Abstract
Objective
To evaluate the authors’ experience with gastric transposition as a method of esophageal replacement in children with congenital or acquired abnormalities of the esophagus.
Summary Background Data
Esophageal replacement in children is almost always done for benign disease and thus requires a conduit that will last more than 70 years. The organ most commonly used in the past has been colon; however, most series have been fraught with major complications and conduit loss. For these reasons, in 1985 the authors switched from using colon interpositions to gastric transpositions for esophageal replacement in infants and children.
Methods
The authors retrospectively reviewed the records of 41 patients with the diagnoses of esophageal atresia (n = 26), corrosive injury (n = 8), leiomyomatosis (n = 5), and refractory gastroesophageal reflux (n = 2) who underwent gastric transposition for esophageal replacement.
Results
Mean ± SE age at the time of gastric transposition was 3.3 ± 0.6 years. All but two transpositions were performed through the posterior mediastinum without mortality or loss of the gastric conduit despite previous surgery on the gastric fundus in 8 (20%), previous esophageal operations in 15 (37%), and previous esophageal perforations in 6 (15%) patients. Complications included esophagogastric anastomotic leak (n = 15, 36%), which uniformly resolved without intervention; stricture formation (n = 20, 49%), all of which no longer require dilation; and feeding intolerance necessitating jejunal feeding (n = 8, 20%) due to delayed gastric emptying (n = 3), feeding aversion related to the underlying anomaly (n = 1), or severe neurological impairment (n = 4). No redo anastomoses were required.
Conclusions
Gastric transposition reestablishes effective gastrointestinal continuity with few complications. Oral feeding and appropriate weight gain are achieved in most children. Therefore, gastric transposition is an appropriate alternative for esophageal replacement in infants and children.
The majority of esophageal procedures performed in infants and children are done for congenital esophageal atresia or acquired caustic strictures. With the former, the vast majority (92–97%) can be corrected without difficulty by primary esophagoesophagostomy. 1,2 Successful esophageal anastomoses may even be performed in those few with “long gap” esophageal atresia, defined as a distance of more than 3 cm between the proximal and distal esophageal remnants, with use of circular myotomies, serial preoperative proximal and distal pouch dilation, and other lengthening techniques. 3–7 Preservation of the native esophagus is desirable and can be achieved in most cases. However, some patients with long gap esophageal atresia will require esophageal replacement. In addition, a number of those patients who are managed with primary repair will require an esophageal substitution as a result of complications of the primary procedure or development of refractory gastroesophageal reflux, persistent stricture, and/or esophageal dysfunction. In those patients, preservation of the esophagus may be futile.
Caustic injuries represent the second most common reason for esophageal replacement in children. Despite enhanced public education, safer packaging, and reduction in the concentration of sodium hydroxide in the most commonly used drain cleaner fluids, caustic injuries to the esophagus continue, especially in the less developed parts of the world, though at a lower rate; the result is the formation of strictures, which can usually be managed with serial dilation. 8 However, 59% of severe caustic injuries will result in long and sometimes multiple strictures that are refractory to serial dilation. 9 The only option in these patients for restoration of esophagogastric continuity is esophageal replacement.
Alternatives for esophageal replacement in infants and children in the past have included a right or left colon interposition, formation of a gastric tube, and a jejunal interposition. All of these have advantages and disadvantages related to short- and long-term complications (Table 1). In 1980, Atwell et al described the use of the stomach as a replacement for the esophagus in six children, all but one of whom were newborns with congenital atresia of the esophagus. 10,11 This was followed in 1987 with a review by Spitz et al, from the United Kingdom, of gastric transpositions performed in 34 infants, 32 of whom had esophageal atresia. 12 Graft survival was 100% and outcome was excellent in 81% of the surviving patients. However, adoption of this technique in the United States has been slow, with few pediatric surgeons performing the procedure.
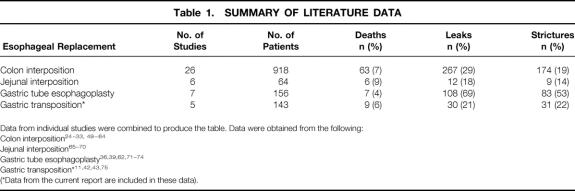
Table 1. SUMMARY OF LITERATURE DATA
Data from individual studies were combined to produce the table. Data were obtained from the following:
Colon interposition 24
Jejunal interposition 65
Gastric tube esophagoplasty 36,39,62,71
Gastric transposition*11,42,43,75
(*Data from the current report are included in these data).
In 1985, we switched from using colon interpositions to gastric transpositions for esophageal replacement in infants and children. Over the last 17 years we have performed 21 of these procedures at the C.S. Mott Children’s Hospital and have been involved in an additional 20 performed at other centers. The purpose of this report is to review our experience with these 41 gastric transpositions and to assess the advantages and disadvantages of this approach compared with alternative conduits.
METHODS
We retrospectively reviewed the records of 41 patients who underwent gastric transposition for esophageal reconstruction between 1985 and 2001 for data regarding demographics, initial esophageal disease, previous treatment, the specifics of the gastric transposition procedure, complications, and follow-up. Internal Review Board (IRB) approval or exemption for IRB review was obtained for all centers in the United States. Approximately half of the operations were performed at the University of Michigan (n = 21). The senior author (A.G.C.) performed or oversaw all operations performed at one of seven additional centers: Hadassah University Hospital in Israel (n = 2), Children’s Hospital of Wisconsin in Milwaukee (n = 4), Children’s Hospital of Los Angeles (n = 2), Baby’s Hospital at Columbia in New York (n = 1), Haemek Hospital in Israel (n = 6), Soroka Medical Center in Israel (n = 2), and Ichilov Medical Center in Israel (n = 3). Data were collected at the individual centers and sent to the University of Michigan without identifiers for analysis.
