Abstract
Objective
To prospectively examine outcomes associated with an aggressive screening protocol for blunt cerebrovascular injury (BCVI), and to compare the accuracy of computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) versus conventional angiography with respect to BCVI diagnosis.
Summary Background Data
In the past 5 years, BCVI (carotid and vertebral arteries) has been recognized with increasing frequency. Initial studies described blunt carotid injuries and their associated morbidity, while more recent reports have established the devastating potential of blunt vertebral injuries. It has been suggested that early diagnosis and anticoagulation will improve outcomes and that less-invasive diagnostic techniques than conventional angiography are desirable for screening. However, there are neither established screening criteria nor studies comparing optimal diagnostic modalities.
Methods
The screened population included all patients with cervical spine fractures, LeFort II or III facial fractures, Horner’s syndrome, skull base fractures involving the foramen lacerum, neck soft tissue injury, or neurological abnormalities unexplained by intracranial injuries. Patients underwent screening with four-vessel cerebral angiography. During the first half of the study, patients also underwent helical CTA. Selected patients during this same period underwent MRA. At the time of diagnosis, anticoagulant or antiplatelet therapy was instituted unless clinically contraindicated. Results of this screening protocol were compared to a previously published cohort with cerebrovascular injuries (1995–1999) from the authors’ institution.
Results
Two hundred sixteen patients were screened over a 2-year period (3.5% of all blunt trauma admissions). Angiography identified 24 patients with carotid artery injuries (CAI) and 43 patients with vertebral artery injuries (VAI) for an overall screening yield of 29%. While the incidence of CAI remained similar between the current study and the previous study group, the incidence of VAI diagnosis increased. Stroke rates in those with CAI were also similar between the two periods. The stroke rate in VAI, however, was markedly lower at 0% as compared to 14% in the previous group. Comparison of CTA and MRA with cerebral angiography in 143 patients demonstrated sensitivities of 47% and 50%, respectively, for CAI; sensitivities were 53% (CTA) and 47% (MRA) for VAI.
Conclusions
Aggressive screening of patients with blunt head and neck trauma identified an incidence of BCVI in 1.03% of blunt admissions. Early identification, which led to early treatment, significantly reduced stroke rates in patients with VAI, but provided no outcome improvement with CAI. More encompassing screening may be required to improve outcomes for patients with CAI. However, less-invasive diagnostic techniques (CTA and MRA) are inadequate for screening. Technological advances are necessary before abandonment of conventional angiography, which remains the standard for diagnosis.
The understanding of blunt cerebrovascular injuries (BCVI) has improved greatly over the past decade of trauma care. Carotid artery injury (CAI) was once felt to be rare, with the possible consequence of cerebrovascular accident unavoidable. It is now clear that these injuries are much more common than once thought, and experimental evidence points strongly to the role of systemic anticoagulation in the prevention of stroke from these lesions. 1–3 The understanding of blunt vertebral artery injury (VAI) is currently undergoing a similar evolution. As with CAI, VAI was thought to be rare and of little consequence. Work from our institution and by others has shown that this is not the case. VAI may be very common in conjunction with certain injury patterns, and there is growing evidence that anticoagulation or antiplatelet therapy plays an important role in stroke prevention. 2,4 Given the evidence that cerebrovascular accident may be prevented if these injuries are diagnosed and treated aggressively, BCVI screening programs are becoming of increasing importance. Although such programs are necessary, many questions concerning screening remain unanswered.
While investigators have identified what appear to be injury patterns with a high risk of associated BCVI, the overall incidence of BCVI in such patterns of injury is not known. Optimal screening criteria have yet to be defined.
Another difficulty with screening for such injuries is that the current diagnostic gold standard is four-vessel cerebral angiography. Angiography produces high accuracy but is cumbersome and carries with it a small but finite risk of complications such as renal failure and cerebrovascular accident. Other less-invasive methods of screening, including computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), have been examined, and early reports as to their accuracy have been encouraging. 5,6 However, the sensitivities of CTA and MRA when compared to the standard of angiography are not yet known.
With this background in mind, the aim of this project was twofold. First, the utility of an aggressive screening protocol for BCVI was prospectively evaluated in a population of blunt trauma victims. In addition, the diagnostic modalities of angiography, CTA, and MRA were directly compared as to accuracy.
METHODS
Patients and Screening Criteria
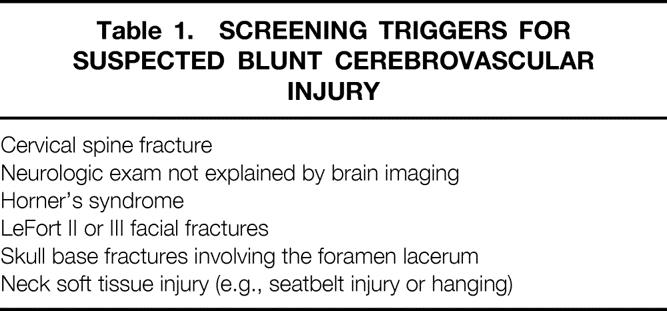
This study was conducted in a prospective fashion at the Presley Regional Trauma Center over a 2-year period. The protocol was approved by the Institutional Review Board of the University of Tennessee at Memphis. All patients who had at least one screening criterion were eligible. Screening criteria are outlined in Table 1. Reasons for exclusion included inability to obtain informed consent, insulin-dependent diabetes mellitus, underlying renal dysfunction, or withdrawal of care.
Table 1. SCREENING TRIGGERS FOR SUSPECTED BLUNT CEREBROVASCULAR INJURY
Comparison of Diagnostic Modalities
All patients were screened with four-vessel cerebral angiography as soon as possible after the screening criteria were diagnosed. During the first half of the study all patients also underwent CTA of the neck using a helical scanner (Siemens Somatom 4). During this same time period, MRA was performed in a subset of patients so that its accuracy could also be examined. Selected patients with diagnosed BCVI but without ferrous medical devices or foreign bodies underwent MRA (Siemens open magnet 0.2 Tesla scanner) after angiography and CTA.
