Abstract
Objective
To determine whether propranolol and growth hormone (GH) have additive effects to combat burn-induced catabolism.
Summary Background Data
Both GH and propranolol have been attributed anabolic properties after severe trauma and burn. It is conceivable that the two in combination would have additive effects.
Methods
Fifty-six children with more than 40% TBSA burns were randomized to one of four anabolic regimens: untreated control, GH treatment, propranolol treatment, or combination GH plus propranolol therapy. Clinical treatment was identical for all groups. Resting energy expenditure was determined by indirect calorimetry and skeletal muscle protein kinetics were measured using stable amino acid isotope infusions before and after each anabolic regimen.
Results
There were no differences in age, sex, or burn size between groups. Tachycardia and energy expenditure were decreased during propranolol treatment (P < .05). The net balance of muscle protein synthesis and breakdown was improved during proprandol and GH plus propranolol treatment (P < .05). There was no significant benefit of GH alone. No additive effect of combination therapy was seen.
Conclusions
Propranolol is a strongly anabolic drug during the early, hypercatabolic period after burn. No synergistic effect between propranolol and GH was identified.
The hypermetabolic response to burn is associated with increased energy expenditure and energy substrate release from protein and fat stores. After severe trauma, protein catabolism is increased, leading to a loss of lean body mass and muscle wasting. 1,2 Even after survival is ensured and the wounds are fully healed, muscle proteolysis continues for at least 9 months after a severe burn. 3 During an acute illness, the degree of catabolism correlates directly with increasing morbidity (including depressed immunity, increased infectious complications, impaired wound healing, and profound generalized weakness) and mortality. 4,5 Throughout convalescence, loss of muscle mass may predispose patients to further complications and may hamper rehabilitation and reintegration of the patient into society.
Endogenous catecholamines are primary mediators of the hypermetabolic response to trauma or burn. 6,7 Levels of these “fight-or-flight” stress hormones are increased approximately 10-fold shortly after severe blunt trauma or a burn of over 30% to 40% total body surface area (TBSA). 8,9 Several investigators have reported modulation of the posttraumatic hypermetabolic response through blockade of β-adrenergic stimulation. Attenuation of supraphysiologic thermogenesis, 10 tachycardia, 11 cardiac work, 12 and resting energy expenditure 13 have all been demonstrated with administration of β-blocking agents after severe thermal injury. Decreased cardiac morbidity and diminished overall mortality have been documented in nontrauma patients given β blockers for control of tachycardia after tissue trauma inflicted by a major surgical procedure. 14
Growth hormone (GH) is known to be a potent anabolic agent and salutary modulator of posttraumatic metabolic responses. 15 After severe burn, it has been shown to decrease whole body catabolism, 16,17 improve muscle protein synthesis, 18 accelerate wound healing, 19,20 attenuate prolonged hyperactivity of the hepatic acute phase response, 21 and promote linear growth. Its side effects when used in burned children are well characterized, and it has been shown to be a safe pharmacologic adjunct to standard excisional therapy following severe burn. 22,23
This study is a continuation of our previous work examining the effects of propranolol and GH on the stress response to injury. We have shown that propranolol (dosed to achieve a 15–20% decrease in tachycardia) decreases energy expenditure and attenuates erosion of lean body mass after severe burn. 24 Using both stable amino acid isotope infusions and whole body composition scans, we documented that long-term β blockade decreased muscle protein catabolism measured both at the substrate level and at the whole body level. The purpose of this study, then, was to determine whether the combination of propranolol with GH would have additive anabolic effects after burn.
METHODS
Subjects
This study was performed under a University of Texas Medical Branch Institutional Review Board-approved protocol. Informed written consent was obtained from each patient’s guardian before enrollment into the study. Inclusion criteria were as follows: children less than 18 years of age, TBSA burns of greater than 40%, and transfer to the Shriners Hospitals for Children within 1 week of injury. Patients with a known history of asthma were excluded.
Within 48 hours of admission, each patient underwent total burn wound excision and grafting with autograft skin and allograft. Patients returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6–8 days from the last operation). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were healed.