Technique of Gastric Transposition
The gastrointestinal tract is prepared so that the colon is available should the gastric conduit prove to be unacceptable. The patient is placed in the supine position with the left chest elevated on a longitudinal roll. The abdomen, chest, neck, and left arm are prepped and draped. A left subcostal incision is performed. If present, the gastrostomy is taken down and closed. Likewise, if a fundoplication has been performed, it is dismantled before proceeding with the transposition. The greater omentum is divided, taking great care to maintain the gastroepiploic arcade. The left gastric artery is test-occluded with a Heifitz clamp and then divided and ligated. The right gastric artery is identified and preserved. An extensive Kocher maneuver is performed to mobilize the duodenum. A pyloromyotomy is then performed. The esophageal hiatus is then opened to allow easy passage of the stomach, and the initial dissection of the lower esophagus is performed bluntly. A left cervical incision is made and the sternocleidomastoid muscle is retracted laterally along with the carotid artery and internal jugular vein in order to identify the esophagus. If an esophagostomy is present, it is mobilized for a distance of 2 to 3 cm. If possible, the recurrent laryngeal nerve is identified and preserved on both sides. The cervical esophagus is encircled, and using blunt dissection, the cervical, thoracic, and abdominal esophagus are mobilized. The cervical esophagus is divided and a 28 French chest tube is sutured to the distal cervical esophagus as the esophagus, along with end of the chest tube, is delivered into the abdomen. In patients with esophageal atresia, in whom there has been no esophageal bed, the mediastinum is bluntly dissected until a path is created between the cervical incision and the esophageal hiatus. The gastroesophageal junction is divided with the stapler and the staple line oversewn. The highest point of the fundus is sutured to the chest tube and then brought up through the hiatus and chest to the cervical incision. The apex of the stomach should be under minimal tension (Fig. 1). Mobilization of the left triangular ligament of the liver to provide a shorter path to the neck may be required if tension on the conduit is excessive. The esophagus is then sutured to the sternocleidomastoid and strap muscles; we believe this is important to prevent slippage of the esophagogastric anastomosis into the mediastinum. A gastrotomy is then created and a single-layer anastomosis is performed between the apex of the fundus and the distal cervical esophagus. A Penrose drain is placed in the cervical incision and the platysma and skin of the neck and the fascia and skin of the abdomen are closed. A contrast study of the conduit is obtained on the seventh postoperative day (Fig. 2).
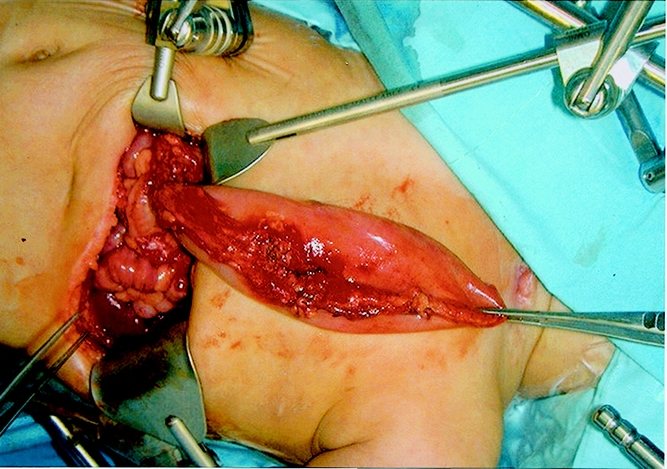
Figure 1. The gastric transposition lying over the chest. Note the length that can be achieved with this conduit. The patient’s neck is to the right in this picture.
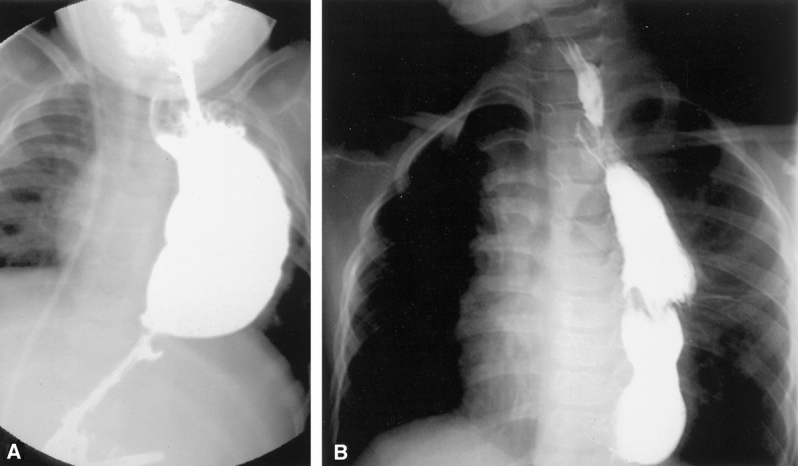
Figure 2. Contrast esophagram after gastric transposition placed through the left chest (A) or via a posterior mediastinal route (B). With the latter, the mediastinum contains the conduit, making it more tubular with less distention.
