Abstract
Objective
To assess the nature of changes in the field of hepatic resectional surgery and their impact on perioperative outcome.
Methods
Demographics, extent of resection, concomitant major procedures, operative and transfusion data, complications, and hospital stay were analyzed for 1,803 consecutive patients undergoing hepatic resection from December 1991 to September 2001 at Memorial Sloan-Kettering Cancer Center. Factors associated with morbidity and mortality and trends in operative and perioperative variables over the period of study were analyzed.
Results
Malignant disease was the most common diagnosis (1,642 patients, 91%); of these cases, metastatic colorectal cancer accounted for 62% (n = 1,021). Three hundred seventy-five resections (21%) were performed for primary hepatic or biliary cancers and 161 (9%) for benign disease. Anatomical resections were performed in 1,568 patients (87%) and included 544 extended hepatectomies, 483 hepatectomies, and 526 segmental resections. Sixty-two percent of patients had three or more segments resected, 42% had bilobar resections, and 37% had concomitant additional major procedures. The median blood loss was 600 mL and 49% of patients were transfused at any time during the index admission. Median hospital stay was 8 days, morbidity was 45%, and operative mortality was 3.1%. Over the study period, there was a significant increase in the use of parenchymal-sparing segmental resections and a decrease in the number of hepatic segments resected. In parallel with this, there was a significant decline in blood loss, the use of blood products, and hospital stay. Despite an increase in concomitant major procedures, operative mortality decreased from approximately 4% in the first 5 years of the study to 1.3% in the last 2 years, with 0 operative deaths in the last 184 consecutive cases. On multivariate analysis, the number of hepatic segments resected and operative blood loss were the only independent predictors of both perioperative morbidity and mortality.
Conclusions
Over the past decade, the use of parenchymal-sparing segmental resections has increased significantly. The number of hepatic segments resected and operative blood loss were the only predictors of both perioperative morbidity and mortality, and reductions in both are largely responsible for the decrease in perioperative mortality, which has occurred despite an increase in concomitant major procedures.
Lortat-Jacob’s report of a true anatomical right hepatectomy for cancer in 1952 ushered in the modern era of hepatic resectional surgery. 1 However, the subsequent experience with hepatic resection was far from encouraging. In 1977, Foster and Berman reported a multicenter analysis of 621 hepatic resections for a variety of indications. 2 In this study, operative mortality was 13% and over 20% for major resections (hepatectomy, extended hepatectomy), with 20% of the deaths resulting from hemorrhage.
Over the past decade, many large series have documented better perioperative results, with operative mortality rates typically less than 5% in high-volume centers. 3–7 As a result, hepatic resection has evolved into the treatment of choice for selected patients with benign and malignant hepatobiliary disease. Also, with improvement in the safety of hepatic resection, indications for its use have broadened, and partial hepatectomy in combination with other major procedures is now performed with greater frequency. 8,9
No single factor is responsible for the marked improvement in perioperative outcome. General improvement in operative and anesthetic technique, better patient selection, and the emergence of hepatobiliary surgery as a distinct area of specialization have all been cited, and probably all play a role. 8 A better understanding of hepatic anatomy and increasing application of anatomically based resections are perhaps the most important factors in this regard.
With this refined appreciation of hepatic segmental anatomy has come an awareness of the feasibility of segment-oriented resections. It has been established that, in the appropriate setting, parenchymal-sparing segmental resections offer the same benefit as classic lobar resections with less risk than is associated with removal of a large volume of functional liver tissue. 3,10,11 In addition, segmental resections are clearly superior to wedge resections with respect to blood loss and tumor clearance. 12
The practice of hepatic resectional surgery thus continues to evolve, but few large, contemporary studies have specifically evaluated the impact of these changes. 8 The present study analyzes consecutive, unselected patients undergoing hepatic resection over the past decade to further define factors associated with morbidity and mortality and to evaluate trends in operative and perioperative variables over the period of study.
METHODS
The hepatobiliary patient database at Memorial Sloan-Kettering Cancer Center (MSKCC) was established in 1991, and all patients undergoing a partial hepatectomy since that time were identified and included in this analysis. Patients who underwent a liver wedge biopsy only were excluded.
Our general approach to patient evaluation and hepatic resection is described elsewhere. 13,14 All patients underwent a thorough history and physical examination. A formal cardiopulmonary evaluation was obtained in patients with comorbid medical conditions suggesting an increased operative risk and in all patients over age 65. A full radiologic extent of disease evaluation was performed on all patients, although the nature of the studies obtained varied depending on the diagnosis. Cases and radiographic studies were reviewed at a twice-weekly multidisciplinary disease management conference attended by surgeons, diagnostic and interventional radiologists, oncologists, and gastroenterologists, and additional studies were obtained as warranted. Patients undergoing operation were explored through an extended right subcostal incision or bilateral subcostal incision with vertical midline extension; a few patients required a thoracoabdominal incision. Since 1997, staging laparoscopy has been used with increasing frequency. 15,16 All patients underwent a full abdominal exploration with bimanual palpation of the liver and intraoperative ultrasound. For most resections, inflow vascular control was obtained before parenchymal transection, which was performed using a crushing technique and with an intermittent Pringle maneuver (porta hepatis clamp). Also, for major resections, hepatic venous outflow control was typically achieved extrahepatically before dividing the liver. Resections were performed with a low central venous pressure (<5 mmHg) and with patients in the Trendelenburg position. Abdominal drains were not used after most resections, except those that included a biliary resection and reconstruction or when there was persistent bile leakage from the cut surface of the liver. 17 Following operation, patients were not sent routinely to the intensive care unit but were monitored overnight in the recovery room and then transferred to the ward, provided they were stable clinically.