Complete diagnostic digital subtraction arteriography (DSA) was performed by a staff neuroradiologist via a transfemoral approach. An 18-gauge single-wall needle was used to gain access to the common femoral artery. A 0.035-inch Bentson guidewire was passed through the needle and the needle removed. A 5F Berenstein or Simmons 3 catheter was used to perform selective single-plane DSA of the aortic arch and each of the subclavian arteries. Biplane DSA of each of the vertebral and carotid arteries was performed, imaging separately over the head and the neck.
The catheter was removed and hemostasis achieved at the access site using a VasoSeal device (Datascope Corp., Montvale, NJ).
CTA was performed with a single contrast bolus of 125 cc at 3 ccs, followed by a 30-second delay before scanning. Helical 1-mm images were then obtained at a pitch of 2:1, including the aortic arch to the skull base. Every three images were printed for review. Sagittal and coronal reconstructions were also created using 1-mm slices.
Two-dimensional time-of-flight MRA angiography without contrast was performed using a 0.2-Tesla open magnet. The aortic arch to the skull base was examined. Studies were interpreted based both on the source axial images as well as the maximum intensity projection reconstructions.
Angiography was performed and interpreted by staff neuroradiologists. All CTAs and MRAs were read by a staff radiologist who was not aware of the status of any of the previous testing done.
Treatment
Patients diagnosed with BCVI were treated with systemic anticoagulation (standard or fractionated heparin) unless contraindicated. Contraindications included severe brain injury or other injury posing a significant bleeding risk. In the case of unfractionated heparin, partial thromboplastin time was maintained at 40 to 50 seconds. Patients unable to be anticoagulated were treated with antiplatelet therapy (aspirin, aspirin/clopidogrel). Aspirin was dosed at 325 mg qd, and clopidogrel was given 75 mg qd. A small number of patients received no treatment due to bleeding risk or withdrawal of care.
Studied data points included patient demographics and injury characteristics, incidence of BCVI with each screening criterion, agreement of CTA and MRA results with initial angiogram, stroke rate, and outcome. The results are compared to historical controls from a previous study group of patients with BCVI diagnosed between 1995 to 1999. 2 Although not applied under a strict protocol, screening criteria in the prior group included cervical spine fracture through the transverse foramen, neurological deficit not consistent with brain imaging, neck hematoma, Horner’s syndrome, basilar skull fracture through the foramen lacerum, and severe complex facial fracture.
Statistical Analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc., Cary, NC). Dichotomous variables were compared using the chi-square or Fisher’s exact test where appropriate. The accuracy of each screening test as compared to angiography was expressed in terms of sensitivity and specificity. Significance was defined as P < .05.
RESULTS
General Population
From January 2000 through March 2002, 241 patients met BCVI screening criteria. Twenty-five were excluded: 15 due to lack of informed consent, 2 due to withdrawal of care, and 8 due to underlying disease such as insulin-dependent diabetes or renal dysfunction making contrast administration potentially unsafe. The remaining 216 underwent screening arteriography. Mechanisms of injury included motor vehicle or motorcycle crash (78%), fall (8%), and pedestrian struck and assault (4% each). The remaining patients had a combination of less-common mechanisms (e.g., hanging injury, diving accident, crush injury, struck by cotton bale). The mean age of this group was 37.6 years, mean Glasgow Coma Scale (GCS) score was 12.9, mean admission systolic blood pressure was 133 mmHg, and the mean admission base deficit was −3.4 mEq/L. One hundred fifteen were male and 101 were female.
In the 216 screening angiograms performed, one patient experienced a complication (0.5%). This patient developed an iatrogenic common carotid dissection during angiography requiring anticoagulation. This resolved on follow-up angiogram.
Injuries
BCVIs were found in 63 people for an overall diagnostic yield of 29%. These included 20 patients with CAI, 39 with VAI, and 4 with both types of injury. The overall incidence of BCVI was 1.03% of all blunt trauma admissions. While the incidence of CAI remained similar between the current study and the prior group, the incidence of VAI diagnosis almost doubled compared to the previous data set (Table 2). CAI occurred in 27 vessels (8 right, 13 left, 3 with bilateral injuries) and the distribution of injury types is shown in Table 3. The majority of these injuries were dissections. Forty-nine vertebral arteries sustained VAI (17 right, 20 left, 6 with bilateral injuries). Again, Table 3 illustrates the distribution and type of injuries seen. These VAIs were roughly equally divided between dissections and occlusions. The mean interval from admission to diagnosis of injury was 29.8 hours.
Table 2. INCIDENCE AND STROKE RATE OF CAI AND VAI IN THE CURRENT STUDY VERSUS THE PREVIOUS STUDY
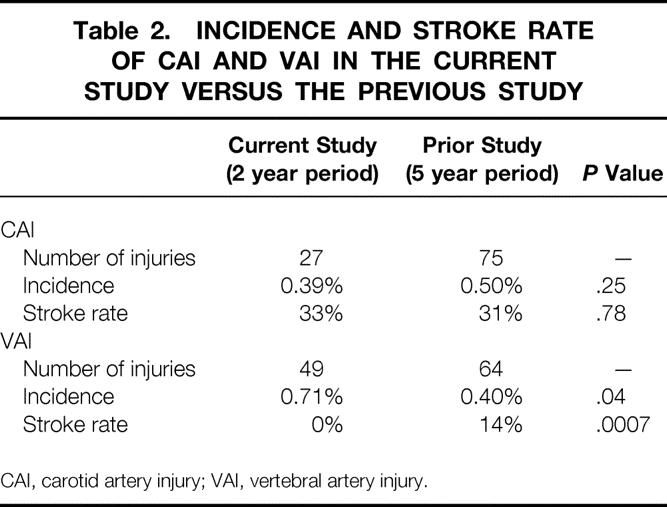
CAI, carotid artery injury; VAI, vertebral artery injury.
