histology is that their patient population included adenocarcinoma arising from the cardia; these patients were excluded from our study and may represent a subset of patients with different survival characteristics and tumor biology. Analysis of our series suggested that there has not been a significant change in the natural history of esophageal cancer over time, because no differences in survival were noted for similar pathologic stages in patients treated with surgery alone. These observations suggest that the increased survival over time was not due to a change in the biology of the disease. Moreover, it suggests that factors other than improvements in perioperative care and preoperative staging were also responsible for the observed improvements in survival. Other authors have reported similar results. In his series of 454 patients treated by surgical resection alone, Ellis et al. reported no significant change in the 5-year survival of similarly staged patients from 1970 to 1994. 15
One possibility for the observed increase in survival over time is that the patients undergoing resection in later time periods may actually represent a group of patients with earlier-stage disease. Although we did not notice a larger number of earlier-stage patients by cTNM (see Table 4), this observation may have been biased by our evolving staging techniques. Attempts to compare the types of patient populations by pathologic TNM criteria are also difficult because of the increasing use of preoperative chemoradiation and subsequent tumor downstaging. As Table 4 shows, the number of patients without viable tumor at surgery (stage 0-Rx) increased from 7% in 1970 to 1985 to 21% in 1997 to 2001, reflecting the increased use of concurrent chemoradiation preoperatively. An increased number of early-stage patients could also have occurred because of increased endoscopic surveillance and better patient selection. One study at UCLA Medical Center reviewed experience over time from the 1970s to the 1990s with resected esophageal cancer and found an increase in survival that appeared to be associated with the resection of a population of earlier-stage tumors. 1 In our series, however, there was a relatively small percentage of stage I patients and a greater increase in patients with clinical stage IIb and III disease over time (see Table 3). The low number of early-stage patients at our institution may have been because we did not participate in an endoscopic surveillance program during this time. Another way that improved preoperative staging could improve overall survival would be by allowing the exclusion of patients with “occult” metastatic disease or more locoregionally advanced disease. This would allow the identification of high-risk patients who might benefit from preoperative neoadjuvant therapies. Our multivariate analysis attempted to control for stage migration due to preoperative testing improvements by excluding all patients with “occult” metastatic disease who underwent surgery. Additionally, we included the type of preoperative staging used and the year of diagnosis as prognostic factors in the multivariate analyses to help control for these time-dependent factors. As Table 5 shows, the type of preoperative staging technique was indeed a significant predictor of long-term survival, suggesting that improved patient selection was one factor in improved long-term survival. This does not imply that staging in and of itself conveys any direct survival advantage to patients, but it will select those patients who are more likely to benefit from neoadjuvant therapy and surgical resection. Staging, therefore, becomes significant in multivariate analysis as a surrogate for patient selection, which is a form of stage migration. Our study clearly shows that the type of preoperative staging technique used is an important factor in long-term survival (see Table 3) and suggests that future reviews evaluating esophageal cancer survival should include this variable in their multivariate analysis to help control for this time-dependent factor.
Although we clearly saw a shift in the type of surgical procedures performed with time (i.e., more transhiatal esophagectomies and fewer two-stage procedures), no association with survival was attributable to operative technique. In the literature, debate continues regarding the efficacy of more extensive “radical” esophageal resections with three-field (abdominal, thoracic, and cervical) as opposed to two-field (abdominal and thoracic) lymph node dissections. 16–18 This study cannot address that debate since all esophagectomies at our institution were performed with a standard two-field rather than an extended three-field lymph node dissection (i.e., lymph nodes from perigastric, celiac, periesophageal, subcarinal, and paratracheal area but not cervical).