Preoperative variables analyzed included patient demographics, diagnoses, comorbid medical conditions, and laboratory values (bilirubin, albumin, creatinine, platelet count). Intraoperative data were obtained from the operative note and the anesthesia record and included operating time, portal triad clamp time (Pringle time), estimated blood loss (EBL), hepatic resections, and any other procedures performed.
The number of hepatic segments resected was determined from the procedure listed: extended hepatectomy, five segments; right hepatectomy (lobectomy), four segments; left hepatectomy (lobectomy), three segments; central hepatectomy, three segments; and left lateral segmentectomy, right anterior or posterior sectorectomy, two segments. Patients who underwent an enucleation were considered to have had zero segments resected, while those submitted to a wedge resection were considered to have had one segment resected. When multiple resections were performed, the number of segments resected represented the total for the entire procedure. A segmental resection was defined as any sublobar, anatomically based resection of one or more segments, either en bloc or as separate procedures. When multiple hepatic procedures were performed, the most extensive resection was considered the main procedure, with the others listed as additional hepatic procedures. Bilobar resections were defined as any procedure that involved resection of segments from both the left and right hemi-livers. A repeat resection was defined as any resection performed in a patient who had previously undergone a partial hepatectomy.
The performance of concomitant major procedures in addition to the principal hepatic resection constituted a “complex hepatectomy.” Patients in this group included those who underwent one or more major extrahepatic procedures (organ resection, biliary resection/reconstruction or bile duct exploration, portal vein and/or vena cava resection/reconstruction, porta hepatis lymphadenectomy, thoracotomy) and/or those who underwent additional hepatic resectional or ablative procedures. Hepatic artery pump placement, cholecystectomy, colostomy/ileostomy reversal, and liver wedge biopsy were not considered additional major procedures. Patients who underwent a single hepatic resectional procedure of any type, without any additional major procedures, were placed in the “hepatectomy only” group.
The postoperative variables analyzed included complications (any), hospital length of stay (LOS), intensive care unit (ICU) admission, and mortality. Operative mortality was defined as any death resulting from a complication of the operation, whenever it occurred. Transfusion of any blood products (packed red cells, whole blood, fresh-frozen plasma or platelets) during the operation or at any time during the hospital stay after operation was also recorded. Data regarding the resected specimen, including number of tumors, size of the largest tumor, and any hepatic parenchymal abnormalities, were obtained from the pathology record. For all specimens, sections of liver were taken from areas distant from the tumor, and underlying parenchymal abnormalities (e.g., steatosis, cirrhosis) were reported according to widely applied and accepted criteria.
The association of variables with mortality and complications was tested using the Fisher exact test for dichotomous covariates and the t test for continuous variables (using a logarithmic transformation as necessary). Stepwise logistic regression was used for multivariate models. Patients were divided into five groups based on the date of operation. Variables related to perioperative outcome were analyzed for each of the five groups, and trends over time were tested for significance using the Cochran-Armitage test. 18 Numerical data are expressed as the mean ± standard error unless otherwise indicated.
RESULTS
Demographics
From December 1991 to September 2001, 1,803 consecutive patients underwent a partial hepatectomy at MSKCC. There were 906 women and 879 men, and the average age was 58.6 ± 0.3 years (range 0.2–89) (Table 1). Seven hundred sixty-seven patients (43%) had at least one comorbid medical condition, most commonly hypertension (23%), cardiac disease (16%), and diabetes mellitus (8%). Preoperative laboratory abnormalities (based on the normal range at MSKCC) were common and included hyperbilirubinemia (>1 mg/dL) in 196 patients (11%), hypoalbuminemia (<4 g/dL) in 468 (26%), thrombocytopenia (<160,000/μL) in 189 (11%), and elevated serum creatinine (>1.3 mg/dL) in 112 (6%).