Table 3. DISTRIBUTION OF INJURY TYPE FOR CAI AND VAI
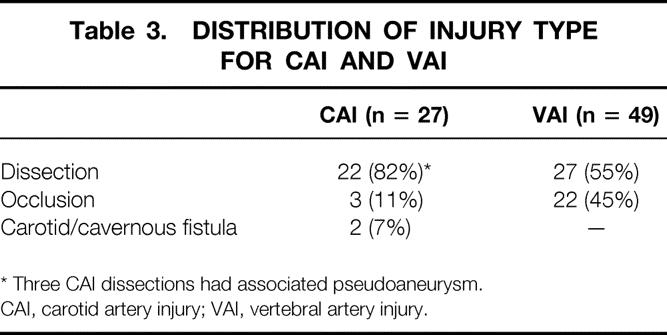
* Three CAI dissections had associated pseudoaneurysm.
CAI, carotid artery injury; VAI, vertebral artery injury.
Stroke Rate
Table 2 shows the rate of stroke in the 27 injured carotid arteries. This does not differ from the stroke rate reported from the prior study group. Stroke occurred in one of two carotid cavernous sinus fistulas (50%), three of three occluded vessels (100%), and 5 of 22 dissections (23%). Of the five strokes seen in dissected vessels, three were associated with significant stenosis, while two had no accompanying stenosis.
No strokes were seen in the 43 patients with VAI. This is significantly lower than the stroke rate seen in the prior study group (see Table 2).
Screening Criteria
Of 216 patients screened during the study period, 212 had a single risk factor, 2 patients had two risk factors each (neck hematoma/cervical spine fracture and facial fractures/neurological examination not explained by brain imaging), and the remaining two patients underwent an arteriogram because the quantity of subarachnoid blood on the admission computed tomography scan raised concern over cerebral aneurysm.
In 214 patients screened based on concern for BCVI, the most common trigger was cervical spine fracture, followed by neck hematoma, facial fracture, Horner’s syndrome, neurological examination incompatible with brain imaging, and basilar skull fracture. Table 4 shows the number screened for each condition and the associated injury rates. The highest risk of these conditions was cervical spine fracture: 33% of these patients had an associated VAI. This was followed by basilar skull fracture and neurological examination incompatible with brain imaging. CAI occurred in almost a third of such patients in both cases. The only injury found in a patient without any screening criteria was diagnosed due to concern over a ruptured cerebral aneurysm based on initial brain imaging.
Table 4. ARTERIAL INJURY RATES ASSOCIATED WITH EACH SCREENING CRITERION
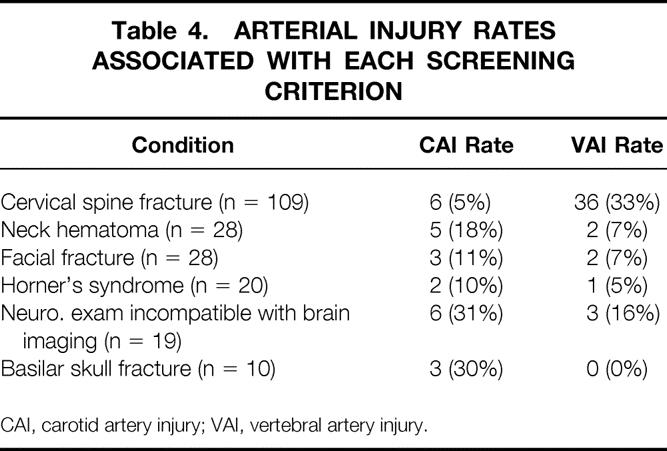
CAI, carotid artery injury; VAI, vertebral artery injury.
Within this aggressive screening protocol, 19 (79%) of patients with CAI were diagnosed before the onset of ischemia. Although higher than the 66% of patients diagnosed before ischemic symptoms in the prior study group, this difference did not attain statistical significance (P = .22). The injury mechanism and associated injuries seen in these five patients not diagnosed before development of ischemic symptoms are shown in Table 5. There is no distinct pattern of injury that might suggest additional screening criteria.
Table 5. ASSOCIATED INJURIES IN PATIENTS DIAGNOSED WITH CAI AFTER THE ONSET OF ISCHEMIA
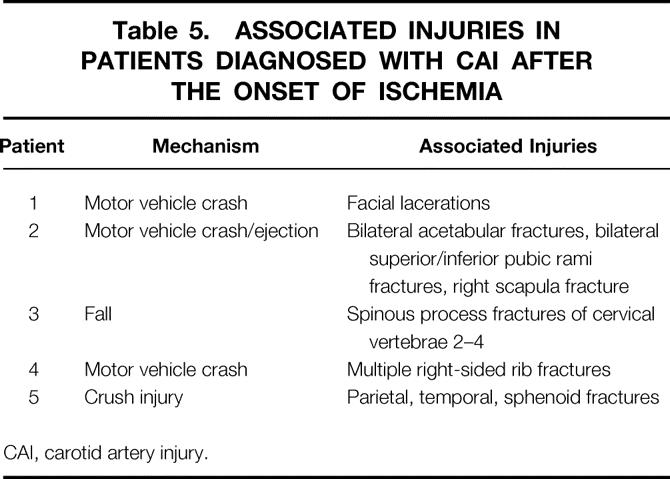
CAI, carotid artery injury.
In contrast to CAI, all VAIs were diagnosed before the development of ischemia. This is also in contrast to the prior study group, in which 12% were diagnosed only after the onset of posterior circulation ischemia (P = .03).
Cervical Spine Fractures and VAI
One hundred nine patients were admitted during the study period with cervical spine fracture after blunt injury. Of these, 46% involved a single cervical level and the remaining 54% involved multiple levels. All of these patients underwent screening for VAI, and arterial injuries were found in 36 (33%). The foramen transversarium was involved in 28 (78%) of these patients with VAI. Of the remaining eight, the majority (n = 5) had evidence of subluxation on imaging. This ranged from 30% subluxation to “locked facets,” with most being more severe in nature. One patient sustained atlanto-occipital dislocation, one had a C4 lamina fracture, and the final injured patient had a nondisplaced C3 facet fracture.