Instead of increasing the number of lymph node fields resected, our institution has focused on evaluating the impact of adding preoperative chemotherapy and/or radiation therapy to surgery in an attempt to improve both the distant and locoregional failure rate seen with surgery alone. 6,19 There have been several different neoadjuvant strategies tried at our institution over the last three decades. In the earliest time period (1970–1985) preoperative radiation therapy alone predominated, from 1986 to 1996 preoperative chemotherapy alone predominated, and from 1997 to 2001 preoperative concurrent chemoradiation strategies with or without induction chemotherapy were most common. These neoadjuvant strategies were evaluated in various protocols and focused on patients who would be at high risk of locoregional or distant relapse with surgery alone. Our series found no improvement in overall survival when preoperative radiation therapy alone or preoperative chemotherapy alone was used. In the literature, no improvement has been seen with preoperative radiation therapy alone, and the results of preoperative chemotherapy alone have been inconsistent, with some studies showing improved survival in responding patients and others showing a reduction in distant metastases that did not translate into an overall survival benefit. 20–25 Our data reinforces that conclusion. In fact, when we analyzed independently or as a group, there was no difference in the hazard ratios of each of the single-modality treatments versus no preoperative treatment, and they were therefore combined and analyzed as a group against our most recent strategy of combined preoperative chemoradiation (see Fig. 3).
In this review we did find a significant improvement in the long-term survival of patients treated with preoperative chemoradiation (see Fig. 3). The reason for this is not clear but may be that preoperative chemoradiation, unlike preoperative radiation alone (predominantly locoregional effect) or preoperative chemotherapy alone (predominantly distant effect), can affect both locoregional and distant disease simultaneously, thereby leading to a survival advantage. In the literature, most randomized studies with preoperative chemoradiation therapy have evaluated squamous cell carcinoma rather than adenocarcinoma, often with a sequential rather than concurrent chemoradiation approach (thereby losing the radiation-sensitizing effect of chemotherapy). 26–28 There have been only two randomized studies performed comparing preoperative concurrent chemoradiation and surgery versus surgery alone in predominantly esophageal adenocarcinoma patients. One of these two trials demonstrated a clear statistical survival advantage with preoperative chemoradiation but suffered from inadequate pretreatment staging. 29 The other study was not statistically significant but may have been underpowered since the survival curves appeared to favor preoperative chemoradiation (P = .15). 30 This observation points out the limitations of the randomized studies done to date, which include low patient numbers; staging inaccuracies, which may bias patient entry; inclusion of only a subgroup of patients, which may not be representative of the whole population; and underpowering of the study, which may yield a negative study even when the treatment differences are clinically meaningful. Although our study is limited by its retrospective nature, with inherent heterogeneity of patient populations, changing preoperative staging techniques, and changing epidemiologies, the large patient population and long temporal perspective of the data are complementary to the randomized studies and can help support evidence-based treatment paradigms and future protocols.
We noted that almost a third of the patients undergoing preoperative chemoradiation demonstrated no viable tumor at the time of surgery, and an additional 20% of patients demonstrated only microscopic disease. 31 Although the multivariate analysis for long-term survival did not demonstrate preoperative chemoradiation to be a significant factor, this may have been due in part to the fact that R0 resection was a stronger predictive factor. It is important, therefore, that the most significant multivariate factor associated with ability to achieve a complete R0 resection was the use of preoperative chemoradiation. The use of preoperative chemoradiation was more important than the type of preoperative testing used, year of surgery, age, or histology. Therefore, improved complete resectability may represent the mechanism through which preoperative chemoradiation is having an impact on survival. Although encouraging, a randomized trial is needed to confirm these observations because of the inherent heterogeneity of the patient populations and the inability of this study to definitively isolate the multiple time-dependent factors associated with esophageal cancer survival.
In summary, our review demonstrated significant changes in the epidemiology of esophageal cancer over time, with a dramatic increase in the proportion of adenocarcinomas of the distal esophagus and gastroesophageal junction. Short-term outcomes such as operative mortality and hospital stay improved with time despite the increasing use of preoperative chemoradiation. The long-term outcomes of surgically resected patients with esophageal cancer, as measured by survival and tumor relapse, have also improved and appear due to multiple factors, including better preoperative staging and selection and an increased ability to achieve a complete R0 resection at the time of surgery. The increasing use of preoperative chemoradiation appears to be the most important factor in the increasing percentage of R0 resections achieved with time. These findings give encouragement to further efforts and studies to improve long-term survival through pathologic downstaging with preoperative chemoradiation strategies. Randomized trials will need to be performed to identify the specific subset of patients affected by these therapeutic strategies.
