Abstract
Objective
To review the authors’ experience with local excision of early rectal cancers to assess the effectiveness of initial treatment and of salvage surgery.
Summary Background Data
Local excision for rectal cancer is appealing for its low morbidity and excellent functional results. However, its use is limited by inability to assess regional lymph nodes and uncertainty of oncologic outcome.
Methods
Patients with T1 and T2 adenocarcinomas of the rectum treated by local excision as definitive surgery between 1969 to 1996 at the authors’ institution were reviewed. Pathology slides were reviewed. Among 125 assessable patients, 74 were T1 and 51 were T2. Thirty-one patients (25%) were selected to receive adjuvant radiation therapy. Fifteen of these 31 patients received adjuvant radiation in combination with 5-fluorouracil chemotherapy. Median follow-up was 6.7 years. One hundred fifteen patients (92%) were followed until death or for greater than 5 years, and 69 patients (55%) were followed until death or for greater than 10 years. Recurrence was recorded as local, distant, and overall. Survival was disease-specific.
Results
Ten-year local recurrence and survival rates were 17% and 74% for T1 rectal cancers and 26% and 72% for T2 cancers. Median time to relapse was 1.4 years (range 0.4–7.0) for local recurrence and 2.5 years (0.8–7.5) for distant recurrence. In patients receiving radiotherapy, local recurrence was delayed (median 2.1 years vs. 1.1 years), but overall rates of local and overall recurrence and survival rates were similar to patients not receiving radiotherapy. Among 26 cancer deaths, 8 (28%) occurred more than 5 years after local excision. On multivariate analysis, no clinical or pathologic features were predictive of local recurrence. Intratumoral vascular invasion was the only significant predictor of survival. Among 34 patients who developed tumor recurrence, the pattern of first clinical recurrence was predominantly local: 50% local only, 18% local and distant, and 32% distant only. Among the 17 patients with isolated local recurrence, 14 underwent salvage resection. Actuarial survival among these surgically salvaged patients was 30% at 6 years after salvage.
Conclusions
The long-term risk of recurrence after local excision of T1 and T2 rectal cancers is substantial. Two thirds of patients with tumor recurrence have local failure, implicating inadequate resection in treatment failure. In this study, neither adjuvant radiotherapy nor salvage surgery was reliable in preventing or controlling local recurrence. The postoperative interval to cancer death is as long as 10 years, raising concern that cancer mortality may be higher than is generally appreciated. Additional treatment strategies are needed to improve the outcome of local excision.
Although local excision for rectal cancer has been practiced for more than 120 years, its proper use for cure of small, localized rectal cancers is still incompletely understood. The appeal of local excision as definitive treatment is considerable. When accomplished by transanal excision, the operation has very low morbidity. There is no need for permanent or even temporary colostomy. Recovery is rapid, and long-term bowel function is excellent. In contrast, radical surgery, even in expert hands, carries a significant risk of perioperative morbidity, may require a stoma, usually necessitates several weeks of recovery, and frequently leaves patients with compromised bowel or sexual function. 1–6
A prerequisite for using local excision as curative therapy in fit patients is the development and validation of selection criteria that can identify those patients who can be treated by local excision alone without compromising cancer cure. Such criteria remain incompletely defined. Tumor size and configuration, imaging properties by endorectal ultrasound (depth of tumor penetration, regional lymph node detection), and pathologic factors (T stage, grade, and vessel invasion) have all been useful in defining patient populations at low versus high risk of tumor recurrence after local excision. 7,8 Despite such selection criteria, local excision carries the unavoidable risk of unresected regional disease and incomplete pathologic staging because regional lymph nodes are not removed and are therefore not pathologically assessed. At present, preoperative imaging modalities cannot accurately predict the presence or absence of regional lymph node metastases. Moreover, there is currently no combination of postoperative clinical and pathologic factors that can completely exclude the risk of occult regional lymph node metastasis. 9,10 The limitation of local excision as a curative operation has been highlighted by recent reports that show a 10% to 40% risk of local recurrence following local excision for T1 and T2 rectal cancers at median follow-up of approximately 5 years. 11,12
Strategies to enhance local control of rectal cancer have included adjuvant chemoradiation therapy as well as close postoperative surveillance with surgical resection of local recurrence. There have been no randomized studies of radiotherapy or chemotherapy to evaluate their efficacy in enhancing local control following local excision. However, the enhanced local control and survival rates achieved by adjuvant chemotherapy and radiation after radical rectal surgery 13,14 provide a strong rationale for their use in combination with local excision. Single-arm studies have shown that postoperative chemoradiation is well tolerated after local excision. However, the benefit in preventing cancer recurrence in this setting is unproven, with mature studies showing local failure rates for T2 cancers after excision and chemoradiation as high as 28%. 12,15–17 Another uncertainty is how often local tumor recurrence can be cured by salvage radical surgery. This is an important question, as at least half of all recurrences are local-regional. Currently, data on surgical salvage are limited, 11,12 and additional reports with long-term follow-up are needed.