Given that foramen fracture and subluxation appear to be high-risk injuries, the rate of VAI was examined in the population of patients with these injury patterns. Indeed, 48% of all patients with transverse foramen involvement (28/58) and 44% of all patients with subluxation (12/27) had arterial injury. There were proportionally more injuries associated with these two high-risk groups combined than in those with other less worrisome cervical spine injuries (P = .0004). Had screening been carried out in only patients with these two conditions, 92% of the injuries would still have been detected, for a screening yield of 47%.
Comparison of Screening Tests
During the first 13 months of the study, 143 patients underwent both angiography and CTA to determine the relative diagnostic accuracy of CTA in diagnosis of BCVI. The mean time elapsed between tests was 37.7 hours. Twenty-one patients in whom 21 BCVIs were known to exist (4 CAI, 17 VAI) also underwent MRA. Injuries in this group included 4 CAI dissections, 7 VAI occlusions, and 10 VAI dissections.
CTA
In these 143 patients, 17 CAIs were diagnosed by angiography. CTA, however, demonstrated only eight (47%) of these. CTA also yielded one false-positive examination. The nine injuries not diagnosed on CTA included one carotid cavernous sinus fistula, one occluded vessel, one dissection with significant stenosis, and six simple dissections. Three (33%) of these nine injuries missed on CTA produced stroke in the affected patients. A similar pattern was seen in the diagnosis of VAI. In the group undergoing both angiography and CTA, 30 VAIs were found. Only 16 (53%) were seen on CTA. Missed injuries on CTA were four occluded vessels and 10 dissections, 2 of which had accompanying stenosis. The overall sensitivity and specificity of CTA compared to conventional angiography for CAI and VAI diagnosis is shown in Table 6. The sensitivity for detection of both types of injuries was poor for this examination.
Table 6. SENSITIVITY AND SPECIFICITY OF COMPUTED TOMOGRAPHIC ANGIOGRAPHY (CTA) AND MAGNETIC RESONANCE ANGIOGRAPHY (MRA) FOR DIAGNOSIS OF CAI AND VAI
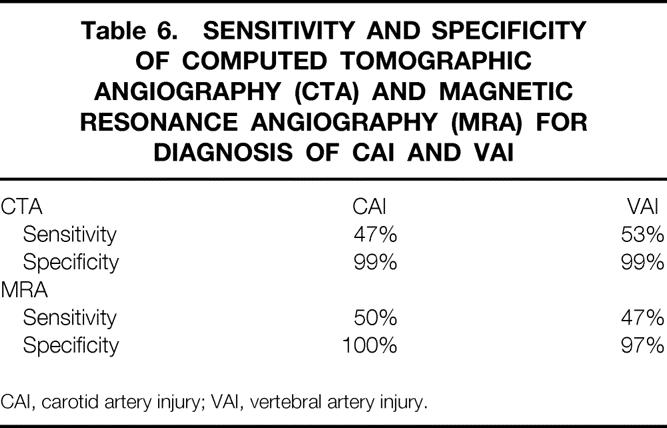
CAI, carotid artery injury; VAI, vertebral artery injury.
MRA
Similar analysis was carried out in the 21 patients undergoing MRA to examine the diagnostic accuracy of this test. Out of four known CAIs in this cohort, MRA diagnosed two (50%), and missed injuries included two dissections. One false-positive MRA occurred. With respect to VAIs, 17 were diagnosed by angiography in this group. MRA findings agreed with angiography in only 8 of 17 (47%) injuries. Nine VAI dissections were missed by MRA. Table 6 demonstrates the sensitivity and specificity of MRA in BCVI diagnosis. As with CTA, MRA sensitivity was not adequate in diagnosis of these injuries.
Treatment
CAI
Ten CAI patients received systemic anticoagulation (nine heparin, one enoxaparin) while nine received antiplatelet therapy (two aspirin/clopidogrel, seven aspirin alone). The two patients with carotid cavernous sinus fistulas underwent coil occlusion of these lesions. The remaining three had no treatment: treatment was withheld in these patients due to devastating cerebrovascular accident and concern for hemorrhagic transformation or withdrawal of care. While the overall stroke rate in the face of CAI was 33%, there was a trend toward stroke risk reduction with treatment. The stroke rate in the subgroup of asymptomatic patients receiving heparin was 11% (1/9, P = .64); it was 17% (1/6, P = .39) in previously asymptomatic patients receiving antiplatelet therapy.
VAI
Of the 43 patients with VAI, 32 received antiplatelet therapy with aspirin (n = 24) or aspirin/clopidogrel (n = 8), while systemic heparin was used in 8. Three patients with VAI were not treated. One of these had care withdrawn due to a severe stroke from concomitant CAI, and two went untreated because the attending neurosurgeon felt that therapy was not indicated. Again, all patients with VAI were diagnosed before development of ischemia, and no strokes were seen in this group.
Complications were uncommon with both systemic anticoagulation and antiplatelet therapy. Anticoagulation led to complications in two (13%) patients receiving it. One patient who received enoxaparin for a carotid dissection died after sustaining a stroke followed by severe intracerebral hemorrhage. The second patient developed significant epistaxis while on heparin. The drug was stopped with no further bleeding episodes. One patient on aspirin therapy (2%) developed bleeding from a gastric ulcer. The bleeding stopped with discontinuation of the aspirin and the patient experienced no further problems.
Mortality
Overall mortality in the group screened was 10 of 216 (5%). In the group with CAI, mortality was 6 of 24 (25%), with 5 deaths being attributable to the injury and sequelae (21%). In those with VAI, 4 of 43 died (9%). There was no stroke-attributable mortality in those with VAI.
DISCUSSION
As the understanding of BCVI continues to develop, the importance of screening programs becomes more evident. These injuries are much more common than once believed, and if undiagnosed, many will produce cerebral ischemia resulting in severe disability or death. Both CAI and VAI appear to be associated with specific injury patterns, and there is most often a latent period of hours to days between injury and ischemia. Finally, treatment has been shown to prevent stroke in most patients. For these reasons, BCVIs lend themselves to screening programs.
While certain high-risk injuries have been associated with BCVI, the optimal screening criteria remain undefined. In addition, although there is current enthusiasm over BCVI screening with less-invasive means such as CTA or MRA, the accuracy of these techniques as compared to angiography is unknown.