RESULTS
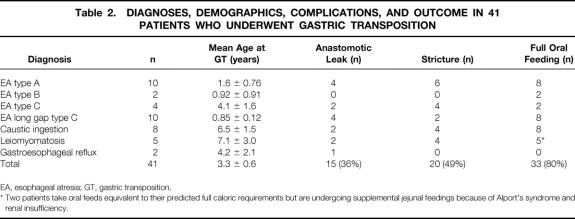
Between 1935 and 2002, 554 patients with esophageal atresia were managed at the C.S. Mott Children’s Hospital/University of Michigan Health System. A gastric transposition was performed in 21 of these patients. Gastric transpositions were performed in an additional 20 patients at other centers. The majority of the patients had a diagnosis of esophageal atresia (n = 26), with other diagnoses including corrosive injury of the esophagus with severe stricture formation (n = 8), leiomyomatosis (n = 5), and refractory gastroesophageal reflux (n = 2) (Table 2). Mean ± SE age at the time of gastric transposition was 3.3 ± 0.6 years (range 1 month to 15 years) for all patients and 1.7 ± 0.4 for those with esophageal atresia (range 1 month to 8.7 years). Of the patients with esophageal atresia, 4 were standard Gross type C with a distal fistula, 10 were long gap type C, 2 were long gap type B with a proximal fistula only, and 10 were type A pure esophageal atresia without a fistula. Table 3 shows the demographic, operative, complication, and outcome data for the patients with esophageal atresia who underwent gastric transposition. The esophagus was abandoned in four patients with standard type C esophageal atresia with a distal tracheoesophageal fistula because of esophageal dehiscence and leak in two patients, multiple anomalies with severe respiratory failure in a third, and a dysfunctional esophagus in a 9-year-old following primary repair of an esophageal atresia and subsequent Nissen fundoplication due to recurrent gastroesophageal reflux. All of the long gap type C patients were referred to the C.S. Mott Children’s Hospital with a cervical esophagostomy after receiving their initial care at another institution. Thus, none of these patients was a candidate for primary repair of the esophagus. Since 1989, our approach has been to perform a gastric transposition in patients with type A pure esophageal atresia without fistula, if indicated, as early as 1 month of age. However, only two type A esophageal atresia patients were primarily managed by us with this approach during that time period; primary anastomosis was performed in the majority of the type A patients. Five of the esophageal atresia patients had previous failed attempts at placement of a conduit (three colon interpositions and two substernal gastric transpositions). The latter two conduits constituted the only ones in our series that were placed in the substernal location. They were initially created at another institution and referred to us because of refractory strictures. These gastric transpositions were approached via a median sternotomy with resection of the esophagogastric anastomosis and successful reestablishment of the esophagogastric anastomosis in the neck. Of the three previous colon interpositions, two were performed at other institutions.
Table 2. DIAGNOSES, DEMOGRAPHICS, COMPLICATIONS, AND OUTCOME IN 41 PATIENTS WHO UNDERWENT GASTRIC TRANSPOSITION
EA, esophageal atresia; GT, gastric transposition.
* Two patients take oral feeds equivalent to their predicted full caloric requirements but are undergoing supplemental jejunal feedings because of Alport’s syndrome and renal insufficiency.
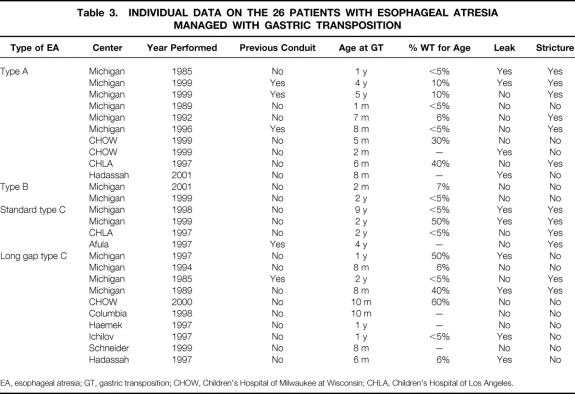
Table 3. INDIVIDUAL DATA ON THE 26 PATIENTS WITH ESOPHAGEAL ATRESIA MANAGED WITH GASTRIC TRANSPOSITION
EA, esophageal atresia; GT, gastric transposition; CHOW, Children’s Hospital of Milwaukee at Wisconsin; CHLA, Children’s Hospital of Los Angeles.
Eight patients underwent gastric transposition because of lye ingestion and the development of long strictures refractory to dilation. One of these patients was managed at the C.S. Mott Children’s Hospital; the remainder were cared for at the various other centers. One of these latter patients had undergone a right colon interposition that failed because of ischemia and graft loss. Five patients were diagnosed with esophageal dysfunction and leiomyomas of the esophagus. Two were brothers, both with accompanying Alport’s syndrome. Finally, two patients with gastroesophageal reflux were managed with esophagectomy and gastric transposition. The first was a patient with congenital diaphragmatic hernia who had four previously failed Nissen fundoplications and developed a dilated, dysfunctional esophagus. The second was a patient with severe esophagitis and a refractory esophageal stricture.
As mentioned above, there were five previous esophageal replacement procedures performed in five patients, including a retrosternal gastric transposition in two who developed refractory strictures, and two right colon interpositions, and a left colon interposition, all three of which failed due to development of ischemia. In addition, six of the patients in whom gastric transposition was performed had a history of a previous esophageal perforation and five had previous empyemas. Previous esophageal operations were performed on 15 (37%) patients. Despite these prior thoracic and mediastinal operations and complications, blunt esophagectomy was successfully performed and the gastric transposition placed via the posterior mediastinum in all of these patients.
Combined abdominal and cervical incisions alone were used in the majority of patients (n = 30), although a thoracoabdominal incision was used in four patients and a separate thoracotomy incision combined with abdominal and cervical incisions in five. The majority of the thoracotomy and thoracoabdominal incisions were used in our early experience with this technique, mostly in patients with previous posterior mediastinal surgery. A median sternotomy approach was used in two patients as mentioned previously. Except for these two latter cases, all conduits were placed in the posterior mediastinum via the left chest (n = 3), the right chest (n = 3), or the resected esophageal bed (n = 33). A cervical esophagogastric anastomosis was performed in 40 patients. In one of the very early patients, an esophagoesophagostomy was formed in the right chest. A pyloromyotomy was performed in 28 patients and a pyloroplasty in 4. Of the remaining nine patients, eight had had a previous gastric drainage procedure. A jejunostomy tube was placed in four patients.