At Memorial Sloan-Kettering Cancer Center, local excision for cure of small rectal cancers has been used selectively for more than 30 years. 18,19 Since 1986 adjuvant radiotherapy and, more recently, adjuvant chemoradiotherapy have been used for selected cases judged to be at high risk for recurrence. 20 We have reviewed our institutional experience to evaluate the long-term cancer treatment results for local excision for primary rectal cancer and salvage surgery for recurrence.
PATIENTS AND METHODS
Patients treated by local excision for rectal cancer at Memorial Sloan-Kettering Cancer Center between January 1969 and September 1996 were identified by a review of hospital databases, office records of attending surgeons, and a prospective database maintained by the Colorectal Surgery Service. Databases were queried for procedure codes, and each potential case was investigated by chart review. Inclusion criteria included a pathologic diagnosis of primary T1 or T2 adenocarcinoma of the rectum and definitive treatment by full-thickness local excision. Eighty-four cases were excluded from analysis for the following reasons: stage IV cancer, T3 cancer, pure adenoma, immediate salvage resection for adverse pathologic features (positive margin, poorly differentiated), pathology slides not available, and insufficient follow-up (<2 years).
In all cases, the preoperative workup included endoscopic examination of the rectum, biopsy of the rectal tumor, and chest x-ray. Tumor location was recorded as the distance from the anal verge to the lower edge of the tumor. In 14 cases, endoscopic snare excision of the rectal tumor was performed before presentation for definitive surgical treatment. CT scan of the abdomen and pelvis was used routinely, although records to document the use of CT were sparse for the 39 patients treated before 1986. Preoperative endorectal ultrasound was obtained routinely starting in 1988, but findings were not recorded for this study.
Tumor excision was performed under general anesthesia using either the dorsal lithotomy or the prone jackknife position. Tumors were generally excised from the rectal wall using electrocautery. Full-thickness excisions were performed in all cases. Tumor grade was assigned as poor, moderate, or well based on the extent of glandular architecture within the tumor. Vessel invasion was scored as present when dysplastic cancer cells were identified within the lumens of blood vessels or lymphatic vessels. Tumor size was assigned as the largest diameter recorded on the original pathology report.
Adjuvant radiotherapy was administered postoperatively to 16 patients, and an additional 15 patients received adjuvant radiotherapy combined with 5-fluorouracil-based chemotherapy. Results in this subset of patients have been previously published. 20 Patients selected for postoperative adjuvant therapy were limited to those with a positive margin, T1 tumors with vascular invasion and/or poorly differentiated histology, or patients with T2 tumors. All patients receiving adjuvant radiotherapy were treated between 1985 and 1996. These patients were treated with 180 to 200 cGy fractions using three- or four-field technique. Anatomical landmarks used to determine the radiation fields were not reviewed. The minimum radiotherapy dose was 4,500 cGy administered to the whole pelvis. Selected patients were treated with doses to the tumor bed as high as 5,400 cGy.
After completion of therapy, patients were followed according to the preference of their physicians, and no attempt was made in this review to document the frequency of surveillance testing. In the vast majority of cases, the excision site was monitored by digital examination and endoscopic visualization. Endorectal ultrasound was not used for postoperative surveillance. Tumor recurrences were documented from clinic notes, radiology reports, and pathology reports. Local recurrence (LR) was defined as any tumor recurrence within the true pelvis. Distant recurrence (DR) was defined as any tumor metastasis identified outside the true pelvis.
The pattern of recurrence was assigned as local, local plus distant, or distant and was based on all sites of disease documented within 6 months from the first evidence of disease recurrence. Salvage resection for LR and the interval from salvage to death or last follow-up were recorded. Patients were evaluated for LR, DR, and disease-specific survival. Outcomes were compared according to treatment and tumor stage. Statistical comparisons of recurrence and survival rates were performed using the Kaplan-Meier method and the log-rank test. Univariate and multivariate analysis of prognostic factors used the Cox proportional hazard method.
RESULTS
One hundred twenty-five assessable patients were identified. The clinical features of the study population are described in Table 1. Tumor size was available for 112 cases. The median tumor size was 2 cm (range 0.8–8). The five largest (>4.5 cm) tumors were invasive adenocarcinomas arising within large villous adenomas. Pathology review confirmed 74 T1 cancers and 51 T2 cancers. There were 4 poorly differentiated cancers, and the remaining 121 were moderately differentiated. Intratumoral vessel invasion was present in 18 cases. Microscopic involvement of the surgical margin was detected in 11 cases.