Each patient received enteral nutrition via a nasoduodenal tube with Vivonex TEN (Sandoz Nutritional Corp., Minneapolis, MN). The composition of Vivonex is 82% carbohydrate, 15% protein, and 3% fat. Daily caloric intake was given at a rate calculated to deliver 1,500 kcal/m2 TBSA burned + 1,500 kcal/m2 TBSA. This feeding regimen was started at admission and continued at a constant rate until the wounds were healed. Caloric intake remained constant throughout the study periods. Insulin was given by continuous infusion to keep the serum glucose level below 200 mg/dL in accordance with standard accepted clinical practice. Insulin doses during the stable isotopic studies were recorded and compared between groups.
Patients were intubated for operations, after which extubation was accomplished as soon as possible. Ventilator settings for those who remained intubated followed ARDSNET recommendations. 25 The presence of inhalation injury at admission and ventilator dependence on study days were recorded and compared between groups. Sepsis, as previously defined, 26 was also recorded and compared between groups.
Subjects were at bed rest after excision and grafting procedures for 5 days. After this, patients ambulated daily until the next excision and grafting procedure. Patients were treated in an identical fashion in terms of mobilization and rehabilitation.
Study Design
We sought to discern whether the addition of propranolol to the anabolic agent GH could further improve skeletal muscle protein metabolism in burned patients. Between September 1998 and March 2000, 56 severely burned (>40% TBSA burn) children were recruited to answer this question. All subjects underwent metabolic evaluation during an initial, untreated baseline period, and then again following a 10-day treatment period. Subjects were randomized to receive GH, propranolol, combination GH and propranolol, or no anabolic therapy during the treatment period. The randomization schedule was designed to include 20 subjects as untreated controls and 12 subjects in each treatment group. The following subjects were dropped from the study: two subjects in the GH-alone group (first for a protocol violation involving administration of a high-fat enteral formula rather than Vivonex TEN and the second because she was given propranolol for 4 days as clinical treatment of severe tachycardia); one subject was dropped from the combination therapy group due to development of a high-output enterocutaneous fistula after electrical injury; and one untreated control subject was dropped post hoc because evaluation of his stable isotope data revealed that his isotopic infusion did not achieve a steady state. GH was delivered as a daily subcutaneous injection, and propranolol was administered orally. β blockade with propranolol was initiated at 0.33 mg/kg q4h and titrated with a goal to decrease tachycardia by 20% to 25%. Growth hormone was dosed at 0.2 mg/kg/d. All metabolic studies were performed in a fed state on the fifth day after the first and third serial excision and grafting operations. Wound healing was not assessed in this study.
Vital Signs
Burned patients were monitored by an Emtek (Eclipsys, Rockville, MD) vital signs tracking system by standard ECG leads. Heart rate was measured hourly and verified by each patient’s nurse. The average heart rate for each entire 24-hour period was determined throughout the hospital stay.
Energy Expenditure
Energy expenditure was measured by indirect calorimetry. Between midnight and 5 am on the day of study, VCO2, VO2, RQ, and resting energy expenditure were determined with a standard metabolic cart (Sensormedics Model 2900, Yorba Linda, CA). All indirect calorimetry measurements were made at 30°C, which is the standard environmental setting for all patient rooms in our acute burn ICU. Inspired and expired gases were sampled and analyzed at 60-second intervals. Values were accepted when they were at a steady state for 5 minutes and their standard deviations were less than 10% of their respective measures.
Stable Isotope Study
The degree of protein catabolism was quantified using stable isotope tracers. Protein kinetic studies were performed beginning between 5 and 7 am on postoperative day 5 after the first excision and grafting procedure. All stable isotope studies consisted of a 5-hour infusion of d5-phenylalanine, as previously described. 27 Because phenylalanine is neither synthesized nor degraded in the peripheral tissues (it is metabolized only in the liver), measurement across the leg reflects the net balance of protein synthesis and breakdown. Blood samples were taken simultaneously from an ipsilateral femoral artery and vein for this determination. Indocyanine green was used to determine leg blood flow. 28
Analysis of Blood Samples
The blood concentration of unlabeled phenylalanine was determined by gas chromatography-mass spectrometry (GCMS) using the internal standard approach and the nitrogen-acetol-n-propyl esters, as previously described. 29 The isotopic enrichment of free amino acids in blood was determined on a HP model 5989 (Hewlett-Packard Co., Palo Alto, CA) by chemical ionization and selected ion monitoring at mass-to-charge ratios of 250:1, 255:1, 256:1. Indocyanine green concentrations were determined spectrophotometrically at λ = 805 mm on a Spectronic 1001 (Bausch & Lomb, Rochester, NY).