Table 1. PATIENT DEMOGRAPHICS AND COMORBID CONDITIONS
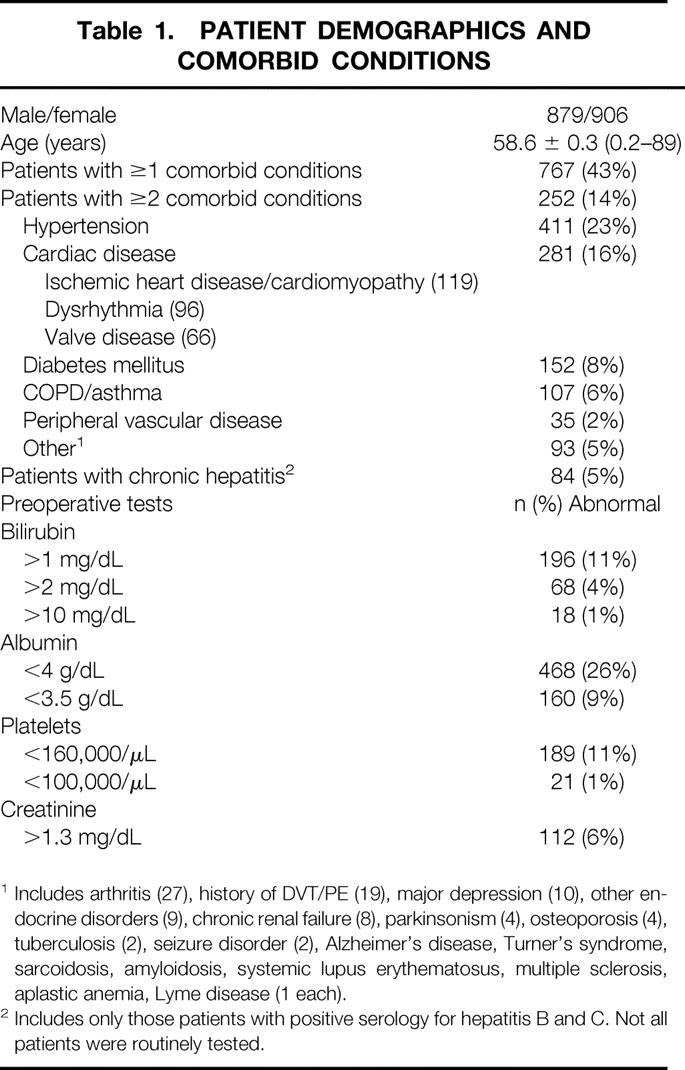
1 Includes arthritis (27), history of DVT/PE (19), major depression (10), other endocrine disorders (9), chronic renal failure (8), parkinsonism (4), osteoporosis (4), tuberculosis (2), seizure disorder (2), Alzheimer’s disease, Turner’s syndrome, sarcoidosis, amyloidosis, systemic lupus erythematosus, multiple sclerosis, aplastic anemia, Lyme disease (1 each).
2 Includes only those patients with positive serology for hepatitis B and C. Not all patients were routinely tested.
Diagnoses
One thousand six hundred forty-two patients (91%) had a malignant diagnosis, with metastatic liver disease present in 1,249 (69%) (Table 2). The most common diagnosis overall was metastatic colorectal cancer (n = 1,021), but there was a wide spectrum of other metastatic tumors resected. Primary hepatic and biliary cancers accounted for 375 cases (21%), of which hepatocellular carcinoma was the most common (188 patients), followed by extrahepatic biliary malignancy (gallbladder carcinoma [77 patients] and hilar cholangiocarcinoma [65 patients]). One hundred sixty-one patients (9%) had benign hepatic or biliary disease, hemangioma being the most common (56 patients); 17 resections were performed for benign biliary strictures.
Table 2. PATIENT DIAGNOSES

1 Abscess (4), fatty infiltration (3)*, regenerative nodule (2)*, solitary fibrous tumor.
2 Cystadenoma (3), abscess (2)*, adenomyomatosis*, porcelain gallbladder*.
3 Germ cell (22), adrenal (17), ovarian (16), renal cell (7), uterine (6), other (5).
4 Lung (9), neuroblastoma (3), adenoid cystic carcinoma (2), thyroid (2), squamous cell carcinoma (2), histiocytoma, paraganglioma, thymoma.
5 Sarcoma (direct extension [6], primary [3]), hemangioendothelioma (4), carcinosarcoma, hemangiopericytoma, lymphoma (primary), neuroendocrine (primary), renal cell (direct extension).
* Unable to exclude malignancy.
Hepatic Resections and Other Procedures
One thousand five hundred sixty-eight patients (87%) underwent anatomically based resections, which were predominantly extended right or left hepatectomy (trisegmentectomy, 544 patients, 30%) and right or left hepatectomy (lobectomy, 483 patients, 27%). Five hundred twenty-six patients (29%) underwent a segmental resection, consisting either of an en bloc resection of more than one segment or resection of multiple discontiguous segments (344) or a unisegmentectomy (182). There were 15 central hepatectomies. Nonanatomical wedge resections or enucleations were performed in 174 patients (10%) and 61 patients (3%), respectively.
The average number of hepatic segments resected was 3.3 ± 0.4 (range 0–6), and 1,113 patients (62%) underwent resection of three or more segments. Seven hundred forty-nine patients (42%) had bilateral resections, 85 (5%) had one or more repeat hepatic resections, and 118 (7%) underwent an en bloc or isolated caudate lobe (segment 1) resection.
In 1,135 patients (63%), a single hepatic resectional procedure was performed with no concomitant major procedures (“hepatectomy only” group), while 668 patients (37%) underwent the principal hepatic resection along with one or more additional major procedures (“complex hepatectomy”) (Fig. 1). The latter group was further divided into those who underwent one or more additional hepatic procedures (267 patients) (single or multiple wedge resections or segmental resections, enucleations, or ablations) or one or more extrahepatic procedures (450 patients). The most common extrahepatic procedure was a concomitant major organ resection (218 patients), followed by major biliary procedures (168; bile duct resection/reconstruction [155] or bile duct exploration [13]) and vascular resections (portal vein or vena cava resection/reconstruction [51]). Other extrahepatic procedures included lymphadenectomy (subhilar, retroperitoneal [38]), chest wall/abdominal wall reconstruction (8), thoracotomy (4), and resection of retroperitoneal tumor (4). Forty-nine patients underwent both an extrahepatic procedure and additional hepatic procedures. It should be noted that patients with hilar cholangiocarcinoma or gallbladder cancer routinely underwent a concomitant subhilar lymphadenectomy, 19 which was not coded as a second major procedure.