These data demonstrate that aggressive screening for BCVI using the criteria defined above will identify injuries in nearly 30% of those screened. Furthermore, all of the VAIs and 79% of the CAIs were found before the development of ischemic symptoms. Current CTA and MRA technology, however, lacks the necessary sensitivity to find such injuries, and until these examinations are shown to be more accurate, we will continue to screen with conventional cerebral angiography.
As it has become clear that CAIs are more common than once believed, centers have developed screening programs and attempted to identify high-risk injuries that might serve as screening triggers. Parikh et al. in 1997 found that head or chest injuries were associated with a 14-fold increase in the risk of CAI. 7 The Denver group has attempted to more closely hone the criteria for CAI screening and in a review in 1999 found a Glasgow Coma Scale score of less than 6, diffuse axonal injury, petrous bone fracture, and LeFort II or III facial fracture to be independent predictors of the presence of CAI. 8 While facial fractures and basilar skull fractures have been included in the current project, it was found that end-of-life issues as well as difficulty with intracranial pressure precluded timely screening of large numbers of patients with severe brain injury. Kerwin et al. recently reported a high rate of diagnostic accuracy using CAI screening criteria synthesized from several other studies, including anisocoria, unexplained paresis or neurological examination, basilar skull fracture, cerebral ischemia, severe facial fracture, and severe epistaxis. 9 The screening criteria in the current study are based on this prior work as well as work from our own institution that utilized screening criteria for CAI identical to the current study. 2
Although these criteria resulted in 79% of the patients with CAI in the current study being diagnosed before ischemia development, diagnosis of CAI before stroke remains a problem. Unfortunately, in most patients with CAI who are screened for a neurological examination not consistent with brain imaging, the neurological deficit is fixed at the time of discovery, a problem echoed by others. 10 Twenty-one percent of the current group of CAIs were found only after the onset of ischemia. While this is less than the 34% reported in the prior study group, the difference did not reach statistical significance.
Closely related to early diagnosis of CAI is the rate of cerebrovascular accident. This is a significant problem, and the stroke rate of 33% in the current data set reflects this. Prospective identification and screening of all patients with CAI risk factors failed to reduce the rate of cerebrovascular accident compared to the prior study group. The studied set of screening triggers is broad and leads to a high rate of diagnosis. However, it appears that to decrease below 21% the rate of diagnosis before ischemia, even more inclusive criteria may be needed. Unfortunately, no common injury characteristics were seen in the group with CAI diagnosed after ischemic onset that would point to directions in which screening criteria could be expanded. Thus, any expanded screening program would likely need to include broad triggers based on mechanism, leading to a decreasing screening yield.
The screening for VAI is closely related to the identification of cervical spine fracture. Willis et al. found that cervical spine fracture with foramen transversarium involvement or with subluxation was associated with a 46% incidence of VAI. 11 Biffl et al. 4 screened all patients with cervical spine fracture and found VAI present in 39%. They found no relationship, however, between VAI and fracture type or level. All patients with cervical spine fractures in the current study were screened based on the Denver data.
Unlike CAI, such aggressive screening significantly improved the rate of VAI diagnosis as compared to the prior study group, with all injuries in the current group being diagnosed before ischemic symptoms. This was coupled with a decreased rate of posterior circulation stroke. In fact, there were no strokes attributable to VAI in the current study, compared to a 14% stroke rate in the prior study group. This improvement in identification was due to the inclusion of all cervical spine fractures (except isolated spinous process fractures) in the screened population. Analysis of fracture types showed, in contrast to the Denver data, 4 a marked relationship between fracture pattern and VAI. Almost half of patients with cervical spine fracture and transverse foramen involvement or subluxation were diagnosed with VAI. Had only this subpopulation been screened, 92% of the diagnosed injuries would have still been found. It appears that forgoing screening in those without foramen transversarium involvement or subluxation is safe, and these patients may not need to be included in screening programs.
The combined screening criteria in this current work led to an overall diagnostic yield of 29% (CAI and VAI). While identification of risk factors for each injury is important, the goal must be to synthesize CAI and VAI criteria into an overall BCVI screening program. This is underscored by the fact that 25% of CAIs were diagnosed in patients having only cervical spine fracture as a screening criterion.
The optimal treatment of these injuries continues to be debated. 1–4,12 While the overall stroke rate in the current data set for CAI was 33%, there was a trend toward a decrease in the rate of stroke in those patients treated with anticoagulation (11%) and antiplatelet therapy (17%) before development of ischemic symptoms. Our policy of aggressive treatment in known CAI led to no asymptomatic patients with CAI in the current study having treatment withheld. For this reason a more in-depth assessment of the effect of treatment on stroke prevention is precluded. There have been several projects from our institution, as well as others, demonstrating the utility of heparin therapy in the treatment of ischemia, and more importantly, prevention of stroke related to CAI. 1–3 Heparin should remain first-line therapy if not contraindicated. If heparin cannot be used, antiplatelet therapy also appears to have a protective effect.
In the case of VAI, treatment following early diagnosis appeared to have a significant effect on patient outcome. No strokes occurred in this group, compared to the 14% stroke rate in the prior study group. Eighty percent of treated patients with VAI were managed with antiplatelet therapy, and the remaining 20% received heparin. These data strongly support the role of treatment in stroke prevention in the face of VAI. This, taken with our previous work as well as that of others, indicates that asymptomatic patients with VAI should be treated with systemic anticoagulation. 2,4 Antiplatelet therapy should be used if heparin is contraindicated.
The overall incidence of BCVI (1.03%) in the current study is similar to that in recent literature. 9 The reported incidence of CAI, however, has varied from 0.08% in earlier studies to 0.86% in a recent series from Denver. 13,14 The incidence in the present data set was 0.39%. It is likely that this increase in incidence over earlier reports represents increased awareness of the condition and its associated injury patterns. However, the reasons for the disparate incidences in the current report compared to the Denver data are less clear. Both centers serve similar populations. Possibilities include overinterpretation of screening angiograms in Denver, angiogram misinterpretation or missed injuries at our institution, or simply regional variations of injury incidence. The current incidence of VAI is among the highest reported, and this is likely a function of the broadening of screening criteria with increasing awareness of the potentially dangerous nature of these injuries.