Calculations
As phenylalanine is neither synthesized nor degraded in the periphery, the difference in concentration of this substrate in the femoral arterial and venous plasma pools reflects the net balance of leg skeletal muscle protein synthesis and breakdown. The net balance (NB) was calculated and standardized for leg volume by NB = (CA - CV) · BF, where CA and CV are the blood free amino acid concentrations of the femoral artery and vein, and BF is leg blood flow in cc/min/100 mL leg. Leg blood flow was determined from the following modification of Fick’s equation: Infusion rate = (CF - CC) · BF, where CF is the femoral venous concentration of ICG and CC is the central (contralateral femoral) venous concentration of ICG. With the infusion rate set at 0.5 mg/min, the equation was solved for leg blood flow (BF). As is indicated above, BF was normalized for each patient by leg volume. Subject weight, leg circumference at prescribed points relative to anatomical landmarks, and the distances between these points were used to mathematically model leg volume.
Two-Pool Protein Kinetic Model
As phenylalanine is neither synthesized nor degraded in the periphery, the rates of appearance (Ra) and disappearance (Rd) of this substrate in the femoral arterial and venous plasma pools reflect leg skeletal muscle protein breakdown and synthesis, respectively. The phenylalanine kinetic rates within the leg were calculated and standardized for leg volume.
NB = (CA - CV) · BF
Rd = (CA · EA - CV · EV)/EA · BF
Ra = Rd - NB
where NB is the net balance of protein synthesis and breakdown across the leg; Rd and Ra are the rates of disappearance and appearance of substrate within the leg, respectively; CA and CV are the blood free amino acid concentrations of the femoral artery and vein; EA and EV are amino acid enrichments (tracer/tracee ratio) in the femoral artery and vein; and BF is leg blood flow in cc/min/100 mL leg. The two-pool model values of Rd and Ra correspond to the rate of incorporation of plasma phenylalanine into muscle protein and the rate of the appearance of phenylalanine in plasma from the process of protein breakdown, respectively.
Data Presentation and Statistical Analysis
Muscle protein kinetics of the propranolol-alone group have been previously reported. 24 Data are presented as means ± SEM. Chi-square analysis was used to compare groups for differences in demographics. Two-way ANOVA followed by Tukey’s test for multiple comparisons was used to analyze the treatment effects of GH and propranolol. The two-way ANOVA was used to test whether GH caused a change in the measured parameters, whether propranolol caused a change in the measured parameters, and whether the combination had an interactive effect. One-way ANOVA was used to detect differences in insulin doses between groups on study days with Tukey’s test for multiple comparisons to assess differences between groups. P < .05 was considered statistically significant.
RESULTS
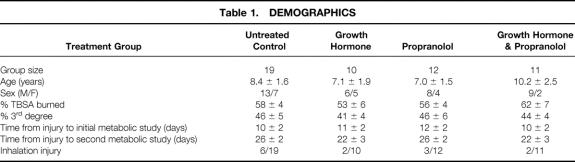
Demographics of the 52 subjects completing this study are shown in Table 1. There were no differences in age, sex, burn size, or inhalation injury between groups.
Table 1. DEMOGRAPHICS
As expected, tachycardia was decreased with β blockade (Fig. 1). In the propranolol group, heart rate was decreased approximately 18% from baseline over the course of treatment. In the combination GH plus propranolol group, only a 15% decrease was achieved, and 5 of these 11 subjects had intermittent doses of propranolol held due to mean arterial pressure between 60 and 65. No changes in mental status or tissue perfusion (specifically, no acidemia or conversion of partial-thickness wounds to full-thickness) were noted.