Figure 1. Breakdown of operative procedures performed. Patients in the “hepatectomy only” group underwent a single hepatic resectional procedure with no additional major procedures. Patients in the “complex hepatectomy” group underwent the principal hepatic resection plus one or more additional major procedures. *49 patients underwent one or more extrahepatic procedures and one or more additional hepatic procedures.
In 997 patients (55%), the non-tumor-bearing liver was histologically unremarkable. Of the remainder, the most common underlying hepatic parenchymal abnormalities were steatosis (325), cholestasis and/or inflammation (253), and cirrhosis/fibrosis (158). Seventy-nine patients had multiple abnormalities described. The liver histology was unavailable in 148 patients.
Perioperative Results: All Patients
The operative and perioperative results for all patients are summarized in Table 3. The median operating time was 4 hours, porta hepatis clamp time (Pringle) was 28 minutes, and EBL was 600 mL. There was a progressive increase in EBL as the number of segments resected increased (P < .001). Eight hundred eighty patients required transfusion of blood products during or after operation, 15% of whom received only fresh-frozen plasma. Median LOS was 8 days, and 112 patients (6%) required ICU care. The perioperative morbidity was 45% (817 patients), with 345 patients (19%) experiencing multiple complications. Infections occurred in 41% of patients with complications. In total, there were 1,350 complications, the most common of which were liver and/or biliary-related (369), although pulmonary complications were nearly as common (344). The most common complication overall was an infected or sterile perihepatic fluid collection (207).
Table 3. OPERATIVE AND PERIOPERATIVE RESULTS: ALL PATIENTS
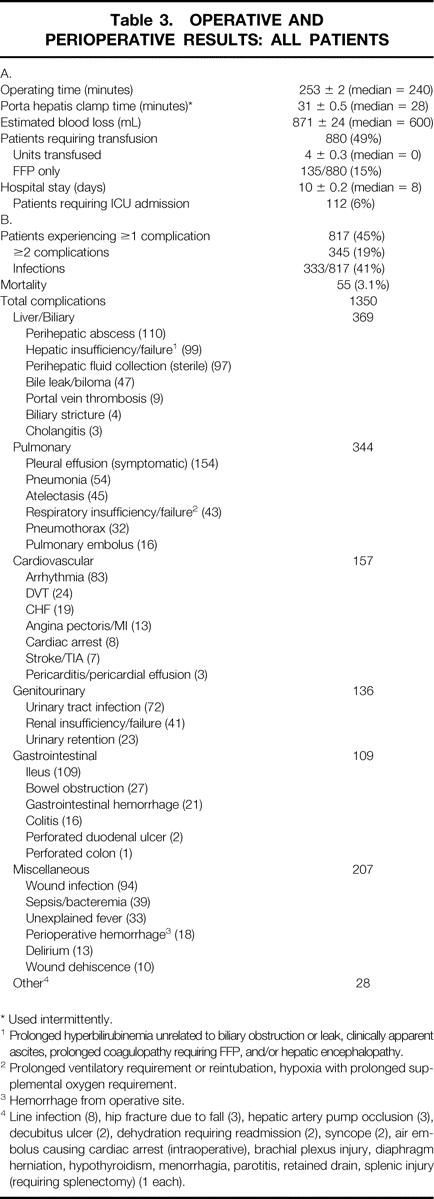
* Used intermittently.
1 Prolonged hyperbilirubinemia unrelated to biliary obstruction or leak, clinically apparent ascites, prolonged coagulopathy requiring FFP, and/or hepatic encephalopathy.
2 Prolonged ventilatory requirement or reintubation, hypoxia with prolonged supplemental oxygen requirement.
3 Hemorrhage from operative site.
4 Line infection (8), hip fracture due to fall (3), hepatic artery pump occlusion (3), decubitus ulcer (2), dehydration requiring readmission (2), syncope (2), air embolus causing cardiac arrest (intraoperative), brachial plexus injury, diaphragm herniation, hypothyroidism, menorrhagia, parotitis, retained drain, splenic injury (requiring splenectomy) (1 each).
The operative mortality was 3.1% (55 patients), and there were no deaths in the last 184 consecutive cases. In the majority of cases, the cause of death was multifactorial, and a single underlying event was difficult to identify. Infections played a prominent role in the deaths of 23 patients (42%). Hepatic failure was observed in 19 patients but was apparently the sole cause of death in only 6 patients. More commonly, liver failure occurred within the context of systemic sepsis and/or multisystem organ failure (11 patients) or after major gastrointestinal hemorrhage (2 patients).
Perioperative Outcome: Extent of Operation
The number of hepatic segments resected had a profound impact on perioperative morbidity and mortality (Fig. 2). In patients who underwent a resection of zero or one segments, the complication rate was 32%; it increased progressively to 75% in patients who underwent resection of six segments (P < .001). There was a similar trend in operative mortality, which increased from less than 1% in patients who underwent resection of less than three segments to 5% and 7.8%, respectively, in those who had five or six segments resected (P < .001).