Means of BCVI diagnosis that involve less risk than conventional angiography are attractive. Complications of angiography are reported to occur in 1% to 3% of patients and include renal failure and cerebrovascular accident. For this reason, other options have been explored. CTA diagnosis of CAI was explored by Rogers et al. in 1999 using a protocol in which those with severe blunt head/neck trauma, evidence of ischemia, or Horner’s syndrome underwent screening. 5 Those with suspicious CTA then underwent four-vessel angiography. These investigators found that the use of CTA increased the rate of CAI detection and shortened the time from injury to detection. Since not all patients underwent angiography, the rate of CAI missed by CTA in this cohort remains unknown.
MRA has also been used by several investigators as a BCVI screening tool. Weller et al. screened 12 patients with cervical spine fracture through the foramen transversarium with MRA and found a 33% incidence of VAI in this group. 15 Zuber et al. performed MRA in 15 patients with angiographically diagnosed BVCI and concluded that routine MRA was a sensitive technique for the diagnosis of BCVI, but that injuries would be missed in up to 20% of patients. 16 MRA has also been studied by several other investigators who concluded that this examination has utility in the diagnosis and screening of patients for CAI and VAI. 17,18
However, the data from this study demonstrate that with the technology used, CTA and MRA lack the sensitivity needed to diagnose both CAI and VAI. The interval between the angiogram and CTA was 37 hours, and a longer period elapsed before MRA in those undergoing this test. Given that these lesions may be dynamic in nature, this may explain some of the missed CAIs or VAIs. This is unlikely to explain the large numbers of false-negative examinations seen with both tests, however. These injuries not seen had important therapeutic implications, with 33% of the CAIs missed on CTA producing stroke. VAIs missed on CTA included both occlusions and dissections, while MRA missed nine VAI dissections. Although no strokes occurred in the current group, 43% of the strokes in the prior study group occurred in simple dissections, and another 43% occurred in the presence of occluded vessels. Thus, it appears that the lesions missed by these methodologies are not inconsequential but can lead to ischemic symptoms and stroke.
Both CTA and MRA involve constantly improving technology. Advances that are beginning to be employed, such as multislice CT scanning, may allow for more accurate imaging of the injured cervical vessels in the near future. Examination of cervical vessels with MRA using gadolinium infusion, dedicated head/neck coils, and novel acquisition sequences is being done in some institutions for the evaluation of cerebrovascular pathology. 19 High-quality images are being obtained, and the techniques continue to advance. These technologic advances may eventually eliminate the need for conventional angiography as a screening tool. Screening with CTA or MRA would likely reduce time to diagnosis, as shown by Rogers et al., 5 and might serve to improve outcome as it relates to early diagnosis and treatment. Currently, however, the use of CTA or MRA for the screening of such patients places them at significant risk of missed injury, which may lead to cerebrovascular accident. Direct comparison of angiography to any new technology to determine accuracy is necessary before such technology can be adopted as a substitute screening examination.
In summary, the criteria examined here represent a prospectively evaluated set of screening triggers that lead to BCVI diagnosis in over 1% of all blunt trauma victims. This screening protocol resulted in a doubling of the rate of VAI diagnosis as compared to the prior study group. With increased diagnosis and therefore treatment, the VAI stroke rate has fallen from 14% to 0% in the current data set. Almost 80% of CAIs were diagnosed before development of ischemia, but the CAI stroke rate remains similar to the prior study group. More encompassing screening may be needed to improve the stroke rate in the face of suspected CAI. This would likely require large-scale screening based on mechanism rather than injury pattern, and we speculate that the screening yield would be much lower for such a screening program. Thus, less-invasive methods of screening would be necessary. However, CTA and MRA are currently inadequate as screening tools, missing roughly half of both CAIs and VAIs when compared directly to angiography. Although less-invasive methods of screening for BCVI are desirable, for the time being, conventional angiography is necessary for adequate screening.
DISCUSSION
Dr. Ronald V. Maier (Seattle, WA): I congratulate the authors on an excellent study. The authors have prospectively confirmed the significance of a previously underrecognized injury in the patient with blunt injuries that is associated with severe morbidity and mortality. However, rather than just raise a red flag, the authors have gone on to more fully define the subset of patients at risk for this injury.
In an era when medicine is being asked—or, rather, being required—to critically assess the utilization of expensive interventions, the authors, while assuring the safety of their patients, have simultaneously been able to decrease the number of patients screened to only 3.5% of their overall blunt-injured population while increasing the diagnostic yield nearly 30-fold from 1% in the overall population to 29% in the group studied. This is extremely appropriate when faced with an expensive and high resource-consuming diagnostic test such as cerebral angiography—assuming one can find a cerebral angiographer at 2 am!
In addition, the authors have compared angiographic intervention to the noninvasive modalities of infusion CT and MRA. Contrary to several recent studies advocating their use based on extremely small numbers and lack of a true denominator (that is, identification of false negatives), the authors demonstrate that these noninvasive tests are currently poor performers compared to the gold standard angiogram and have only an approximate 50% sensitivity.
Thus, I applaud the authors for giving us the evidence to base our clinical care decisions in these difficult patients and would ask a number of questions.
In the manuscript, very few patients with vascular injury were missed by the screening based on their clinical criteria. However, did the authors identify any patterns of injury that better define this patient population to enable capture of 100% of the patients at risk for suffering a cerebral injury?
The onset of neurologic deficit occurs at a variable time after injury, and the current data suggest that early diagnosis and prevention are critical in this process. The average time to diagnosis in this study was 30 hours. With these new data, do the authors believe that earlier diagnosis and therapy may have prevented the vascular complication in some of their patients who went on to stroke after admission? Should angiography become an emergency procedure in these patients defined by their risk characteristics and thus become a much higher priority in the workup of these patients?