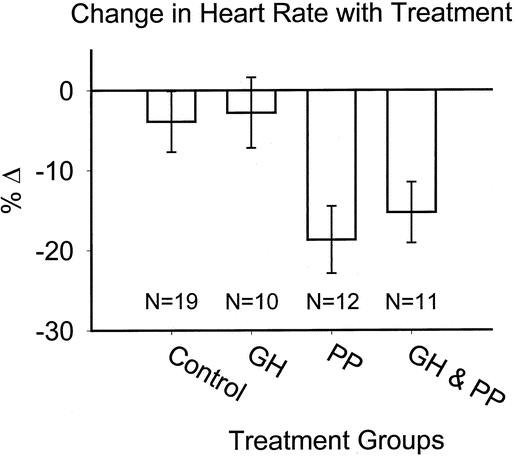
Figure 1. Change in heart rate over treatment period.
Effects of the anticatabolic agents on energy expenditure are shown in Figure 2. We found a significant decrease in REE during β blockade (P < .05 by two-way ANOVA). GH exerted no effect on energy expenditure after burn. No differences were found between groups for sepsis or ventilation differences on study days.
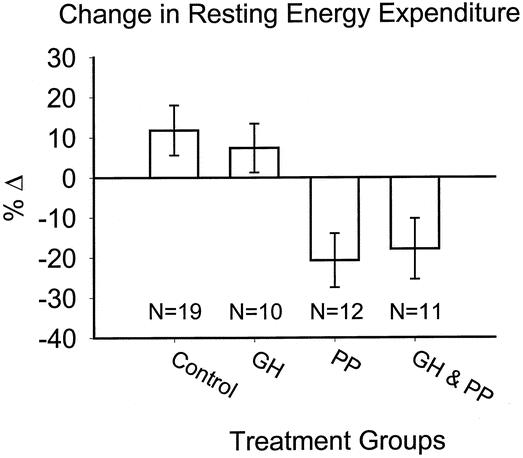
Figure 2. Change in resting energy expenditure over treatment period.
Muscle protein kinetics are depicted in Figures 3 and 4. The effects of treatment on the net balance of muscle protein synthesis and breakdown were essentially identical during propranolol administration and during combination GH plus propranolol therapy. In both of these treatment groups, anabolism was achieved and the protein net balance was significantly greater after anabolic treatment than during the untreated, baseline period (see Fig. 4, P < .05 by repeated measures ANOVA). No alteration of muscle protein kinetics was found in the GH group or in the untreated control group. Two-way repeated measures ANOVA revealed a significant improvement in net balance associated with propranolol administration (P < .05). In both the propranolol and the combination GH plus propranolol groups, β blockade was associated with a large increase in measured Rd, suggesting that muscle protein synthesis was augmented (Fig. 4, P < .05).
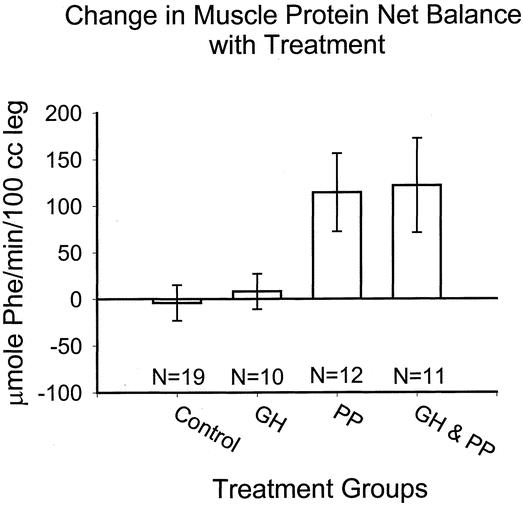
Figure 3. Change in muscle protein net balance over treatment period.

Figure 4. Change in Rd, approximating protein synthesis, and Ra, approximating protein breakdown, over treatment period.