DISCUSSION
Dr. John G. Hunter (Portland, OR): I would like to thank Drs. Hofstetter, Swisher, and yourself for the early submission of your manuscript for thorough review, in anticipation of this discussion. This paper demonstrates, quite elegantly, the progress of esophageal surgery over the last 30 years at M.D. Anderson. One of the striking findings is the exponentially increasing surgical volume: there are more cases in the last 4 years than in the first 15 years. Clearly, you believe that the improved outcomes are not solely related to your surgical expertise (a modest conclusion, not entirely supported by the data). You believe that neoadjuvant chemoradiation therapy has made the difference, but the regression model supports that conclusion only because preop chemoradiation allowed more R0 resections.
Have you analyzed survival as a function of pathologic staging? It appears in the manuscript that 43% of patients in the last time period were staged as 0 or 1, as opposed to 15% in the first time period and 14% in the second time period. Might this provide an alternate hypothesis for the difference in survival?
The randomized data don’t support preop chemoradiation, but most of us believe that there must be some benefit to rendering at least a quarter of patients pathologically disease-free at the time of resection. Is there room for another randomized trial of neoadjuvant therapy for stage 2 and 3 tumors? In the meantime, what should we be doing with our stage 2 and 3 patients?
I know this paper does not address the functional status of your patients, but this is a tremendously important end point as more individuals become survivors of this terrible disease. Have you performed an “era-dependent” or procedure-dependent assessment of symptoms, nutrition, or quality of life following esophagectomy?
I would like to thank the society for the opportunity to discuss this paper, and congratulate Dr. Brennan on his well-deserved ascendancy to the premier surgical organization in the world, as reflected by our international visitors and our increasingly international leadership. Thank you.
Presenter Dr. Jack A. Roth (Houston, TX): Thank you, Dr. Hunter, for those very insightful comments and questions.
The role of neoadjuvant chemotherapy still remains uncertain in the treatment of this disease. You mentioned the randomized studies that have been done to date, and indeed some of those have been positive. However, there have been problems with these studies in that patients have been inadequately staged, the studies themselves may only be applicable to a subgroup of patients with esophageal cancer, and in many cases the studies are underpowered. However, the majority of studies have still shown an increased survival with the administration of some form of neoadjuvant therapy. It is because of this confusing state that we decided to go back and look at this series and thought that perhaps it might provide some additional supportive data one way or the other. Indeed, neoadjuvant therapy does increase the ability to do an R0 resection. It turns out the R0 resection is such an important and powerful determinant of ultimate survival that it to some degree overshadows the contribution of the neoadjuvant therapy. But when one does this careful multivariate analysis, in fact, the contribution of the neoadjuvant therapy comes out.
It may be that what is happening is that in fact we are improving local control of this disease, which is relatively poor with surgery alone, with about 30% of patients developing local recurrence in our experience, and that eliminating the local recurrence factor may in fact actually improve survival. There is also a modest decrease in those patients developing metastatic disease when neoadjuvant chemotherapy is given.
So we think that the randomized data is still somewhat controversial, and that perhaps this additional type of analysis can still contribute something to determine what we should do for patients with esophageal cancer.
In terms of the survival by pathologic stage, the pathologic stage is, of course, altered by induction of chemoradiotherapy. I think that is what we are seeing in terms of the increased number of stage I patients in the later series. It is almost impossible to look at pathologic stage without this bias. However, we have looked at clinical stage, which is now much more accurate than it was 20 or 30 years ago, and in fact there appear to be more patients in the higher clinical stages in the more recent group. But again this may be due to the improved staging modalities. We also have to realize that the staging system itself is quite inaccurate and very heterogeneous.
In terms of the functional status, many of these patients do have good functional status. We are currently looking at that prospectively now in terms of their swallowing function, using modified barium swallows to look at this and other functional measures, but I don’t have any data to report on that at this time.
Dr. Joseph LoCicero, iii (Chicago, IL): Dr. Roth, your group is to be congratulated for a wonderful series. Periodically we need to reassess the expected standards for the surgical management of diseases which many surgeons see only occasionally. Your group presented a series from a single institution that sets the current standard against which we all must be measured. I would like to make an observation and get your thoughts concerning neoadjuvant therapy. We have the same experience that you do. Our caseload is increasing. We were stable at about a dozen cases per year, rising to two dozen and eventually to three dozen cases per year. So we felt like maybe in about 40 years we might be able to approach your series.