There were no deaths and no loss of the gastric conduit despite previous fundoplications performed in eight patients (20%), including one Collis-Nissen. Small leaks from the esophagogastric anastomosis were noted in 15 patients (36%), and all uniformly resolved without intervention. Mediastinitis did not occur in any patient. Anastomotic strictures (defined as requiring one or more dilations) formed in 20 patents (49%). However, none of these patients currently require dilations. Only eight patients required more than three dilations, and none of the anastomoses were revised. Immediate postoperative complications included three patients with vocal cord paresis, two with effusions, and one with pneumonia.
Follow-up was from 5 months to 18 years with a mean of 6.5 ± 0.8 years. Delayed gastric emptying was initially observed in five (12%) patients. Feeding intolerance necessitating jejunal feeding at last follow-up was observed in eight patients (20%) and was due to delayed gastric emptying in three, severe neurological impairment in four, and feeding aversion related to esophageal atresia in one patient. Weight at last follow-up was available for 20 of the 26 esophageal atresia patients. Eight of these patients were below the fifth percentile for weight for age. Two patients with leiomyomatosis take oral feeds equivalent to their predicted full caloric requirements but are undergoing supplemental jejunal feedings because of Alport’s syndrome and renal insufficiency. Otherwise, all patients with leiomyomatosis or lye ingestion are on full feeds and thriving. Only one patient demonstrated symptoms compatible with dumping syndrome, which has resolved. There were no respiratory symptoms encountered, and only one case of pneumonia occurred in the postoperative period. Almost all patients have undergone endoscopy of the cervical esophagus and stomach. Esophagitis has not been noted in any of these patients.
DISCUSSION
The findings in this study confirm that gastric transposition is an effective replacement for the esophagus. Short-term complication rates are relatively low, and there are few long-term complications. There were no respiratory physiologic problems associated with performance of a gastric transposition, even in infants.
The approach to the patient with long gap esophageal atresia is controversial and without a perfect solution. As such, a number of strategies for management have been developed. Most surgeons agree that the native esophagus serves as the best conduit and should be salvaged whenever reasonable. Studies have suggested that this can be accomplished in most newborns with long gap esophageal atresia. Mahour et al applied the technique of bougienage of the proximal pouch once or twice daily along with periodic radiographic evaluation of the distance between the upper and lower esophageal segments, demonstrating growth of both esophageal segments over a 4- to 13-week period. 6 Successful esophagoesophagostomy was achieved in all 12 of their patients. The incidence of anastomotic leak, stricture, and gastroesophageal reflux with this approach was high, with subsequent frequent fundoplication and occasional anastomotic stricture resection. Nevertheless, most patients ultimately did well. 2,13–16 The high incidence (almost 100% in patients with long gap disease) of gastroesophageal reflux observed in these patients was often managed with a Nissen fundoplication, which can aggravate the already present swallowing difficulties seen in these patients with a dysfunctional esophagus. 17 In fact, esophageal replacement was performed in one patient in our series because of the development of refractory esophageal dysfunction following a Nissen fundoplication for gastroesophageal reflux.
A number of techniques are used to aid in primary repair of the widely separated proximal and distal esophageal segments. Proximal and distal circular myotomies may help to achieve primary anastomosis in the setting of long gap esophageal atresia, although ballooning of the myotomy, diverticulum formation, and altered esophageal motility may necessitate esophageal replacement. 18–20 Foker et al demonstrated successful approximation in those patients with esophageal atresia and gaps as long as approximately 7 cm by placing temporary sutures in the esophageal ends and applying increasing external traction over 6 to 10 days. 16 Kimura et al applied a multistaged extrathoracic esophageal prolongation technique in which the proximal esophagus was translocated to the subcutaneous tissues of the anterior chest wall and serially elongated. 5 Successful anastomosis was achieved in all patients. Scharli recommends a transverse stapling of the body of the stomach to allow elongation of the lesser gastric curve, thus allowing transposition of the lower esophagus into the chest for esophagoesophagostomy. 21 However, the Kimura and Scharli techniques have been done in a small number of patients with a large number of complications and even the necessity for subsequent replacement (personal communication).
Although the long gap esophagus can usually be successfully salvaged, a few cannot be put together primarily. Even if a primary anastomosis is accomplished, often under significant tension, complications of the initial procedure may result in severe stricture formation and refractory gastroesophageal reflux ultimately leading to esophageal dysfunction. Our series represents a few of the most complicated cases from eight centers. In fact, only 5 of the 14 patients with esophageal atresia managed at the C.S. Mott Children’s Hospital underwent their primary operation at our center; all others were referred following an initial operation. In reality, therefore, referral centers treating newborns with complex esophageal atresia will be faced with managing patients with complex forms of this anomaly, many of whom will require esophageal replacement.
In adults, esophageal replacement is performed for malignancy in 74% of cases. 22 In contrast, such a procedure is applied to children uniformly for benign disease. Therefore, the conduit must maintain excellent function for a lifetime. The colon interposition as initially described by Waterston et al has been the most popular operation for esophageal replacement in children. 23 Colonic conduits are effective when placed through the left chest, the bed of the resected esophagus, or in the substernal position (see Table 1). 24–33 In most of these series, however, significant graft loss or death, along with the problems of redundancy of the distal colon, has been seen. Even adenocarcinoma in the conduit has been observed. 34 The colon is also prone to gastroesophageal reflux. Finally, a colon interposition is a more complex endeavor than a gastric transposition.