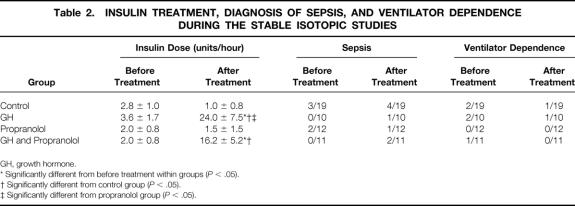
There were no negative clinical consequences resulting from β blockade. No clinically relevant hypotension or bronchospasm occurred in any propranolol subject in this study. Fifteen of the 21 subjects receiving GH manifested hyperglycemia during anabolic treatment. In all cases this was easily controlled by administering insulin (nine subjects controlled with insulin drip, six controlled with sliding-scale insulin), with a goal to keep the serum glucose level below 200 mg/dL. Two of 19 untreated control subjects received insulin during the study periods and only 1 of the 12 propranolol-alone subjects required insulin. Mean doses are listed in Table 2 where significantly more insulin was given in the groups receiving GH (P < .05).
Table 2. INSULIN TREATMENT, DIAGNOSIS OF SEPSIS, AND VENTILATOR DEPENDENCE DURING THE STABLE ISOTOPIC STUDIES
GH, growth hormone.
* Significantly different from before treatment within groups (P < .05).
† Significantly different from control group (P < .05).
‡ Significantly different from propranolol group (P < .05).
DISCUSSION
Both GH and propranolol have been attributed anabolic properties after severe trauma and burn. While the effects of GH have been repeatedly verified since Cuthbertson’s seminal works of the 1940s, 15–23 propranolol has only recently been reported to improve skeletal muscle protein kinetics in the setting of severe burn. 24 This recent report by our group stated that oral propranolol dosed to achieve systemic efficacy (documented by decreased tachycardia) resulted in preservation of lean body mass and improved skeletal muscle protein kinetics following a severe burn. These conclusions were based on data derived from 12 subjects who underwent metabolic study before and after long-term β blockade at our institution in 1998 and 1999. Here, these data are confirmed with data derived from subjects concurrently treated at our institution and randomized to one of three other anabolic regimens: GH alone, combination GH plus propranolol, or no anabolic therapy (control). Data from these additional 41 subjects, along with the statistical analysis of control–drug and drug–drug interactions (only possible through evaluation of all four treatment groups), are novel.
Our presumption was that GH and propranolol would be additive or synergistic in combination, as we suspected that these anabolic agents had different mechanisms of action. While this latter premise is still probable (though not proven), we found no effect of combination anabolic therapy. After GH plus propranolol treatment, there was a large increase in the rate of cellular disappearance of amino acids (approximating protein synthesis), just as we had previously found and reported during propranolol treatment alone. In fact, the changes in protein synthesis and overall net protein balance were entirely consistent among the 23 subjects receiving either propranolol or propranolol plus GH.
We found no anabolic effect attributable to GH in analysis of its administration both alone and in combination with propranolol. This is contrary to numerous studies from our institution as well as from other groups active in the study of surgical metabolism. 16–18,30,31 GH has been shown to stimulate wound healing, 19,20 increase linear growth, 32 stimulate hepatic albumin synthesis, 33 and accelerate accretion of both lean mass and bone mineral content in convalescing burn survivors when administered over several months. 16 Rather than signifying pharmacologic impotency, we assume this implies some flaw in our study design, perhaps insufficient length of GH administration, as we have previously shown that long-term administration over several months accelerates accretion of both lean mass and bone mineral content in convalescing burn survivors. 16 It is our impression in light of this study that GH is likely to be more weakly anabolic than propranolol, and it is probable that this could be extrapolated to other agents such as oxandrolone and insulin. Further studies should be done to corroborate this. With these conditions in mind, we can only conclude that there is no anabolic synergism between GH and propranolol in the short term. Perhaps other anabolic agents might be more appropriate for combination anabolic therapy in relatively short-term cycles during the early, hypercatabolic period after severe burn (i.e., insulin, 34 oxandrolone 27).
Of course, the alternative hypothesis must also be entertained, which would state that optimally dosed combination anabolic therapy is insufficient to improve net muscle protein synthesis. This would imply a ceiling of anabolic effect that could be reached with a number of agents. If this were found to be true, the safest and most economical agent then should be chosen as the standard of care in patients with severe catabolism associated with injury. This notion, of course, requires much more study before it can be espoused.