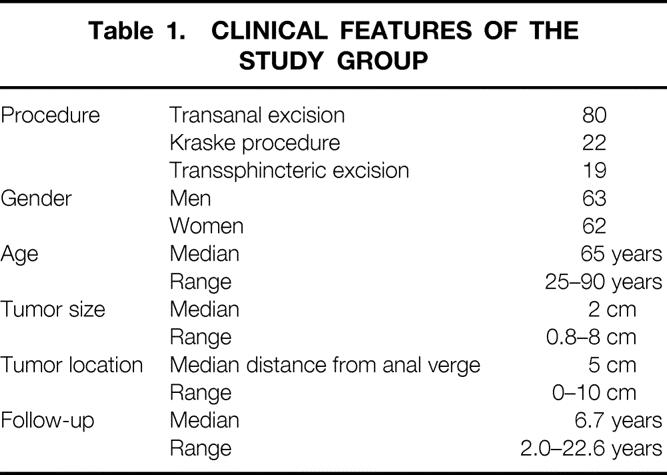
Table 1. CLINICAL FEATURES OF THE STUDY GROUP
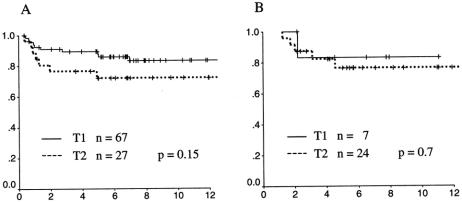
The actuarial rates of local recurrence for T1 and T2 tumors are shown in Figure 1. Sixty-seven T1 cancers and 27 T2 cancers were treated by local excision alone. Among the T1 patients, 10 patients experienced local recurrence at a postoperative interval ranging from 4 months to 7 years. At 10 years the actuarial LR rate was 17%. For T2 patients, seven patients developed LR at 0.8 to 4.9 years postoperatively, with an LR rate at 10 years of 28%. Although there was a trend toward higher local failure for T2 carcinomas compared to T1 carcinomas, this trend did not reach statistical significance (P = .15).
Figure 1. Actuarial local control rates for T1 (solid line) and T2 (dotted line) rectal cancers. (A) Cancers treated by local excision alone. (B) Cancers treated by local excision and radiotherapy.
LR rates for patients treated by local excision plus radiotherapy differed little from nonradiated patients. Ten-year LR rates for T1 and T2 cancers were 17% and 24%, respectively. All local failures occurred within 5 years of surgical excision. However, time to LR was somewhat longer in patients receiving adjuvant pelvic radiotherapy: median time to LR was 2.1 years in radiated cases compared to 1.1 years for nonradiated cases. In evaluating the LR data, it should be noted that patients receiving adjuvant radiotherapy had a higher risk profile than nonradiated patients. Cancers treated with adjuvant radiation were more often T2 (77% vs. 29%, P = .001) and had a higher proportion of vessel invasion (26% vs. 11%, P = .05) and positive surgical margin (23% vs. 5%, P = .002) than those treated by local excision alone. It is clear that patients were selected to receive adjuvant radiation because of these high-risk features. Therefore, direct comparison of the treatment results between radiated and nonradiated patients does not fairly assess the impact of radiotherapy, and interpretation of the results must be done with caution.
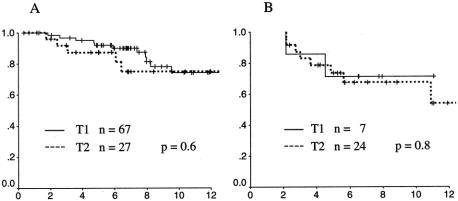
Actuarial survival rates for patients with T1 and T2 cancers were remarkably similar (Fig. 2). At 10 years the disease-specific survival rate for nonradiated cases was 74% for T1 cancers and 75% for T2 cancers (P = .6). For radiated cases, the 10-year survival rates were 71% for T1 cancers and 68% for T2 cancers (P = .8). In contrast to LR, death from disease was observed in a time frame extending well beyond 5 years. A total of 26 deaths from disease were observed at follow-up times ranging from 1.7 to 10.9 years. Median time to cancer death was 4.0 years. Time to cancer death was indistinguishable for patients with T1 versus T2 cancers. For the 10 radiated patients who died of disease, there was a slightly shorter median survival (median 3.3 years, range 2.1–10.9) compared to the 16 nonradiated patients who died of disease (median 5.3 years, range 1.7–9.5), but the range of postoperative survival times was identical.
Figure 2. Actuarial disease-specific survival rates for T1 (solid line) and T2 (dotted line) rectal cancers. (A) Cancers treated by local excision alone. (B) Tumors treated by local excision and radiotherapy.
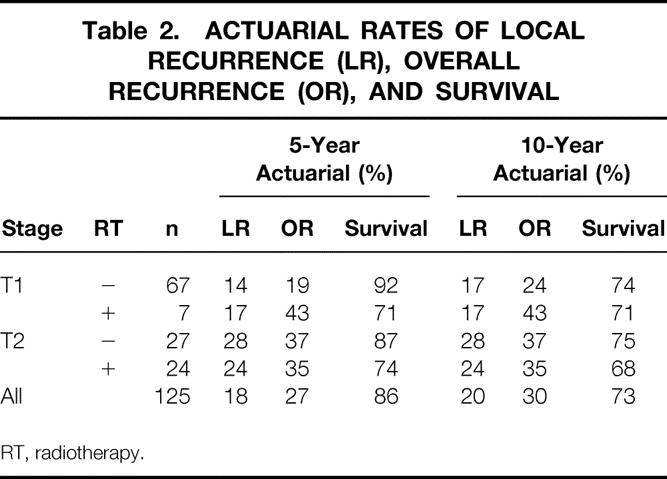
The maturity of the outcome data for this series allowed us to compare oncologic outcomes at 5 and 10 years of follow-up (Table 2). LR rates and overall recurrence rates were nearly identical at 5 years and 10 years for each cohort of patients, as 22 of 23 local failures and 18 of 22 distant failures occurred within 5 years of local excision. On the other hand, 8 of 28 cancer deaths (28%) occurred beyond 5 years of local excision. It is interesting in this series that the T1, nonirradiated cohort accounted for the one patient with LR and the four patients with distant recurrence beyond 5 years. It is unclear if this is simply a random finding because this cohort is the largest treatment group or whether this cohort is at higher risk for late tumor failures because of early diagnosis and lead-time bias.