An interesting solution to the discontinuous esophagus is the reverse gastric tube, which was popularized by Anderson and Randolph 35 and Burrington and Stephens. 36 The gastric tube remains narrow, does not become redundant, and serves most children well. However, gastric tubes are associated with a significant incidence of graft failure as well as deaths related to pulmonary aspiration and leaks from the esophagogastric anastomosis. 37 Development of mediastinitis from anastomotic leaks and the need for stricture resection exist in most series. 35,36,38,39 Gastroesophageal reflux and peptic ulcer formation are additional problems. 37,39,40 The gastric tube in infants is also associated with decreased capacity of the stomach, which appears to resolve over the first 3 postoperative months. 41
In 1987, Spitz et al reported their experience with 34 infants who underwent a gastric transposition for esophageal replacement, 32 of whom had esophageal atresia. 12 Twenty-seven of the infants had a long gap that prevented initial primary anastomosis and five infants had disruption of the anastomosis such that the native esophagus had to be abandoned. The authors demonstrated excellent results, with a mortality of 9%. Although two of the three deaths occurred within 48 hours of the gastric transposition, all of the deaths were respiratory-related and in patients with severe preoperative respiratory insufficiency. There was no graft failure. Four children developed esophagogastric anastomotic strictures that resolved with dilation. Two small anastomotic leaks were noted and resolved spontaneously. An excellent result was noted in 25 children; in 4 there was mild dysphagia. The majority of the children had excellent weight gain. A similar experience with gastric transposition was reported by Valente et al 42 and Marujo et al. 43 Based on these successful reports and our initial success, we began to use the gastric transposition for esophageal replacement. The majority of our patients (n = 26, 63%) had esophageal atresia.
We did not experience any respiratory symptoms in our patients. Because of the potential for the stomach in the chest to compromise respiratory status, Davenport et al evaluated respiratory status in 17 children 5 years after gastric transposition. 44 All but 1 of the 17 children had lung function values that were lower than the predicted values; median total lung capacity was 68% and median forced vital capacity was 64%. Interestingly, the pulmonary function in children who had a primary gastric transposition was better than in those who had complicated thoracic procedures before the gastric transposition, suggesting that the underlying lung disease, rather than the stomach itself, might be the cause for the observed decrease in pulmonary function. Early in our experience we used either the right or left chest for the gastric conduit. Subsequently, we have preferred the transmediastinal route. This is likely advantageous from a respiratory point of view since the stomach is confined within the mediastinum. Figure 2 demonstrates the difference in the size of the gastric conduit in two children who underwent a left transpleural and a transmediastinal gastric transposition. We found esophagectomy with transmediastinal gastric transposition to be safe despite a history of multiple previous esophageal operations, development of empyema following failed colon interpositions, perforations as a complication of esophageal dilation, and the absence of a native esophagus, such as in pure esophageal atresia, treated with a primary gastric transposition. Thus, our experience suggests that transhiatal esophagectomy is relatively safe even in the scarred mediastinum.
Previous gastric procedures have often been considered a contraindication to gastric transposition. However, in our series, eight patients underwent previous fundoplications for reflux. One of these eight had a Collis-Nissen performed after a previous Nissen fundoplication. Yet in all, the stomach proved to be a viable and effective conduit despite multiple operations and questions of adequate blood supply and associated scarring.
A vagotomy is an inherent part of an esophagectomy and gastric transposition. Likely as a result of the vagotomy, we initially experienced delayed gastric emptying after gastric transposition in five patients. However, only three patients had persistent delayed gastric emptying at last follow-up. Davenport et al demonstrated that rapid emptying occurred from the intrathoracic stomach within 5 minutes of ingestion. 44 Erythromycin may enhance the early postoperative function of the transposed stomach. 45 Eight of 20 patients (40%) with esophageal atresia had weights less than 5% predicted for age. None of the patients with other etiologies for their esophageal dysfunction demonstrated low weights for age except the two patients with Alport’s syndrome.
One unusual feature of our series is the five patients with leiomyomatosis of the esophagus. Successful esophagectomy with gastric transposition was performed in all. Only 24 cases of diffuse esophageal leiomyomatosis have been reported in children less than 14 years of age. 46 Most patients have required resection of the esophagus.
Although we have not observed esophagitis in any of our patients, the long-term risk of neoplasia and development of cervical esophageal malignancy is unclear. The only study addressing this issue is the one by Lindahl et al, in which they systematically biopsied the cervical esophagus in 14 patients more than 2 years following gastric tube reconstruction of the esophagus. 47 Barrett’s esophagus was found in 10 patients and was confirmed histologically in 8. As such, long-term follow-up with routine surveillance is required, especially into adulthood. Guidelines for patients with Barrett’s esophagus without dysplasia suggest performance of endoscopy every 2 to 3 years. 48 Extrapolation of these guidelines to the patient with a gastric transposition would not be unreasonable.
In conclusion, the gastric transposition establishes effective gastrointestinal continuity with few long-term complications. Oral feedings and appropriate weight gain are achieved in most children. Therefore, the gastric transposition is an appropriate alternative for esophageal replacement in infants and children. 49–75
DISCUSSION
Dr. Moritz M. Ziegler (Boston, MA): Dr. Hirschl and the Ann Arbor group have once again contributed significantly to our understanding of esophageal surgery in children. It has been literally more than 60 years since Dr. Haight successfully did the first esophageal atresia repair. Now we have heard another addition to the armamentarium of esophageal replacement: namely, gastric transposition or interposition. I wanted to ask Dr. Hirschl several questions regarding both the approach and the technique.