Importantly, here we report another 11 victims of serious burn who safely underwent continuous β blockade and experienced a decrease in energy expenditure and attenuation of muscle protein catabolism. This corroborates our previous report. Both gross physiologic mechanism (increased protein synthesis) and overall outcome (improved muscle protein net balance) are entirely consistent between the propranolol-alone and the propranolol plus GH groups. The cellular processes through which propranolol accelerates muscle protein synthesis remain unknown; in fact, our results are counterintuitive to previous reports in burned animals for anabolic effects of β-receptor agonists. 35,36 We speculate that blockade of catecholamine receptor activation leads to decreased intracellular protein catabolism by diminishing effects of inflammation-associated signal transduction pathways. We are actively performing studies based on this notion. Yet to be determined as well is the mechanism by which propranolol damps systemic energy expenditure.
While we freely admit a lack of mechanistic understanding, we just as freely promote clinical administration of propranolol in the setting of serious burn. When titrated to achieve a 15% decrease in tachycardia in hypermetabolic, catabolic burn victims, propranolol is a safe, inexpensive, and strongly anabolic agent. Further study of its mechanism of action and potential clinical interactions with GH or other anabolic agents seems justified. In summary, when dosed to achieve a 15% to 20% decrease in tachycardia after severe burn, propranolol dramatically improved skeletal muscle protein kinetics through an acceleration of protein synthesis. Addition of GH during acute burn care did not further stimulate muscle protein accretion.
DISCUSSION
Dr. Basil A. Pruitt, jr. (San Antonio, TX): Yesterday our president noted that improved burn care was one of the signal advances of the past century. This paper is the latest installment of an impressive body of work that has contributed to that improvement by defining postinjury hypermetabolism and developing effective programs of metabolic and nutritional support.
This study confirms that β-adrenergic blockade with propranolol has a salutary metabolic effect. That is the good news. But the disappointing bad news is that the addition of growth hormone did not exert any synergistic effect, and, in fact, the patients receiving both propranolol and growth hormone had a lesser increase in protein synthesis and an actual increase in protein degradation when compared to the patients receiving only propranolol.
That leads to the possibility that growth hormone actually antagonized the effect of propranolol. The authors need to address that puzzling discordance with their earlier studies and provide us with additional information to help us evaluate these findings.
The patients were at bed rest for 5 days after each operation, but after that were allowed to ambulate. Could differences in activity level account for your findings? And just how did you ensure that activity was comparable in all groups?
You note that REE was measured on the day of study but don’t state how well those measurements matched caloric intake. Since the positive effect of growth hormone on nitrogen balance is evident only when calorie supply equals or exceeds metabolic rate, inadequate calorie support could explain the lack of effect of growth hormone in these studies.
Is it possible that the propranolol dose in the propranolol plus growth hormone group was inadequate? In that group there was only an average 15% decrease in heart rate, and 5 of 11 intermittent doses of propranolol were withheld because of blood pressure measurements of 60 to 65 torr. Were these patients sufficiently hypovolemic to reduce the delivery of growth hormone to the skeletal muscle mass?
You note that 15 of the 21 patients receiving growth hormone were hyperglycemic and required insulin to keep the blood glucose below 200 mg/dL. Was the hyperglycemia associated with elevation of catecholamine levels and a disproportionate increase in metabolic rate in those patients?
Lastly, and of particular concern, is the information on page 13 which indicates that the propranolol-only group is a group of patients studied previously. If that noncontemporaneous group were excluded, the group given both propranolol and growth hormone would show a synergistic effect as compared to the contemporaneous growth hormone-only group. That would support a quite different conclusion.
I enjoyed the paper and compliment the group upon this contribution, which expands our understanding of postinjury hypermetabolism.
Presenter Dr. David N. Herndon (Galveston, TX): The activity levels in all groups were similar, the interval to study was exactly the same in all groups, and the patients were all ambulated on the fifth postoperative day through the time of the study. Resting energy expenditure was measured in all patients. The caloric delivery, as indicated, was 1.4 times the REE in all patients who were studied. And this is a continuous infusion of Vivonex throughout the study, which would eliminate the caloric delivery question. We have previously shown that at 1.2 times REE-delivered glucose, there is weight loss; at 1.6 times REE-delivered calories, there is a weight gain; and at 1.4 there is maintenance.