As you have already mentioned, neoadjuvant therapy is very controversial. The various trials that are out there at the current time have been severely flawed. The two European trials are severely underpowered. The most recent U.S. trial was equivocal and showed no specific advantage for neoadjuvant therapy. While in Boston, we developed a very aggressive neoadjuvant trial, and we found only about half of our patients qualified for the neoadjuvant trial. Of the half who qualified, only about half could complete the neoadjuvant trial, and we really saw no difference between the groups. This reminds me of a pregame statement by a famous athlete who once said, “If we do all right, we’ll be okay.”
So are there pieces of information that you can give us from this database, possibly cytologic information, that we can use to identify a group of patients specifically that we should give neoadjuvant therapy to rather than taking all of these patients?
Dr. Jack A. Roth (Houston, TX): You raise two critical questions. We have had a fair amount of success in terms of being able to treat these patients with a neoadjuvant therapy and have them complete their treatment and receive the appropriate amount of chemotherapy and radiation. The way it is given currently in our institution is to give two cycles of induction therapy. We have reduced this from three cycles, and this may make a difference. The radiation therapy is given at a dose of 5,040 Gy, and this again is an important reduction in dose that may make this type of therapy more tolerable. In terms of identifying patients for the neoadjuvant treatment, clearly the patients who achieve a complete response are those patients who benefit. However, at the present time we don’t have any information as to the best way to identify these patients. I believe that the future of this will probably rely on some type of more precise molecular staging techniques which will allow us to pick out those patients that have more responsive tumors.
Dr. Murray F. Brennan (New York, NY): The possibility of achieving an improved R0 section associated with the chemoradiation therapy is obvious. You got a complete response and therefore came to what must be an R0 resection because the tumor is gone. But the other possibility is that you did not come to operation because the patient progressed on therapy. Were there any patients like that? Because that would also account for an improved R0 resection as the denominator falls.
Dr. Jack A. Roth (Houston, TX): Actually, the percentage of patients that progressed on therapy was relatively low, less than 10%. I think the point you raise is an excellent one, and that is, is the RO resection itself a self-fulfilling prophecy resulting from the response to neoadjuvant therapy? Local failure in our earlier experience was extremely high in this disease, approaching 30%. I think anything that we can do to reduce that local failure rate is likely to have an impact on survival, as a significant percentage of patients die with only local disease. I believe that is what happened here.
Dr. Carlos A. Pellegrini (Seattle, WA): Can you tell us what would you do today for a patient that comes in with the most common lesion, which would be an adenocarcinoma 3-cm-long transmural with a T3N1?
Dr. Jack A. Roth (Houston, TX): We currently have ongoing induction therapy protocols for that patient. Generally it involves two cycles of chemotherapy. Cisplatin and 5-fluorouracil are important agents in the regimen. CPT-11 and Taxol have recently been used. After two cycles, which generally encompass approximately 6 weeks, that patient then begins concurrent chemotherapy and radiation. The chemotherapy is generally 5-fluorouracil and cisplatin. This has varied with some of the protocols to date. Following completion of the concurrent chemoradiation therapy, which takes approximately 5 weeks, we wait about 3 or 4 weeks and then the patient comes to surgery. Depending on the location of the lesion, we will tailor the operation for tumor location. I still favor a Lewis approach for a distal-third cancer with an open thoracotomy and resection and lymph node dissection, although some of my colleagues will do a transhiatal resection for that as well. To date we have not seen any difference in the outcome between the two operations.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Stephen G. Swisher, MD, The University of Texas M.D. Anderson Cancer Center, Department of Thoracic and Cardiovascular Surgery, Director, Esophageal Cancer Program, 1515 Holcombe Blvd., Box 445, Houston, TX 77030.
E-mail: sswisher@mdanderson.org
Accepted for publication April 24, 2002.