Table 2. ACTUARIAL RATES OF LOCAL RECURRENCE (LR), OVERALL RECURRENCE (OR), AND SURVIVAL
RT, radiotherapy.
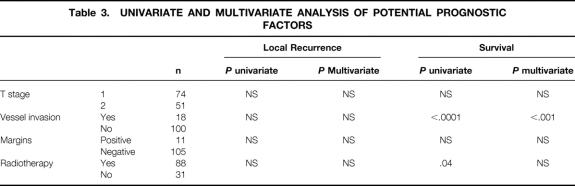
A multivariate analysis was performed to identify factors that might contribute to LR and cancer mortality after local excision (Table 3). No factors were significantly associated with LR. The presence of vessel invasion was, however, strongly associated with survival (P < .001). A survival curve demonstrating the impact of vessel invasion on survival is shown in Figure 3.
Table 3. UNIVARIATE AND MULTIVARIATE ANALYSIS OF POTENTIAL PROGNOSTIC FACTORS
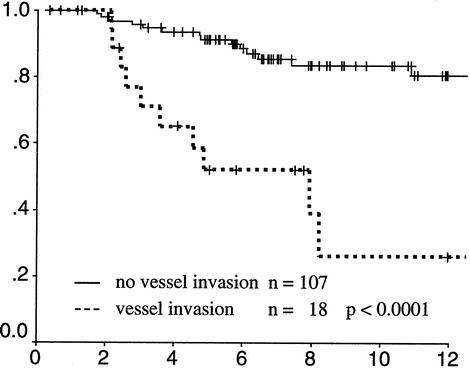
Figure 3. Actuarial survival of rectal cancers with (dotted line) and without (solid line) intratumoral vessel invasion identified on histopathology.
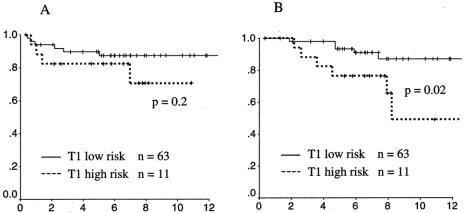
To evaluate the treatment results of local excision in a clinically favorable cohort, patients with T1 cancers were divided into a high-risk group (n = 11) and a low-risk group (n = 63) based on the presence or absence of adverse features (high grade, vessel invasion, positive surgical margin). Patients with low-risk T1 cancers were found to have a local failure rate of 13% and a disease-specific survival rate of 87% at 10 years (Fig. 4). Patients with high-risk T1 cancers had significantly worse survival (49%, P = .02) and a trend toward higher local failure (29%, P = .2).
Figure 4. Local recurrence-free survival and disease-specific survival of patients with T1 rectal cancers with (high risk, dotted line) and without (low risk, solid line) adverse features on histopathology (high grade, vessel invasion, or positive margin). (A) Local recurrence. (B) Survival.
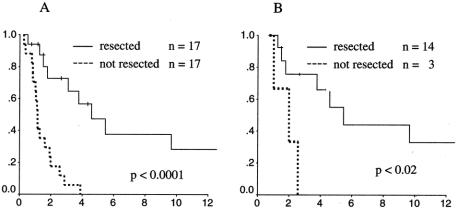
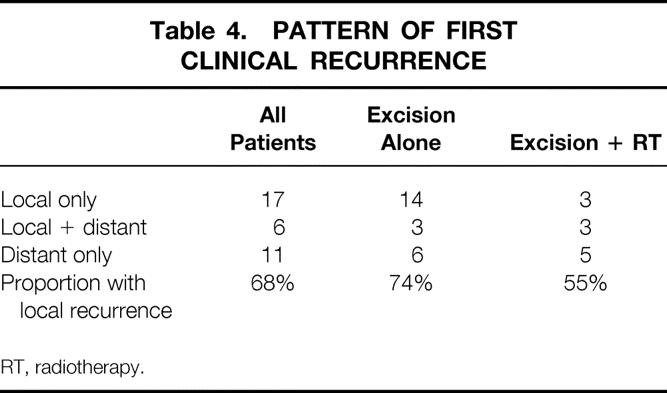
A total of 34 patients developed recurrence of tumor during the follow-up period (Table 4). The predominant site of initial recurrence was local in radiated as well as nonradiated patients. Among the 34 patients who developed tumor recurrence, half were able to have a salvage operation with complete resection of their recurrent tumors. Fourteen of 17 local failures (82%) were successfully resected, whereas only 3 of 11 distant recurrences could be resected (Table 5). The subsequent survival rates of patients from the time of recurrence are shown in Figure 5. Patients whose recurrence could not be resected fared quite poorly, with all patients dying of disease within 4 years. However, resection did not confer a durable remission for most patients. Only 7 of the 14 resected patients had no evidence of disease at last follow-up. The 6-year actuarial survival rate for resected patients was approximately 40% when all recurrences were considered and 45% when isolated LRs were considered.