The propranolol dose in the combined group was similar to the propranolol dose in the propranolol-alone group. The decrease in heart rate of 15% versus 18% is not statistically significant. The 5 patients out of 11 who had withheld doses, only had episodic doses one or two doses held for a mean arterial pressure between 60 to 65. No patient had a mean arterial pressure of less than 60. So I don’t think this is attributable to a dose effect.
The hyperglycemia relative to catecholamines in the growth hormone group with propranolol is probably not due to catecholamine effect. Only 2 of 12 patients in the propranolol-alone group had similar elevations in glucose. So I think the elevation in glucose is due to growth hormone, as has previously been demonstrated in multiple other studies.
The question as to whether the propranolol group was noncontiguous with the other groups—all of these patients were enrolled in a four-way study that began in 1998 and extended until 2001. The propranolol patients had been reported in part with some crossover in a previous paper, but were contiguous with this group. So there was no difference in time between the groups.
Dr. William G. Cioffi (Providence, RI): Drs. Herndon and Hart today have sought to determine whether treatment with growth hormone and β blockade have an additive benefit in mitigating the hyperdynamic and hypermetabolic effects of thermal injury. The presentation today suggests there is not.
The study is well performed. But not surprisingly, the groups are small. Consequently, intergroup differences may exist. To assure that the groups are similar, do you have more data on issues such as intubation and ventilatory requirements, work of breathing, the presence of infection, the amount of physical therapy, and other issues which may have a profound effect on the metabolic data?
Many patients received insulin, as pointed out by Dr. Pruitt. This is a potent anabolic agent. How did this affect your results?
Most troubling, as pointed out by Dr. Pruitt, is the lack of effect of growth hormone alone. The authors have previously reported that growth hormone is capable of blunting the catabolic effects of burn injury. How do you explain this?
In previous studies you have included wound healing data. Do you have this kind of clinical data for your β-blockade group showing a true clinical effect?
This is short-term treatment. Is the effect of β blockade long term and durable?
Finally, I would like to you speculate on the mechanism. β blockade affected both anabolism and protein degradation. I ask this because of previously published data in animal burn models suggesting treatment with β agonists was capable of improving protein synthesis.
If duplicated in a larger series, this is an exciting and important finding. β blockade could be used in many other types of patients in our intensive care units who are affected by the often deleterious hypercatabolic response to injury. Again I congratulate Dr. Herndon and colleagues and thank the Association for the privilege of the floor.
Dr. David N. Herndon (Galveston, TX): These are small groups, especially for a four-group study analyzed by two-way ANOVA. The variables in regards to intubation, work of breathing, ventilation, and physical therapy are no different in such small groups.
Insulin is a potent anabolic agent. However, insulin was given to the growth hormone-treated group in 7 of 11 patients. Normally three of the other groups, only three individuals in the other groups, propranolol and control, received insulin at any time, and then only for very small amounts of time. So the anabolic effect of insulin would have worked against our study conclusions instead of for them.
Wound healing data was not obtained in this short-term study. And that may explain why the growth hormone effects that we have previously defined were not seen in this particular study. Growth hormone studied over a prolonged period of time showed improvements in wound healing, improvements in anabolism, improvements in bone accretion, and improvements in growth. But this study perforce was a short study comparing individuals as their own control to an treatment period of 10 days. In all probability that amount of time is insufficient to show protein synthetic effects. In some previous studies, short infusion studies have shown effects of growth hormone. Those were with diets that do not induce hyperinsulinemia, such as the one that we gave, which has been demonstrated to increase endogenous insulin production well above that.
β blockade effects are durable. We have studied this through the hospital course and are now studying it well into convalescence.
The final question about when the finding that as cienbuterol as a β1 agonist improved protein synthesis in animal studies. Propranolol is a β1 β2 antagonist. It is altogether possible that β1 agonists may be anabolic, whereas the primary effect against catabolism is the β2-blocking effect of propranolol. Further studies with β1 and β2 blockers will be required to elucidate that issue. β3 blockers also need to be studied.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Supported by SHC Grant #8660, SHC Grant #8490, NIH Training Grant #2T32GM0825611, NIH Center Grant #1P50GM60338-01, NIH Grant #GM56687-02.