Next, while angiography remains the gold standard, its interpretation is still operator-dependent. The authors noted a doubling in the incidence of vertebral artery injuries compared to their previous study. Since this is a difficult study to interpret, are they convinced that this is not merely an overinterpretation of the disease by their radiologist?
Also, do they obtain accurate readings of their films at 2 am? This is extremely pertinent if one is going to start patients with multiple injuries on potentially dangerous anticoagulant therapy based on a wet read in the early morning hours. Do the authors do subsequent dry reads to back-check on the early reads to identify discrepancies? Was there a frequent change in the interpretation?
The authors do not describe in the manuscript how they define their main end point: stroke. Head CT or better diffusion MRI data would be needed to quantify the stroke type. Were their strokes embolic or ischemic watershed events? If watershed, one could argue that revascularization may have been considered to limit the impact of the insult. Or, in contrast, was there evidence that their anticoagulant therapy actually caused hemorrhagic conversion and worsening of the ischemic damage caused by the initial insult?
The authors do not comment on the use of transcranial Doppler monitoring in asymptomatic patients to detect microemboli. The presence of emboli would provide an indication that the preventive therapy is not working and argue for more aggressive intervention, including potential operative approaches. Doppler screening could also be used to determine if antiplatelet therapy alone was sufficient, particularly for vertebral artery injuries, or if in an individual patient full heparinization would be indicated.
And lastly, why were there no stents, direct vascular repair, or bypass performed for any of the injuries? I agree that most are very high and difficult, but some are lower and reachable through a surgical approach, and the higher lesions can be reached by stents. What indications do the authors use for stenting or operative intervention?
Presenter Dr. Timothy C. Fabian (Memphis, TN): To address the issue of carotids missed by the screening process: there were five patients who were found to have carotid injury only by development of neurologic insult and who did not meet screening criteria. There were a few other patients who met screening criteria but developed neurologic insults after admission and prior to angiography. It is anticipated that if there were a rapid screening tool such as CT, we could substantially reduce the number of patients who developed insults by providing early therapy. Since this is primarily a hyperextension/flexion injury, a rather ubiquitous mechanism, I think the only way you are going to get most patients diagnosed early is with a rapid large-scale screening procedure. Conventional four-vessel cerebral angiography is not going to be able to accomplish that due to the somewhat complex technical demands to obtain arteriography relative to institutional resources with availability of manpower and equipment. I believe the solution will be future-generation technology of CT. The multislice scanners that have been clinically utilized very recently may be the answer to the problem. The current study clearly shows that helical/spiral scanning technology is not adequate. MR technologies will not likely be appropriate as they will not permit ferrous devices in the examination area and, similar to angiography, will not be widely available on an acute basis. A study similar to this needs to be repeated in the future with the advanced-generation CT technology and, hopefully, that will be the solution. If not, I suspect that further development of high-resolution CT scanning will ultimately solve the problem of early blunt carotid injury diagnosis.
There was a question suggesting that the vertebral artery injury incidence has grown due to overinterpretation of angiography. That may be true, but I seriously doubt that that explains it. I believe the increased incidence is from recognizing true pathology. The studies were performed and interpreted by neuroradiologists as well as interpreted by our neurosurgeons trauma faculty. So, I believe nearly all of these are true injuries. Perhaps the most important finding in the entire study was the identification of the blunt vertebral injuries. We were able to eliminate posterior fossa strokes, which are often catastrophic.
It was questioned whether the strokes are embolic or ischemic. I believe the majority of the strokes that we have seen in our series are flow-related due to either advanced narrowing of the carotids or total obstruction associated with dissection. However, there clearly are some embolic phenomena that occur from the injured intimal surfaces and the resultant platelet adhesion and aggregation. That is, in fact, the rationale for using anticoagulation and antiplatelet therapy in these patients. The rationale is similar to that of the management of spontaneous dissections of the carotid system. Are we concerned that heparin can increase neurologic complications in patients with strokes? There is a small risk, but I think it is uncommon. We had one patient who had an advanced stroke and did have further hemorrhage associated with heparin in this particular study. When treating with heparin, we keep the partial thromboplastin time in the 40 to 50 range. Heparin is used to prevent further propagation of clot and, at the same time, allow the internal fibrinolytic system to promote resolution of the formed clot.
We have not used transcranial Dopplers up to this time. There have been some sporadic reports of its usage in this area. It is a technology that deserves investigation in a more organized fashion in the future.
Relative to the question concerning use of carotid stents for management of these lesions, we currently believe that stenting is appropriate for patients with pseudoaneurysms. In fact, 25% to 35% of patients with dissections will ultimately deteriorate into pseudoaneurysm formation, while approximately 5% to 10% of patients initially present with pseudoaneurysms. We also occasionally place stents in dissections that have over 50% reduction of internal carotid flow. The Denver group appears to have similar indications and have reported such. The use of stents remains an area that deserves close clinical observation and investigation as most of these patients are rather young and, at this time, it is very difficult to know what will happen over the course of decades with carotid stents. It is clear that stent technology is rapidly improving, spurred primarily by carotid stenting. It is anticipated that the stents will be coated in the near future, which will substantially decrease the risk of neointimal hypoplasia.
Nearly all of the pseudoaneurysms of the carotid injuries potentially present serious problems. They are related to embolization from the pseudoaneurysm sac as well as gradual expansion of the pseudoaneurysm with compression of surrounding structures and the risks of rupture and thrombosis.
Dr. A. Brent Eastman (La Jolla, CA): Dr. Fabian, I congratulate you on this study of a potentially lethal injury that has often been underdiagnosed. My question is the role of the interventional radiologists, particularly the neural radiologists who are now so skillful, particularly at embolizing intracranial aneurysms. I gather from your last comment that with screening and an early diagnosis, you would utilize the invasive radiologist.
My second question is, if the girl who was hit with the hockey puck had come to your trauma center, would she have qualified for this screening?