This has been predominantly a remedial series of taking a group of patients who had previous complications of their esophageal surgery in the majority of cases, some 70% I think, and then done this gastric interposition. Now that the technique is so standardized, if you had a child with pure atresia without a fistula, would you consider doing such a gastric interposition without first doing a cervical esophagostomy by simply doing this procedure primarily in a younger age group? Would this technique actually be amenable to the current vogue in pediatric surgery: namely, the minimally invasive approach to esophageal atresia repair?
Though you have reported that the patients eventually achieve oral feedings, there in fact is about a third of the patients in this series that have a prolonged problem with either feeding aversion or their complete transition to oral feeds. Has that changed your approach to the operation such that you would consider placing a feeding tube jejunostomy at the time of the original procedure?
The final question is related to the long-term follow-up. As you correctly stated in the manuscript, this is an esophageal replacement that has to serve the child for their life, maybe 70-plus years. In the face of doing an emptying procedure and in the face of doing a potential vagotomy, what would you recommend for the long-range follow-up for these patients? If they are achlorhydric or if they reflux, do they require additional esophagogastric surveillance as they grow older?
Presenter Dr. Ronald B. Hirschl (Ann Arbor, MI): With regard to creating a cervical esophagostomy, our approach has changed over the last decade. A number of centers are now performing daily bougienage over the first 3 months in an attempt to lengthen the proximal esophagus in hopes of performing a primary anastomosis. We use that approach as well and thus are avoiding a cervical esophagostomy. We take the patients to the operating room in the first 3 months and try to achieve a primary anastomosis. If we find that, in fact, we cannot safely accomplish a primary anastomosis, then we would perform a gastric transposition.
Should we perform a gastric transposition using a minimally invasive approach? The procedure is fairly extensive and technically demanding, especially with regard to the dissection of the esophagus. At the University of Michigan we apply minimally invasive approaches to as many situations as are possible. However, at this stage I do not believe that our technology is ready for us to perform this technique in a minimally invasive fashion in newborns and infants.
Feeding aversion is a problem, especially in the infants and children who have had esophageal atresia and have not been feeding over the first months or years of life. I would tend to place a feeding tube if I had a patient who was not going to be feeding in the near term. In fact, there were four patients in the series who had jejunostomy feeding tubes placed during the esophageal replacement. There were also a number of patients who required placement of jejunostomy feeding tubes after the gastric transposition. So, a feeding tube should be considered at the time of the gastric transposition.
There are studies which have suggested, especially in adults, that there may be development of esophageal metaplasia or Barrett’s esophagus in the esophageal remnant. As such, long-term follow-up is important. In childhood it is critical to follow these patients to ensure that they are growing and developing well. In our series approximately 40% of the esophageal atresia patients were at less than the fifth percentile for their weight for their age. So, close follow-up is required to ensure that appropriate growth occurs.
Dr. John E. Foker (Minneapolis, MN): We agree completely that the goal should be 70-plus years, but that is a very stringent requirement. When the esophagus is to be replaced in children, there are four major considerations. The first is that the operation can be done relatively free of early, compromising complications The gastric pull-up appears to meet this requirement. But under the constraints of the 70-year goal, ease of doing an operation does not provide sufficient justification for its use. The next three requirements will be harder to meet. The operation should produce no bad physiological consequences. For children, the main requirement is adequate nutrition for good growth and development. As presented by DeMeester and colleagues earlier in this meeting, the gastric pull-up produces the unfortunate combination of dumping and gastric retention. A pyloroplasty seems necessary and the displaced stomach becomes a conduit with a rapid transit time, all of which makes nutrition difficult. And as your data show, at least 20% are below the fifth percentile in weight.
Your abstract stated that 32% of these children are on jejunal feedings. What percentage of them have feeding tubes in place? What prevents these children from eating sufficiently? What is the average number of feedings per day? What do you predict the percentage will be that will be eventually free of tube feedings?
Pulmonary consequences also occur. As you know, Davenport found all of the children had significantly decreased lung volumes. In addition, aspiration appears to be common. Do these children sleep with the head of the bed elevated? Are you concerned about the effects on the lungs of chronic aspiration?
Another main requirement is to minimize the adverse psychological consequences. Your patients apparently have spit fistulas and G-tubes for 2 to 3 years. After repair, the feeding tubes, the continuing supplemental feedings, as well as medications and surveillance, contribute to a state of chronic therapy, if not outright illness. This is not conducive to optimal emotional development.
The final and very important requirement is that no pathological processes should be set in motion by the procedure. The lack of a functional GE junction means there will be obligatory reflux into the upper esophagus. Although no long-term pediatric study has surfaced for gastric transposition, there are data on the physiologically similar gastric tube. Lindahl looked at 14 patients who had a gastric tube for an average of 7 years and found 10 of 14 already had the precancerous Barrett’s changes in their upper esophagus.
As Dr. DeMeester has so elegantly defined, it is not just the acid reflux that is the problem, but the contribution of bile. Antacid therapy reduces the symptoms but not its most serious consequence.
My questions are: What percentage of these children remain on antacids? Are you treating for H. pylori? Because there is no good antireflux procedure for the gastric transposition, can you prevent these changes? What method of surveillance are you going to be using for Barrett’s esophagitis? And what do you plan to do if you find Barrett’s changes?
Another important consequence of stomach denervation will likely be atrophic gastritis. Lam, who looked at adult patients from 1 to 9 years after gastric pull-up, found 68% had moderate to severe atrophic gastritis. What are you doing in this regard to prevent anemia? For the longer term, do you have a surveillance strategy for evaluating changes in these stomachs?
The gastric pull-up would merit consideration if there were no good alternatives. Part of the pediatric surgical litany, however, is that one’s own esophagus is best. Five years ago, we presented to this Association a flexible approach which allows a true primary repair to be achieved throughout the entire esophageal atresia spectrum. A primary repair could be done even after failed prior operations and the bailout of a cervical esophagostomy.