Table 4. PATTERN OF FIRST CLINICAL RECURRENCE
RT, radiotherapy.
Table 5. SALVAGE SURGERY FOR FIRST CLINICAL RECURRENCE
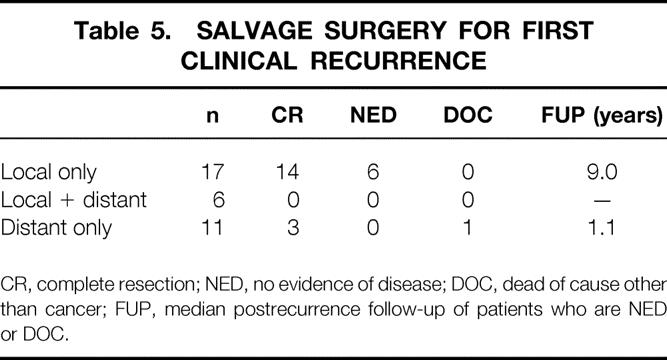
CR, complete resection; NED, no evidence of disease; DOC, dead of cause other than cancer; FUP, median postrecurrence follow-up of patients who are NED or DOC.
Figure 5. Actuarial survival rates of patients who developed recurrence. Each graph compares patients whose recurrence was treated by surgical resection (solid line) to patients whose recurrence was not resected (dotted line). (A) Patients with any recurrence. (B) Patients with isolated local recurrence.
DISCUSSION
Review of the published literature shows great variation in the oncologic results of local excision for small T1 and T2 cancers of the distal rectum. 21 Several groups have reported local failure rates that are lower (0–15%) and survival rates that are higher than those observed in the present study. 15,18,22–24 Although more stringent patient selection may be the difference, it is concerning that the crucial differences in patient selection criteria are difficult to define. Moreover, the small number of patients and the variable follow-up of individual patients reported in most published reports raise the question of which data are most representative and useful. Several recent reports from experienced surgical groups suggest that high local failure rates after local excision (15–30%) are the norm when follow-up is sufficient. 11,12,25 Recently published studies that include patients who received postoperative adjuvant chemoradiation have equally variable results. 17,20 At present, the extent to which local control is enhanced by postoperative adjuvant radiation is unknown.
Two prospective studies have been reported. The RTOG randomized 60 patients over 8 years to three treatment arms. 26 The LR rates for T1 and T2 cancers were 39% and 29%, respectively (median follow-up 6 years). The CALGB/Intergroup study reported 59 patients treated by local excision for T1 cancers and 51 patients treated for T2 cancers by local excision with postoperative chemoradiation. 27 LR data were favorable, with 5% local failures for T1 cancers and 14% for T2 cancers. It should be recognized that because 38% of patients registered for this trial were excluded from analysis based on a variety of protocol violations, among which were positive resection margins, the study group may represent an optimized series that is more favorable than a consecutive series of patients encountered in practice. In addition, the median follow-up of 4 years is insufficient for final assessment. Nevertheless, when the mature CALGB/Intergroup results are reported, this will provide benchmark data for this field.
The essential finding of the current study is that tumor recurrence rates are higher than those observed with radical surgery, and a limited number of tumor recurrences can be treated successfully at the time of first clinical recurrence. These findings are similar to the results of a recently published large, relatively mature, retrospective study, in which local failure rates for local excision without radiotherapy were 18% for T1 cancers and 47% for T2 cancers, and salvage therapy yielded only 50% survival with short follow-up. 11 A second important observation in the current study is that death from cancer can occur at quite long intervals after excision. In our series 28% of cancer deaths occurred after 5 years, and the longest postoperative interval to cancer death was 11 years. These findings suggests that long-term cancer survival rates may be lower than generally appreciated in the literature and raise concerns about the use of local excision for young, fit patients.
What is the reason for the high LR rates? We were unable to identify any factor that was strongly associated with LR. However, the circumstantial evidence seems to favor occult, unresected local-regional tumor dissemination as the major factor. We observed a preponderance of local failure, with 67% of recurrences including a local component. Most local failures developed relatively quickly (median interval 1.4 years), whereas there was a more delayed appearance of distant metastases (median interval 2.1 years). This pattern of recurrence might be expected with unresected regional lymph node metastases. In contrast, the failure pattern for rectal cancers of all stages treated at our institution by radical surgery without radiotherapy is predominantly distant failure, with local failure being documented in only 6% to 10% of cases. 2,28,29 Our data strongly implicate unresected disease in regional lymphatics as a cause of local failure after local excursion.