Correspondence: Steven E. Wolf, MD, Shriners Hospitals for Children, 815 Market Street, Galveston, TX 77550.
E-mail: swolf@utmb.edu
Accepted for publication April 24, 2002.
References
- 1.Monk DN, Plank LD, Franch-Arcas G, et al. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg 1996; 223: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessey PQ, Jiang ZM, Johnson DJ, et al. Posttraumatic skeletal muscle proteolysis: the role of the hormonal environment. World J Surg 1989; 13: 465–470. [DOI] [PubMed] [Google Scholar]
- 3.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery 2000; 128: 312–319. [DOI] [PubMed] [Google Scholar]
- 4.Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock 1998; 10: 155–160. [DOI] [PubMed] [Google Scholar]
- 5.Moore FD. Response to starvation and stress. In: Moore FD, ed. Metabolic Care of the Surgical Patient. Philadelphia: WB Saunders, 1959: 202–275.
- 6.Harrison TS, Seaton JF, Feller I. Relationship of increased oxygen consumption to catecholamine excretion in thermal burns. Ann Surg 1967; 165: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 1974; 180: 653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodall M, Stone C, Haynes BW Jr. Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg 1957; 145: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am 1978; 58: 1173–1187. [DOI] [PubMed] [Google Scholar]
- 10.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg 1988; 208: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minifee PK, Barrow RE, Abston S, et al. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg 1989; 24: 806–810. [DOI] [PubMed] [Google Scholar]
- 12.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil 1997; 18: 223–227. [DOI] [PubMed] [Google Scholar]
- 13.Breitenstein E, Chiolero RL, Jequier E, et al. Effects of beta-blockade on energy metabolism following burns. Burns 1990; 16: 259–264. [DOI] [PubMed] [Google Scholar]
- 14.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335: 1713–1720. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson DP, Shaw GB, Young FG. The anterior pituitary gland and protein metabolism. II. The influence of anterior extract on the metabolic response of the rat to injury. J Endocrinol 1941; 2: 468–474. [Google Scholar]
- 16.Hart DW, Herndon DN, Klein G, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg 2001; 233: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg 1993; 218: 400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 19.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg 1990; 212: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilpin DA, Barrow RE, Rutan RL, et al. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994; 220: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarrar D, Wolf SE, Jeschke MG, et al. Growth hormone attenuates the acute-phase response to thermal injury. Arch Surg 1997; 132: 1171–1175. [DOI] [PubMed] [Google Scholar]
- 22.Knox J, Demling R, Wilmore D, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma 1995; 39: 526–530. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez RJ, Wolf SE, Barrow RE, et al. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 1998; 228: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 2001; 345: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 25.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed]
- 26.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg 2000; 232: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg 2001; 233: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biolo G, Chinkes D, Zhang XJ, et al. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN 1992; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 29.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 1995; 268: E514–520. [DOI] [PubMed] [Google Scholar]
- 30.Gamrin L, Essen P, Hultman E, et al. Protein-sparing effect in skeletal muscle of growth hormone treatment in critically ill patients. Ann Surg 2000; 231: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman RS, Harrison LE, Pearlstone DB, et al. Growth hormone, alone and in combination with insulin, increases whole body and skeletal muscle protein kinetics in cancer patients after surgery. Ann Surg 1999; 229: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet 1999; 354: 1789. [DOI] [PubMed] [Google Scholar]
- 33.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med 2000; 28: 1578–1584. [DOI] [PubMed] [Google Scholar]
- 34.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martineau L, Little RA, Rothwell NJ, et al. Clenbuterol, a beta 2-adrenergic agonist, reverses muscle wasting due to scald injury in the rat. Burns 1993; 19: 26–34. [DOI] [PubMed] [Google Scholar]
- 36.Chance WT, von Allmen D, Benson D, et al. Clenbuterol decreases catabolism and increases hypermetabolism in burned rats. J Trauma 1991; 31: 365–370. [DOI] [PubMed] [Google Scholar]