The definition of a true primary repair remains an intact esophagus without myotomies and with the stomach and GE junction below the diaphragm where they belong. The definition is not satisfied by pulling the stomach into the chest and joining it to the upper esophageal pouch.
We have achieved a primary repair in 30 infants with gaps of 4 cm or longer, 14 with gaps of 6 cm or longer, and two with gaps of longer than 10 cm. All have been satisfactorily repaired, and no patient has been turned down.
There are two main components to our approach. First, we have shown that a well-constructed anastomosis will reliably withstand considerable tension. But with the finding that esophageal growth can be stimulated to occur in days, even more flexibility is achieved. For segments that can almost be brought together initially, 2 to 3 days of internal traction can be used. On reoperation, this will have induced sufficient growth to allow a primary anastomosis.
For the most severe gap lengths, including all of those who had a cervical spit fistula that had to be closed, external traction was used. Again, the growth response surprised us and within 8 to 18 days, these relative nubbins of tissue had grown to allow a very satisfactory true primary repair.
By using these techniques, we have achieved a true primary repair in all patients. The long gap repairs are begun when the infants weigh 3 to 4 kg. None have been excluded, no matter their previous history. The true primary repair has even been used for a long caustic stricture. An interposition graft has not been used since 1983. All of these children swallow satisfactorily, and only the second-order problems of oral aversion and in two cases food allergies have slowed the progression to a normal diet. We anticipate, in the absence of significant CNS problems, that all will eat normally and be without G-tubes.
We also stressed in our report to the Association that to achieve the 70-year good result, these patients would have to be followed for reflux. If the reflux is significant, and it usually is after a long gap repair, we believe an effective fundoplication is preferable to long-term medication. The ability to control reflux also sharply distinguishes a true primary repair from a gastric transposition.
My concluding question would be that given the ability to do a true primary repair across the entire EA spectrum, even after failed repairs, how can one justify setting in motion all of the issues associated with gastric transposition? A much better alternative is available, and the benefits of using the child’s own esophagus will only increase with time.
Dr. Ronald B. Hirschl (Ann Arbor, MI): We agree that one should try to salvage the native esophagus; that is the primary goal of all pediatric surgeons who manage these children. However, if you examine our series, the majority of the patients were first managed operatively at other institutions, followed by abandonment of the esophagus for one reason or another. For instance, in one patient an anastomotic dehiscence led to performance of a cervical esophagostomy. With another, the initial surgeon felt that the tension was too great for a successful esophagoesophagostomy. Finally, there were patients who developed long-term complications of a primary esophagoesophagostomy such as refractory stricture or severe esophageal dysfunction. So, although we agree that one should try to salvage the esophagus, those who are serving as a referral center for complicated esophageal atresia will end up managing patients in whom an esophageal replacement will likely be required. In fact, if you look at our series of 200 patients over the last 20 or 25 years, only 8 of the patients that required an esophageal replacement received their primary treatment at our institution.
We only observed dumping in one of our patients, and that has resolved. It is true that we observed failure to thrive, as defined by predicted weights less than 5%, in 40% of our esophageal atresia patients. But those patients are continuing to grow, most without supplemental feeds, and are following an appropriate growth curve.
Eight of our patients have feeding tubes in place to provide baseline caloric needs. Four of these are a result of neurologic delay and inability to adequately take oral feeds. Three patients have delayed gastric emptying and one has feeding aversion associated with an esophageal atresia. I don’t know the average number of feedings per day, but most patients are on full oral feedings.
The respiratory status has not appeared to be an issue in our patients. By bringing the stomach through the mediastinum, the stomach is constrained by the surrounding tissues. When we look at contrast studies, the stomach in the mediastinum is much more tubularized than is the stomach in the left or right chest. This may play a role in decreasing the respiratory effects that have been observed previously with gastric transpositions in children.
We have not had problems with aspiration. Pneumonias were limited to a couple of instances which occurred in the immediate postoperative period. I should note that Dr. Orringer has reviewed 1,085 adults with replacement of the esophagus using a gastric transposition due to nonmalignant disease. In 30% reflux was observed only if they lay down after a full meal, 7% required that the head of their bed be elevated, while only 1% had pulmonary pathology from aspiration.
Four of our patients were on H2 blockers. I believe that I already addressed the issue of long-term follow-up and surveillance.
Dr. John E. Foker (Minneapolis, MN): One quick comment. Among those patients that I was talking about, five came to us with left and four with right cervical fistulas. A number of these cases had abandoned attempts at a primary repair. All of that is part of what comes to a major referral center for esophageal atresia.
Dr. Tom R. DeMeester (Los Angeles, CA): I rise to discuss the issue that has surfaced regarding the long-term follow-up of these patients and the development of reflux disease. Often when the torch is passed from one discipline to another, much is lost in the transition. The principle is sound that you cannot put gastric acid-secreting mucosa next to squamous mucosa anywhere in the body and get away with it for long. We have seen carditis develop in the cervical esophagus in 24% of patients 3 or more years after esophagogastrectomy. This goes on to intestinal metaplasia in about 14% of the patients. I personally had a patient whose esophagus was replaced with his stomach at age 5 and subsequently developed an adenocarcinoma in the cervical esophagus later in life. I concur with you that you can use the stomach for reconstruction, and I commend you on your technical expertise. But is it wise to do so? Having heard the flavor of the three previous discussants, will you return home to scope your patients? It might be important to do so before you publish.
Dr. Ronald B. Hirschl (Ann Arbor, MI): As you note, the “torch-passing” is likely the most important part of the process. The complications that arise will likely be long term and will probably arise in adulthood. We have performed endoscopy on a number of children and have not observed esophagitis. However, there have been studies in which children underwent esophageal mucosal changes. So, these patients may be at risk for malignancy and need to receive appropriate long-term follow-up.