References
- 1.Swisher SG, Hunt KK, Holmes EC, et al. Changes in the surgical management of esophageal cancer from 1970 to 1993. Am J Surg 1995; 169: 609–614. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265: 1287–1289. [PubMed] [Google Scholar]
- 3.Siewert JR, Stein HJ, Breucher LDM, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lesions from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001; 234: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swisher SG, Holmes EC, Hunt, et al. The role of neoadjuvant therapy in surgically resectable esophageal cancer. Arch Surg 1996; 131: 819–825. [DOI] [PubMed] [Google Scholar]
- 5.Muller J, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990; 77: 845–857. [DOI] [PubMed] [Google Scholar]
- 6.Fahn H-J, Wang L-S, Huang B-S, et al. Tumor recurrence in long-term survivors after treatment of carcinoma of the esophagus. Ann Thorac Surg 1994; 57: 677–681. [DOI] [PubMed] [Google Scholar]
- 7.Forastiere AA, Orringer MB, Perez-Tarnayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol 1993; 11: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 8.Orringer MB. Transhiatal esophagectomy without thoracotomy for carcinoma of the thoracic esophagus. Ann Surg 1984; 200: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digestive system: the esophagus. In: AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 1997:65–67.
- 10.DeMeester SR, DeMeester TR. Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann Surg 2000; 231: 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putnam JB Jr, Suell DM, McMurtrey MJ, et al. Comparison of three techniques of esophagectomy within a residency training program. Ann Thorac Surg 1994; 57: 319–325. [DOI] [PubMed] [Google Scholar]
- 12.Swisher SG, DeFord L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000; 119: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 14.Matthews HR, Powell DJ, McConkey CC. Effect of surgical experience on the results of resection for oesophageal carcinoma. Br J Surg 1986; 73: 621–623. [DOI] [PubMed] [Google Scholar]
- 15.Ellis FH Jr, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia: a comparison of findings and results after standard resection in three consecutive eight-year intervals with improved staging criteria. J Thorac Cardiovasc Surg 1997; 113: 836–846. [DOI] [PubMed] [Google Scholar]
- 16.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–859. [PubMed] [Google Scholar]
- 17.Law S, Wong J. Two-field dissection is enough for esophageal cancer. Dis Esoph 2001; 14: 98–103. [DOI] [PubMed] [Google Scholar]
- 18.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma. Ann Surg 2001; 234: 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mafune KI, Tanaka Y, Takubo K. Autopsy findings in patients with esophageal carcinoma: comparison between resection and non-resection groups. J Surg Oncol 2000; 74: 196–200. [DOI] [PubMed] [Google Scholar]
- 20.Launois B, Delarue D, Campion JP, et al. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol Obstet 1981; 153: 690–692. [PubMed] [Google Scholar]
- 21.Arnott SJ, Duncan W, Kerr GR, et al. Low-dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol 1992; 24: 108–113. [DOI] [PubMed] [Google Scholar]
- 22.Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the EORTC. World J Surg 1987; 11: 426–432. [DOI] [PubMed] [Google Scholar]
- 23.Roth JA, Pass HI, Flanagan MM, et al. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg 1988; 96: 242–248. [PubMed] [Google Scholar]
- 24.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998; 339: 1979–1984. [DOI] [PubMed] [Google Scholar]
- 25.Clark P, MRC Clinical Trials Unit. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: an updated analysis of a randomised controlled trial conducted by the UK Medical Research Council Upper GI Tract Cancer Group. Proc Am Soc Clin Oncol 2001; 20: 126a. [Google Scholar]
- 26.Bosset J-F, Gignoux M, Triboulet J-P, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997; 337: 161–167. [DOI] [PubMed] [Google Scholar]
- 27.Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994; 73: 1779–1784. [DOI] [PubMed] [Google Scholar]
- 28.Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized multicenter study of preoperative radiotherapy and chemotherapy: the Second Scandinavian Trial in Esophageal Cancer. World J Surg 1992; 16: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 29.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery of esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–467. [DOI] [PubMed] [Google Scholar]
- 30.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001; 19: 305–313. [DOI] [PubMed] [Google Scholar]
- 31.Ajani JA, Komaki R, Putnam JB Jr, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer 2001; 92: 279–286. [DOI] [PubMed] [Google Scholar]