Dr. Timothy C. Fabian (Memphis, TN): Concerning the tragic case of the child that died following the hockey puck injury, this is obviously a very rare mechanism. The vertebral injury was undoubtedly caused by hyperextension and flexion with subsequent thrombosis over the course of a day and a half following the injury. She likely had an inadequate circle of Willis to allow for compensation from the anterior to posterior circulation. From my review of the literature on vertebral injuries, it would appear that children might be at an increased risk compared to adults with hyperextension. I suspect that this could be associated to the relative elasticity of the spine in these young individuals compared to adults.
Dr. Lewis M. Flint, jr. (Tampa, FL): Dr. Fabian, I enjoyed this very much. I have a question about the decision process leading to the use of anticoagulation. You said that a contraindication to anticoagulation is significant brain injury. In looking at a small group of patients amounting to about 15 patients with positive angiography for extracranial vascular injury, we found that positive brain computed tomography was present in about 60% of those patients if you looked at all brain injury patterns. So could you tell us more about what significant brain injury is? We currently think that anticoagulation is probably possible unless there is intracranial hemorrhage.
Number 2, have you looked at the anatomy of the angiograms to see if there is some anatomic pattern that might alleviate the need for anticoagulation? In other words, if the patient has good collateralization and fills from the other side, does that indicate a patient that might not have to be anticoagulated?
Dr. Timothy C. Fabian (Memphis, TN): Returning to the question of which patient population would we not heparinize: certainly those with significant intracerebral hematomas would not receive heparin. However, we generally will give heparin to patients with punctate hemorrhages. Concerning the issue of the adequacy of the circle of Willis for potential collateral flow, there is an intact circle of Willis in only 20% of adults. The remaining 80% have several different anatomic configurations, but most of these result in a compromise for potential collateralization between the anterior and posterior circulations. As we know from chronic vascular disease, there is an occasional patient who can live off of one cerebral vessel because of a wide-open circle of Willis, but this is very uncommon. In patients who have incomplete circles, issues such as shock correlate with the development of neurologic defects. We have not angiographically correlated the circle of Willis anatomy with outcomes. It would be an interesting thing to do, but it is often difficult to angiographically clearly demonstrate inadequate communications with the posterior and anterior arteries in the circle of Willis. However, this question of marginal collateralization is clinically important relative to patient management in that I believe maintenance of normal to slightly elevated systemic pressure will likely improve the outcomes in patients with carotid dissections, preventing sluggish flow and decreasing the progression to thrombosis. Therefore, we keep the patients well hydrated and occasionally will use pressors to maintain normal pressure to slightly elevated pressures and presumably maintain high flow. However, this approach is anecdotal and ideally would be the subject of controlled study, but such studies would be difficult due to the heterogeneity of anatomy and physiology which occurs with blunt cerebrovascular injuries.
The authors would like to thank all of the discussants for their interest and the Association for the privilege of presenting this information.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Timothy C. Fabian, MD, Professor and Chairman, Department of Surgery, University of Tennessee Health Science Center, 956 Court Avenue, Suite G228, Memphis, TN 38163.
E-mail: tfabian@utmem.edu
Accepted for publication April 24, 2002.
References
- 1.Fabian TC, Patton JH, Croce MA, et al. Blunt carotid injury: importance of early diagnosis and anticoagulant therapy. Ann Surg 1996; 223: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: Diagnosis and treatment. J Trauma 2001; 51: 279–286. [DOI] [PubMed] [Google Scholar]
- 3.Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: Early diagnosis improves neurologic outcome. Ann Surg 1998; 228: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffl WL, Moore EE, Elliot JP, et al. The devastating potential of blunt vertebral artery injuries. Ann Surg 2000; 23: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers FB, Baker EF, Osler TM, et al. Computed tomographic angiography as a screening modality for blunt cervical arterial injuries: preliminary results. J Trauma 1999; 46: 380–385. [DOI] [PubMed] [Google Scholar]
- 6.Zuber M, Meary E, Meder J-F, et al. Magnetic resonance imaging and dynamic CT scan in cervical artery dissections. Stroke 1994; 25: 576–581. [DOI] [PubMed] [Google Scholar]
- 7.Parikh AA, Luchette FA, Valente JF, et al. Blunt carotid artery injuries. J Am Coll Surg 1997; 185: 80–86. [PubMed] [Google Scholar]
- 8.Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999; 178: 517–522. [DOI] [PubMed] [Google Scholar]
- 9.Kerwin AJ, Bynoe RP, Murray J, et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma 2000; 51: 308–314. [DOI] [PubMed] [Google Scholar]
- 10.Carrillo EH, Osborne DL, Spain DA, et al. Blunt carotid artery injuries: difficulties with the diagnosis prior to neurologic event. J Trauma 1999; 46: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 11.Willis BK, Greiner F, Orrison WW, et al. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery 1994; 34: 435–441. [DOI] [PubMed] [Google Scholar]
- 12.Eachempati, SR, Vaslef SN, Sebastian MW, et al. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma 1998; 45: 997–1004. [DOI] [PubMed] [Google Scholar]
- 13.Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: incidence, associated injuries, screening and treatment. J Trauma 1990; 14: 967–973. [PubMed] [Google Scholar]
- 14.Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: Early diagnosis improves neurologic outcome. Ann Surg 1998; 228: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller SJ, Rossitch E Jr, Malek AM. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J Trauma 1999; 46: 660–666. [DOI] [PubMed] [Google Scholar]
- 16.Zuber M, Meary E, Meder J-F, et al. Magnetic resonance imaging and dynamic CT scan in cervical artery dissections. Stroke 1994; 25: 576–581. [DOI] [PubMed] [Google Scholar]
- 17.Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: Three-dimensional time of flight MR angiography and MR imaging versus conventional angiography. Radiology 1997; 28: 97–103. [DOI] [PubMed] [Google Scholar]
- 18.Bok AP, Peter JC. Carotid and vertebral artery occlusion after blunt cervical injury: the role of MR angiography in early diagnosis. J Trauma 1996; 40: 968–972. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc X, Pruvo JP. Recent advances in magnetic resonance angiography of carotid and vertebral arteries. Curr Opin Neurol 2000; 13: 75–82. [DOI] [PubMed] [Google Scholar]