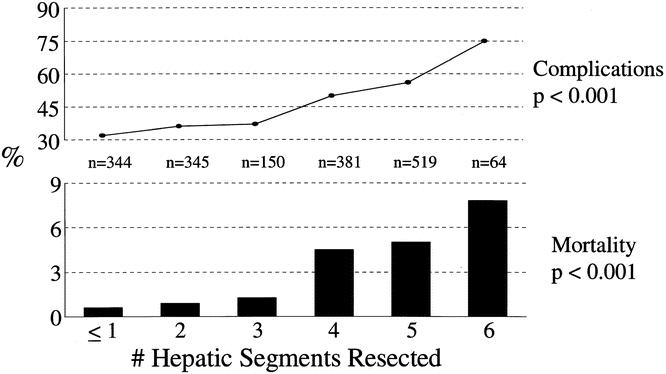
Figure 2. Perioperative complications and mortality stratified by the number of hepatic segments resected.
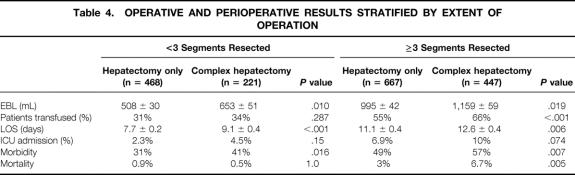
The influence of a complex hepatic resection on perioperative outcome, stratified by the number of segments resected, is shown in Table 4. When a limited hepatic resection was performed (<3 segments), concomitant additional major procedures increased EBL, LOS, and morbidity but did not affect transfusion requirements or ICU admissions and had no impact on mortality. By contrast, in patients who underwent resection of three or more segments, a complex procedure significantly increased all of these variables (except ICU admission). The most notable finding was a more than twofold increase in operative mortality, from 3% to 6.7% (P = .005).
Table 4. OPERATIVE AND PERIOPERATIVE RESULTS STRATIFIED BY EXTENT OF OPERATION
Factors Associated with Perioperative Morbidity and Mortality
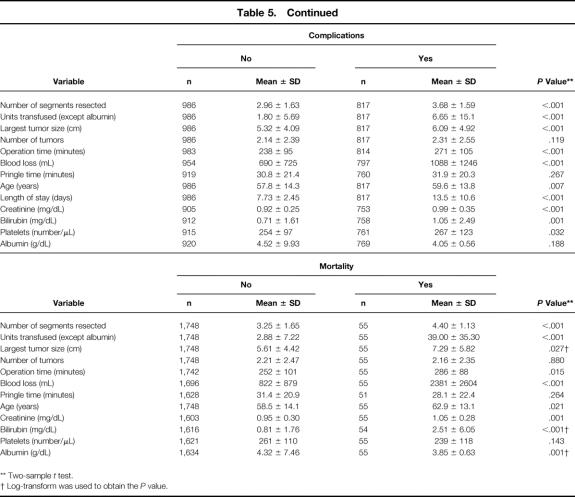
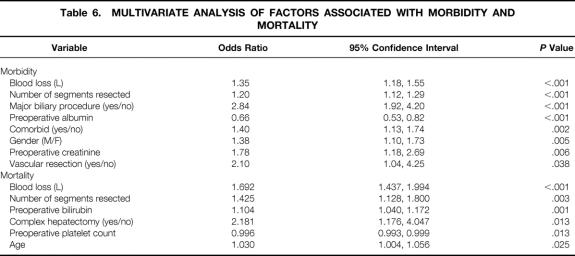
Several preoperative and intraoperative variables were associated with a greater likelihood of perioperative morbidity and mortality on univariate analysis (Table 5). On multivariate analysis, eight independent predictors of morbidity emerged: EBL, number of segments resected, a concomitant major biliary procedure or vascular resection, preoperative hypoalbuminemia or elevated serum creatinine, male gender, and one or more comorbid medical conditions (Table 6). EBL and number of segments resected were also independent predictors of mortality and were the only two common variables in both analyses. Additional factors predictive of mortality were preoperative hyperbilirubinemia or thrombocytopenia, a complex hepatectomy, and age. Patients with extrahepatic biliary malignancy had the highest morbidity and mortality of any other patient group, but neither the primary diagnosis nor the presence of underlying hepatic parenchymal disease was an independent predictor of increased morbidity or mortality.
Table 5. UNIVARIATE ANALYSIS OF FACTORS ASSOCIATED WITH PERIOPERATIVE MORBIDITY AND MORTALITY
* Fisher exact test.
1 Patients with gallbladder carcinoma, hilar cholangiocarcinoma, or preoperative jaundice.
2 Cirrhosis and steatosis were not significant when analyzed independently.
Table 5. Continued
** Two-sample t test.
† Log-transform was used to obtain the P value.