It is clear that surgeons have difficulty predicting the presence or absence of lymphatic dissemination, even when the final pathologic features of the excised or resected primary rectal cancer are known. 9 The problem of predicting lymph node spread has been shown to be true even for small T1 and T2 rectal cancers, a group in which the overall rate of lymph node spread remains in the 10% to 20% range. 10 Of particular concern is the recent report that among 353 resected T1 colorectal cancers, in which the overall lymph node metastasis rate was 13%, T1 cancers located in the lower third of the rectum were at particularly high risk for lymph node involvement (10 of 29 cases [34%], sixfold relative risk, P < .001). 30 The published data are convincing that for resected cancers using conventional clinical and pathologic parameters, it is exceedingly difficult to define a group whose risk of lymph node metastases is consistently less than 10%. We believe it is this difficulty that underlies the uncertainty and the compromise involved in offering local excision as curative therapy for rectal cancer.
We were somewhat surprised to discover in our series that microscopic involvement of the surgical margin was not associated with LR. This may be explained by the fact that at our institution, a positive excision margin is regarded as an indication for either immediate radical resection or delivery of adjuvant radiotherapy. In the present study, microscopic involvement of the surgical excision margin was observed in only 11 cases, and all 11 received postoperative radiotherapy. Only one (9%) developed LR, and this patient was successfully treated by abdominoperineal resection at the time of recurrence. Thus, it is not surprising that surgical margin was not a dominant risk factor for recurrence or survival in our data. We, of course, believe in the importance of clear surgical margins and feel that clear margins should be achieved in the vast majority of cases. Yet despite clear margins, LR is still seen. Among the 114 patients with clear surgical margins in our series, 22 (19%) developed LR. We speculate that efforts to achieve wider surgical margins will yield little improvement in tumor recurrence rates.
Despite frequent arguments for the curative potential of salvage resection at the time of LR, there are few data to support its effectiveness. In a recent review, the data from 15 studies reporting salvage surgery for LR following local excision were described. 21 The median number of salvage operations was four. Follow-up time was not stated in seven studies, and in the remaining studies the follow-up ranged from a few months to 10 years among individual papers. The three largest studies reported disease-free survival rates of 20%, 42%, and 50%. 11,12,31 In our own institutional experience, we have previously reported that immediate salvage for high-risk features yields a better outcome than salvage at the time of first clinical recurrence. Data from the present study indicate that surgical resection of recurrence is marginally effective as a salvage strategy. Only half of tumor recurrences are resectable, and less than one half of those resected appear to be cured of their disease. When all patients with recurrence are considered, only 15% to 20% are alive at 6 years after resection.
We conclude that local excision for T1 and T2 rectal cancers is associated with recurrence rates that are higher than those reported for radical surgery. Postoperative adjuvant therapy does not appear to be reliable in preventing local tumor recurrence, and surgical salvage of recurrent cancers has a low cure rate. The data suggest that local excision for T1 and T2 cancers does not offer cure rates equivalent to radical resection. New strategies are needed to improve the oncologic outcome of local excision for rectal cancer.
DISCUSSION
Dr. Stanley M. Goldberg (Minneapolis, MN): Thank you very much for the privilege of commenting on this paper that Dr. Paty and his colleagues from Sloan-Kettering have presented. As a strong advocate of local excision for early rectal cancers, I was pleased to see that you have utilized this approach at your institution.
Small vessel and lymphatic invasion was found to be a poor prognostic indicator in your series. Dr. Charlie Freil, who is now at the University of Virginia, last year looked at our results and found the identical thing; namely, that a poor prognosis is associated with bad histology, which leads me to my first question: How today would you handle a T1 N0 or T2 N0 patient with bad histology that you obviously could resect transanally?
Dr. Charles Finne III at our institution has shown that transanal microsurgery results in a better margin of resection. This leads me to my next question: Were any of your patients resected by the TEM technique? If so, were the results any better?
We all know that lesions in the posterior rectal wall can be resected with a wider margin than those on the anterior rectal wall. Did you look at your recurrence rates in regard to their location within the rectum?
Your low survival rate with reoperation makes me wonder about the preoperative evaluation of your patients. Did all of your patients have CT scanning before definitive curative surgery? Your data showed no benefit with postoperative chemoradiation. And this leads me to my last question: Based on this study, what is your approach today to the T2 N0 lesion found on endorectal ultrasound?
Presenter Dr. Philip B. Paty (New York, NY): Thank you, Dr. Goldberg, for some very good questions.
Your first question was: how do we handle T1N0 or T2N0 cancers with bad histology? I presume you are referring to ultrasound N0, since you wouldn’t have pathologic staging. Our practice has been, in patients who have been treated by local excision and found to have unfavorable histology, whether it is high-grade elements or vascular invasion, to offer the patient either adjuvant radiation with chemotherapy or return to the operating room for radical resection. I don’t have outcome data to compare these two management options. But in 1995 Dr. Warren Enker did publish a paper from our institution indicating that when high-risk features are found in the local excision specimen, the patients who return to the operating room immediately for radical surgery have a significantly improved disease-free survival compared to patients who have radical surgery at the time of local recurrence. So, these are tough questions in tough cases. I think it boils down to the informed consent issue. I try to present the options to the patient and make a decision on an individual basis.
Regading the use of transanal endoscopic microsurgery, we have not used this technique in our institution, and I can’t really comment on whether it would achieve better results. My sense, though, is that achieving a wider surgical margin in the bowel wall is not the problem. From our data, I feel that early lymphatic dissemination is more likely to be the problem and I am not sure TEM selves that.