Table 6. MULTIVARIATE ANALYSIS OF FACTORS ASSOCIATED WITH MORBIDITY AND MORTALITY
Trends in Operative and Perioperative Results Over Time
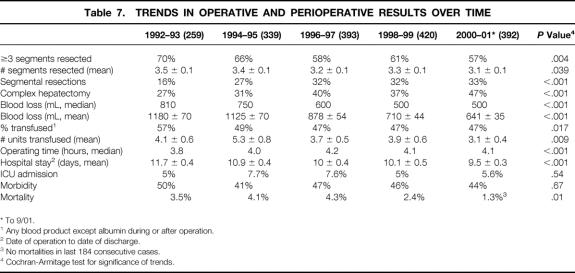
Changes in several operative and perioperative variables over the period of study were analyzed by stratifying patients into five groups based on the date of operation (Table 7). There was a progressive and significant increase in the proportion of segmental resections performed (16% to 33%, P < .001), with a corresponding decline in the number of hepatic segments resected (3.5 ± 0.1 to 3.1 ± 0.1, P = .039). On the other hand, the proportion of complex hepatectomies increased from 27% to 47% (P < .001). The mean EBL decreased by 46% (P < .001), with a corresponding decrease in transfusion requirements. The average LOS decreased by over 2 days (P < .001), and while the proportion of patients requiring ICU admission and the perioperative morbidity did not change significantly, there was a sharp decline in operative mortality (P = .01). In the last 812 consecutive cases (from Jan. 1., 1998, to present), there were 15 perioperative deaths (1.8%) and no deaths in the last 184 cases. Over the study period, there was a small but progressive increase in the proportion of patients with extrahepatic biliary malignancy, but patient demographics and the proportion with comorbid conditions did not vary significantly.
Table 7. TRENDS IN OPERATIVE AND PERIOPERATIVE RESULTS OVER TIME
* To 9/01.
1 Any blood product except albumin during or after operation.
2 Date of operation to date of discharge.
3 No mortalities in last 184 consecutive cases.
4 Cochran-Armitage test for significance of trends.
DISCUSSION
In a remarkably short period, hepatic resection has evolved from a high-risk, resource-intensive procedure at the fringe of surgical practice to a mainstream operation with broad indications. Hepatic resection is now firmly established as the most effective treatment for selected patients with primary and secondary hepatobiliary malignancy and the only effective treatment for a number of benign conditions. 3–7,19,20 This evolution is due in large part to improvements in perioperative morbidity and mortality over the past 20 years.
A notable advance has been the increased use of parenchymal-sparing, segmental resections, which has contributed to the improved perioperative results. 3,10,11 This in turn has fostered efforts to expand the indications for hepatic resection and to use it more frequently in combination with other major procedures. 8,9 Several studies have documented better results after hepatic resection for selected indications, but few have specifically evaluated perioperative outcome and resource utilization within the context of this changing practice. 8 The present study addresses these issues by analyzing a large number of consecutive patients treated in a single center over a short time period.
The data illustrate the importance of resection extent and EBL in determining outcome. Several variables were found to be independent predictors of morbidity and mortality, but only EBL and the number of hepatic segments resected were predictive of both. As the extent of the hepatic resection increased, there was a nearly linear escalation in perioperative morbidity and mortality. Also, a complex hepatectomy was associated with an increased risk of death, while components of this variable, namely a major biliary procedure or a vascular resection, increased the risk of postoperative complications.
The results of the multivariate analysis fit with the trends in perioperative outcome. Over the study period, there was a progressive increase in the proportion of segmental resections and a decrease in the number of segments resected. Despite these changes, however, 62% of all patients underwent resection of at least three segments. In addition, there was a significant reduction in EBL, which was in part related to the increase in parenchymal-sparing resections. These two factors appear to be primarily responsible for the marked reduction in perioperative mortality, from approximately 4% in the first 5 years to 1.3% in the last 2 years, with no deaths in the last 184 consecutive resections.
The reduction in operative mortality occurred despite a significant increase in the number of complex resections, an observation that initially appears paradoxical. However, the adverse impact of performing additional major procedures was dependent on the number of segments resected. Patients who underwent resection of less than three segments had no increase in transfusion requirements or mortality if a complex procedure was performed, while additional procedures in combination with a major hepatic resection (≥3 segments) had a much more profound impact, significantly increasing EBL, transfusion requirements, morbidity, and mortality. The increase in complex resections may explain the greater operating time and stability in ICU admissions and morbidity over the study period. The increase in segment-oriented resections probably also contributed to the longer operating times. Fan et al observed a similar correlation in patients undergoing partial hepatectomy for hepatocellular carcinoma. 3 Segmental resections, although commonly classified as “minor” hepatectomies, are often more demanding technically than classical lobar resections and should not be regarded as simple procedures.
The present study found an increased risk of hepatic resection in the setting of jaundice. Most of these patients had either hilar cholangiocarcinoma or gallbladder carcinoma and required an extended hepatectomy for complete tumor clearance. Morbidity and mortality were higher in these patients than for any other group, in part because of infectious complications associated with preoperative biliary stents. 19,21,22 By contrast, underlying liver disease (cirrhosis, fibrosis, steatosis) did not affect perioperative outcome, probably because of the small number of such patients and the much larger proportion with normal parenchyma in this study. However, preoperative thrombocytopenia, most common in patients with HCC and likely reflecting some degree of portal hypertension, was associated with an increased risk of death.
Belghiti et al recently reported an analysis of 747 hepatic resections in the 1990s. 8 A major finding of this study was that a concomitant extrahepatic procedure was the only independent predictor of operative death in patients with no underlying liver disease. The authors observed an increase in the proportion of major hepatic resections and additional major procedures over time but found no significant change in morbidity or mortality. It should be pointed out, however, that the proportion of major resections and additional major procedures was much greater in the present study.
In summary, the present study documents the change in hepatic resectional surgery over the past decade, with more parenchymal-sparing segmental resections and more frequent complex procedures. The number of hepatic segments resected and EBL were the only two predictors of both perioperative morbidity and mortality, and reductions in both appear to be largely responsible for the marked reduction in perioperative mortality. Other factors, such as overall improvement in operative and anesthetic technique and postoperative management, probably also contributed. With respect to utilization of hospital resources, LOS was reduced by over 2 days and the use of blood products decreased significantly, although ICU stay did not change and there was some increase in operating time. The results suggest that complex resections can be performed safely, provided a limited hepatic resection is anticipated; however, caution must be used if a more extensive resection is required, and future studies should be aimed at further clarifying the risk in this group.
Acknowledgments
The authors are deeply indebted to Jessica Bodniewicz, MPH, without whose assistance this project would not have been possible. Special thanks also to Miranda Youssef, Christina Bilsky, RN, Nancy Heffernan, RN, Anne Culkin, RN, and Maria Reyes.
DISCUSSION
Dr. David M. Nagorney (Rochester, MN): Dr. Jarnagin and Dr. Blumgart, to succinctly highlight the experience garnered from over 1,800 liver resections is truly impressive. The team effort required to achieve these results is both laudable and noteworthy. You have set the standards which all of us are going to have to match. I have several questions prompted by your talk and your paper.
First, the categorization of wedge resections as segmental may potentially bias the outcome. Some segmental resections or wedge resections are big, but most are very small. Including the wedge to segmental resection could result obviously in segmental sparing and could potentially bias your multisegmental groups if you had multiple small wedge resections. How many of your patients had multiple wedge resections without concomitant anatomical polysegmentectomies? Could this number affect the outcome of your larger group?
Second, your complex resection included both patients with major biliary and major non-biliary resections. Was there a difference in the morbidity and mortality between the major complex biliary and non-biliary resections, and do you recommend staged resections for the non-biliary major resections?
Our studies have shown that steatosis, especially severe steatosis, does affect operative morbidity and mortality. Though not addressed in your talk, your data did not. How did you measure steatosis? Was it quantitative? And did severity affect outcome?
Finally, one technical question. The anterior segments are easy to excise anatomically because the main segmental portal pedicles are accessible at the hilar or the umbilical plates. Pedicle ligation before parenchymal transection of this group delineates the area to resect and makes an anatomical resection quite clear. The posterior segments 4-A, 7, and 8 are much harder to access. Do you do a hepatotomy to get the pedicle to delineate the resection area or simply get the pedicle after you go through the liver during resection?
Presenter Dr. Leslie H. Blumgart (New York, NY): Dr. Nagorney, thank you very much. There are a lot of questions there, and I will try to answer them in the order they were asked.
We didn’t actually categorize wedge resections as segmental. We simply analyzed them within this study as equivalent to one segment so that we could have some reasonable way of analyzing the data. However, I don’t believe this caused a shift in the data. Even if all such patients were categorized, the number is small. The figure you asked for, the multiple wedges on their own, were only 43 patients (or 2.4%), and that certainly wouldn’t change the results in such a large series.
You suggested that wedge resections carried less risk. I don’t think that is really true. In a study that Ron DeMatteo published from our department, there was no difference in mortality and morbidity, blood loss, or length of stay between wedge and segmental resections. What we did find was that for wedge resection, the positive margin rate was 16%, which is in line with Scheele’s findings in Germany. This compared to a 2% positive margin rate for anatomical wedge resections.
You are quite right regarding the mortality data. Yes, we had a higher mortality in the presence of vascular resection. It was actually 17%. For biliary-related procedures, in the presence of jaundice, it was 7%. However, that figure is relevant to your second question as to whether we use drainage, since the deaths in that group were nearly all infective and all resulted from the presence of previous drains. So it is very difficult, and I don’t know the answer as to whether we should drain these patients before we operate on them or not. As you know, I did a study years ago which suggested that it wasn’t necessary. In general we don’t do preoperative drainage because I would like to avoid the possibility of biliary infection. But I think that is an unanswered question. At the moment, we don’t routinely drain them.
You asked about steatosis. Well, I know you are interested in this and you are conducting a proper study. We have simply relied on the pathology report, and this is a very subjective assessment. However, I am not sure how relevant it is, because we didn’t assess liver function other than with Child’s-Pugh assessment in cirrhotic patients. Almost all patients that we operated on were Child’s A. And there were only 6 of 55 deaths due to liver failure alone. So we are looking at a small problem here. I am not sure that steatosis is that relevant. Perhaps conservation of blood loss is more important, particularly in these patients with soft livers.
As to your final technical point, I don’t find posterior sectorectomy much more difficult than anterior sectoral anterior. Yes, it is true, you can get the pedicle easily for the anterior sector. But I think what you meant by hepatotomy was the pedicle ligation technique described by Launois. Yes, we do that if the tumor is far enough away from the pedicle to allow tumor clearance. I have, however, found it increasingly possible to dissect the posterior sectoral vessels extrahepatically and get a very nice line of demarcation. In short, this has not been a major problem.