Regarding posterior versus anterior lesions, we did not have that information for the early cases in our series and we didn’t look at location. We found it hard in retrospect to convincingly assign tumors as anterior or posterior because many were lateral.
Regarding CT scans, we didn’t report the CT data except to note that the patients were not felt to have metastatic disease at the time of surgery. At our institution from the mid-1980s on, virtually all patients did have preoperative CT scans as part of their workup for rectal cancer. I believe the vast majority of patients in this study did have CT scans. Endorectal ultrasound has also been used routinely for rectal cancer at our institution since 1988.
Your final question was: how are we handling a patient with a T2N0 tumor on endorectal ultrasound? Again, I think these cases are some of the most difficult to manage. We take an individualized approach based on the tumor location, the biopsy features, and the gross configuration. If the tumor is amenable to a low anterior resection, even a very low coloanal, we generally recommend radical surgery for fit patients with T2 tumors. When the lesion might require a permanent colostomy, then the option of local excision and adjuvant chemoradiation is given greater consideration.
Dr. Bruce G. Wolff (Rochester, MN): As of today, to put it in New York City terms, local excision has become the third rail of rectal cancer surgery. Drs. Paty and Wong and their colleagues are to be commended for bringing these somewhat disturbing results to our attention, adding further impetus to the previously reported data from the University of Minnesota referenced by Dr. Goldberg. I have three questions.
First, only 31 patients had chemoradiation or radiation therapy, and there was no improvement in local recurrence or survival. In fact, it seemed to be somewhat worse. Last year, Dr. Timothy Yeatman and his colleagues reported, at this very meeting, work that showed much better results with local excision in patients who had preoperative chemoradiation therapy in a similar group of patients. Is preoperative chemoradiation therapy one of the techniques, or strategies, as you refer to it, to be added in the future to improve local excision results? What other modifications or strategies would you suggest?
Second, had these patients initially undergone more radical resective surgery, what is the evidence that survival or local recurrence rates would have been improved? Would a case-matched series have been feasible? And would this give you the answer?
Finally, how has your practice at Memorial-Sloan Kettering changed regarding local excision? Preoperative staging has improved tremendously in recent years, and, at your institution, is as accurate as it is anywhere in the world, and undoubtedly some of your patients in this series were understaged. And that would not occur today. So does that make these results somewhat invalid? To put it more specifically, and to follow up with Dr. Goldberg’s question, if I can try to nail you down on this, if I have a 48-year-old man with a 1.5-cm lesion, uT2 N0 at the dentate line, do I recommend an abdominal peritoneal resection on that man or a local excision?
Dr. Philip B. Paty (New York, NY): Thank you, Dr. Wolff. Very good questions.
Your first question concerned the role of preoperative chemoradiation therapy or other strategies to improve the outcomes for local excision. I think strategies to improve the results are either going to relate to better patient selection or better treatment.
With regard to preoperative radiation, I do think there is a theoretical advantage. When patients are radiated up-front, you can then select patients for local excision based on the response to radiotherapy. You might be more inclined to locally excise those tumors that respond well and radically resect those that do not. In addition to sterilizing microscopic metastases in the pelvis, reoperative radiation may also reduce the likelihood of tumor cell implantation and subsequent recurrence in the local excision site. So, I do believe there is some advantage for the more high-risk cases. On the other hand, the flip side of the coin of local excision is that the majority of patients we operate on are cured. We just can’t identify them beforehand. So, why radiate everybody, make it more complicated and add to the cost, when what we really need is better information: who can be cured by local excision? In that sense, I think the most compelling strategy for the future is to get more information through molecular markers or other highly sensitive imaging techniques to better select patients.
Your second question was regarding whether radical surgery would have changed the outcome and did we have a case-matched series. There has never been a true randomized study. And there is no question that patients treated by local excision are a different group when compared to those treated by radical surgery.
In our institution, we have not yet done a matched case control comparison. But we have reviewed our results of T1N0 and T2N0 cancers treated by radical surgery. The local failure rate was 6% and the five-year survival rate was 85%, which I think is superior to the results I presented today. I would argue that the radical surgery group is actually a more advanced group of T1 and T2 tumors, since it is the very small ones we treat by local excision.
Although there is no randomized trial and no proof, I think the evidence is compelling that local excision is a compromise treatment. The failure pattern is reversed. After local excision, there is local failure; whereas after radical surgery, failures are primarily distant. Although many patients do well after local excision, we can’t confidently identify those patients who will have outcomes equivalent to radical surgery.
Regarding our practices, particularly the use of preoperative imaging and endorectal ultrasound, are the results we presented today relevant to 2002? I think that is an excellent question. Obviously, in order to accumulate enough cases to analyze, a more than twenty-year retrospective review was required and this is reflective of the past. And I think that you are absolutely right—we now do many things differently. We don’t often do the trans-sphincteric or Kraske approaches anymore. We tend to limit ourselves to transanal excision, we are more selective about who we operate on, and we have higher-quality endorectal ultrasound. It may be true that today’s results are better and I think we have to be cautious about overreacting to nonrandomized historical data. However, until better data are reported, we are left with the data we have.