Dr. William C. Chapman (Nashville, TN): I would like to thank the American Surgical Association for the privilege of discussing this very important paper, reviewing one of the largest consecutive series of major liver resection from a single institution. This group has been a leader in the field of liver surgery and today has established a new benchmark for hepatic resection, even in the most complex cases. I have several questions for the authors to clarify.
In this series, 18 patients underwent tumor ablation, presumably with cryo- or radiofrequency ablation in combination with liver resection. Given that your data suggest that parenchymal sparing was a significant factor in your improved results over time, should tumor ablation play an even greater role in patients with metastatic and primary liver tumors? I would be interested in your view regarding the recent marked expansion and availability of radiofrequency ablation systems to many centers in the U.S.
Second, what approach do you currently follow for patients with early-stage cirrhosis and small hepatocellular carcinomas who otherwise would receive favorable scoring under the new UNOS MELD-based organ allocation system? While your results suggest that perioperative risks may be reasonable in your hands for standard resection, data from a number of centers suggest that these patients may have better long-term results with liver transplantation.
Dr. Leslie H. Blumgart (New York, NY): Thank you very much, Dr. Chapman. I don’t quite know how to answer your question on RFA ablation. When you said “market expansion,” I thought you were going to say “market driven.” That is part of the problem. And this is very much an industry-driven technique at the moment. It is not proven. It has a mortality, as recorded in one of the recent papers, of up to 3% in the management of metastatic colorectal cancer, which is not acceptable. It is often used, unfortunately, inappropriately. Everybody in this audience running a specialist hepatobiliary unit knows that we receive patients with large tumors, quite inappropriately treated lying close to major vessels. There is no appropriate study as yet. That is our fault and in our program we haven’t yet done a proper comparative study with resection. We are busy setting that up right now aiming to compare RFA with surgical approaches.
We have used RFA mainly for recurrent disease in some cases where resection was inappropriate. We have been using it in irresectable disease in combination with resection and intraarterial infusion pump chemotherapy, and that study is ongoing. So as you see we have made very restricted use of RFA. It probably has a place, but it needs definition. And it should be used in specialist units and not by everybody who can afford a machine.
The other question you asked opens up a Pandora’s box. There are serious questions, at least in my mind, as to the suitability of liver transplantation for primary hepatocellular cancer in patients with Child’s A liver function. I am particularly concerned about living donors for transplantation for HCC in adults with Child’s A liver function.
However, you are quite right, there are some transplant series which suggest that the results of transplantation may be modestly better. On the other hand, there are surgical series which show up to a 65% 5-year survival after resection for small tumors, or indeed after ablative techniques for treatment of small lesions. I don’t think that this question is resolved as yet, and further study is needed.
I notice Ronnie Poon and John Wong are in the audience from Hong Kong. I very much liked their study where they actually took a group of such patients (small HCC potentially resectable), resected them, and then reserved transplantation for failure and used that as a way of limiting the need for transplantation. I can’t quite remember the figures, but certainly the need for transplantation, selected in that way, was very much less than subjecting all such patients to initial transplantation.
References
- 1.Lortat-Jacob J, Robert H. Hepatectomy droite reglee. Presse Med 1952; 60: 549–551. [PubMed] [Google Scholar]
- 2.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg 1977; 22: 1–342. [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999; 229: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong Y, Fortner JG, Sun R, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: Analysis of clinical and pathological risk factors. Surgery 1994; 116: 703–711. [PMC free article] [PubMed] [Google Scholar]
- 6.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 7.Scheele J. Liver resection for colorectal metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000; 191: 38–46. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Detroz B, Lasser P, et al. Is simultaneous hepatectomy and intestinal anastomosis safe? Am J Surg 1995; 169: 254–260. [DOI] [PubMed] [Google Scholar]
- 10.Billingsley KG, Jarnagin WR, Fong Y, et al. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg 1998; 187: 471–481. [DOI] [PubMed] [Google Scholar]
- 11.Scheele J, Stangl R. Segment oriented anatomical liver resections. In Blumgart LH, ed. Surgery of the Liver and Biliary Tract. London: Churchill Livingstone, 1994.
- 12.DeMatteo RP, Palese C, Jarnagin WR, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg 2000; 4: 178–184. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham JD, Fong Y, Shriver C, et al. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg 1994; 129: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 14.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998; 187: 620–625. [DOI] [PubMed] [Google Scholar]
- 15.Jarnagin WR, Bodniewicz J, Dougherty E, et al. A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg 2000; 4: 34–43. [DOI] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 2001; 91: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 17.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection: results of a randomized trial. Am J Surg 1996; 171: 158–162. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. New York: Wiley; 1990.
- 19.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001; 234: 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charny CK, Jarnagin WR, Schwartz LH, et al. Management of 155 patients with benign liver tumours. Br J Surg 2001; 88: 808–813. [DOI] [PubMed] [Google Scholar]
- 21.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg 1999; 134: 261–266. [DOI] [PubMed] [Google Scholar]
- 22.Povoski SP, Karpeh MS Jr, Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg 1999; 3: 496–505. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: William R. Jarnagin, MD, FACS, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: jarnagiw@mskcc.org
Accepted for publication April 24, 2002.