Finally, your last question regarding the 45-year-old male with a T2N0 lesion at the dentate line: would I do an APR or local excision? Although I would consider APR to be the gold-standard therapy, this is an informed consent issue. You have two options in front of you and I don’t believe there is a right or wrong answer. Your recommendation might depend on where the tumor is located, how deep it is, whether there is an option for preoperative radiation to shrink it, and how confidently you believe you can perform a local excision with clear margins and maintain good function. Also, patient preference is always an important factor.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Philip B. Paty, MD, Colorectal Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: patyp@mskcc.org
Accepted for publication April 24, 2002.
References
- 1.Enker WE, Havenga K, Polyak T, et al. Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg 1997; 21: 715–720. [DOI] [PubMed] [Google Scholar]
- 2.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg 1999; 230: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothenberger DA, Wong WD. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg 1992; 16: 478–485. [DOI] [PubMed] [Google Scholar]
- 4.Averbach AM, Chang D, Koslowe P, et al. Anastomotic leak after double-stapled low colorectal resection. Dis Colon Rectum 1996; 39: 780–787. [DOI] [PubMed] [Google Scholar]
- 5.Williamson ME, Lewis WG, Finan PJ, et al. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: myth or reality? Dis Colon Rectum 1995; 38: 411–418. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli NJ, Nagel S, Rodriguez-Bigas M, et al. Morbidity and mortality following abdominoperineal resection for rectal adenocarcinoma. Am Surg 1993; 59: 400–404. [PubMed] [Google Scholar]
- 7.Bleday R. Local excision of rectal cancer. World J Surg 1997; 21: 706–714. [DOI] [PubMed] [Google Scholar]
- 8.Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut 1977; 18: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodsky JT, Richard GK, Cohen AM, et al. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 1992; 69: 322–326. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg D, Paty PB, Guillem JG, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum 1999; 42: 881–885. [DOI] [PubMed] [Google Scholar]
- 11.Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum 2000; 43: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg 1999; 230: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000; 92: 388–396. [DOI] [PubMed] [Google Scholar]
- 14.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–715. [DOI] [PubMed] [Google Scholar]
- 15.Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum 1997; 40: 388–392. [DOI] [PubMed] [Google Scholar]
- 16.Bouvet M, Milas M, Giacco GG, et al. Predictors of recurrence after local excision and postoperative chemoradiation therapy of adenocarcinoma of the rectum. Ann Surg Oncol 1999; 6: 26–32. [DOI] [PubMed] [Google Scholar]
- 17.Ota DM SJ, Rich TA. M. D. Anderson Cancer Center experience with local excision and multimodality therapy for rectal cancer. Surg Oncol Clin North Am 1992; 1:147–152.
- 18.DeCosse JJ, Wong RJ, Quan SH, et al. Conservative treatment of distal rectal cancer by local excision. Cancer 1989; 63: 219–223. [DOI] [PubMed] [Google Scholar]
- 19.Baron PL, Enker WE, Zakowski MF, et al. Immediate vs. salvage resection after local treatment for early rectal cancer. Dis Colon Rectum 1995; 38: 177–181. [DOI] [PubMed] [Google Scholar]
- 20.Wagman R, Minsky BD, Cohen AM, et al. Conservative management of rectal cancer with local excision and postoperative adjuvant therapy. Int J Radiat Oncol Biol Phys 1999; 44: 841–846. [PubMed] [Google Scholar]
- 21.Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum 2001; 44: 1345–1361. [DOI] [PubMed] [Google Scholar]
- 22.Bailey HR, Huval WV, Max E, et al. Local excision of carcinoma of the rectum for cure. Surgery 1992; 111: 555–561. [PubMed] [Google Scholar]
- 23.Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of Radiation Therapy Oncology Group protocol 89–02. Int J Radiat Oncol Biol Phys 2000; 46: 313–322. [DOI] [PubMed] [Google Scholar]
- 24.Hager T, Gall FP, Hermanek P. Local excision of cancer of the rectum. Dis Colon Rectum 1983; 26: 149–151. [DOI] [PubMed] [Google Scholar]
- 25.Varma MG, Rogers SJ, Schrock TR, et al. Local excision of rectal carcinoma. Arch Surg 1999; 134: 863–867. [DOI] [PubMed] [Google Scholar]
- 26.Russell AH, Harris J, Rosenberg P, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of Radiation Therapy Oncology Group protocol 89–02. Int. J. Radiation Oncology Biol Phys 2000; 46: 313–322. [DOI] [PubMed] [Google Scholar]
- 27.Steele GD, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol 1999; 6: 433–441. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg D, Paty PB, Picon AI, et al. Stage I rectal cancer: identification of high-risk patients. J Am Coll Surg 1998; 186: 574–580. [DOI] [PubMed] [Google Scholar]
- 29.Paty PB, Enker WE, Cohen AM, et al. Treatment of rectal cancer by low anterior resection with coloanal anastomosis. Ann Surg 1995; 219: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nascimbeni R, Burgart L, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002; 45: 200–206. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg 1998; 175: 360–363. [DOI] [PubMed] [Google Scholar]