Dr. Arnold G. Coran (Ann Arbor, MI): I want to stand to just make two points to respond to Jack Foker and Tom DeMeester. There is no perfect substitute for the esophagus. We are not saying that the stomach is better than the colon, just that it works. With due respect to Jack, if one surveys most of the pediatric surgeons around the world, there is not one center in the world that is able to put every single esophageal atresia together. I wish that were true. So I commend you on being able to do that. But the rest of us can’t technically do that. As a result, we need something to replace the esophagus in a small number of cases. The question is: Is it going to be colon, which we have experience with in several centers around the country and around the world with lots of complications, even death, or stomach, which seems to work better? We are well aware of the long-term risks of doing this. But overall, if one weighs the pluses and minuses, in our minds, stomach is still better in this group of patients than colon.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Ronald B. Hirschl, MD, Section of Pediatric Surgery, 1500 East Medical Center Drive, F3970 CS Mott Children’s Hospital, Ann Arbor, MI 48109-0245.
E-mail: rhirschl@umich.edu
Accepted for publication April 24, 2002.
References
- 1.Manning P, Morgan R, Coran A, et al. Fifty years’ experience with esophageal atresia and tracheoesophageal fistula: beginnning with Cameron Haight’s first operation in 1935. Ann Surg 1986; 204: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healey P, Sawin R, Hall D, et al. Delayed primary repair of esophageal atresia with tracheoesophageal fistula. Arch Surg 1998; 133: 552–556. [DOI] [PubMed] [Google Scholar]
- 3.Livaditis A. Oesophageal atresia: a method of over bridging large segmental gaps. Z Kindershir 1973; 13: 298–306. [Google Scholar]
- 4.Brown A, Gough M, Nicholls G. Anterior flap repair of oesophageal atresia: A 16-year evaluation. Pediatr Surg Int 1995; 10: 525–528. [Google Scholar]
- 5.Kimura K, Nishijima E, Tsugawa C, et al. Multistaged extrathoracic esophageal elongation procedure for long gap esophageal atresia: Experience with 12 patients. J Pediatr Surg 2001; 36: 1725–1727. [DOI] [PubMed] [Google Scholar]
- 6.Mahour G, Woolley M, Gwinn J. Elongation of the upper pouch and delayed anastomotic reconstruction in esophageal atresia. J Pediatr Surg 1974; 9: 373–383. [DOI] [PubMed] [Google Scholar]
- 7.Brown A, Tam P. Measurement of gap length in esophageal atresia: A simple predictor of outcome. J Am Coll Surg 1996; 182: 41–45. [PubMed] [Google Scholar]
- 8.Hugh T, Kelly M. Corrosive ingestion and the surgeon. J Am Coll Surg 1999; 189: 508–522. [DOI] [PubMed] [Google Scholar]
- 9.Panieri E, Rode H, Millar A, et al. Oesophageal replacement in the management of corrosive strictures: when is surgery indicated? Pediatr Surg Int 1998; 13: 336–340. [DOI] [PubMed] [Google Scholar]
- 10.Lewis I. The surgical treatment of carcinoma of the esophagus. Br J Surg 1946; 34: 18–31. [DOI] [PubMed] [Google Scholar]
- 11.Atwell J, Harrison G. Observations on the role of esophagogastrostomy in infancy and childhood with particular reference to the long-term results and operative mortality. J Pediatr Surg 1980; 15: 303–309. [DOI] [PubMed] [Google Scholar]
- 12.Spitz L, Kiely E, Sparnon T. Gastric transposition for esophageal replacement in children. Ann Surg 1987; 206: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Hunt M, Fleet M, Wagget J. Delayed primary anastomosis for wide-effect esophageal atresia: a 17 year experience. Pediatr Surg Int 1994; 9: 21–23. [Google Scholar]
- 14.Puri P, Ninan G, Blake N, et al. Delayed primary anastomosis for esophageal atresia: 18 month to 11 years follow-up. J Pediatr Surg 1992; 27: 1127–1130. [DOI] [PubMed] [Google Scholar]
- 15.Ein S, Shandling B, Heiss K. Pure esophageal atresia: Outlook in the 1990s. J Pediatr Surg 1993; 28: 1147–1150. [DOI] [PubMed] [Google Scholar]
- 16.Foker J, Linden B, Boyle E, et al. Development of a true primary repair for the full spectrum of esophageal atresia. Ann Surg 1997; 226: 553–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheatley M, Coran A, Wesley J. Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair. J Pediatr Surg 1993; 28: 53–55. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl H, Louhimo I. Livaditis myotomy in long-gap esophageal atresia. J Ped Surg 1987; 22: 109–112. [DOI] [PubMed] [Google Scholar]
- 19.Ricketts R, Luck S, Raffensperger J. Circular esphagomyotomy for primary repair of long-gap esophageal atresia. J Pediatr Surg 1981; 16: 365. [DOI] [PubMed] [Google Scholar]
- 20.Otte J, Gianello F, Wese D, et al. Diverticulum formation after circular myotomy for esophageal atresia. J Pediatr Surg 1984; 19: 68–71. [DOI] [PubMed] [Google Scholar]
- 21.Scharli A. Esophageal reconstruction by elongation of the lesser gastric curvature. Pediatr Surg Int 1996; 11: 214–217. [DOI] [PubMed] [Google Scholar]
- 22.Orringer M, Marshall B, Iannettoni M. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999; 230: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman C, Waterston D. Oesophageal reconstruction in children using intrathoracic colon. Arch Dis Child 1957; 32: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azar H, Chrispin A, Waterston D. Esophageal replacement with transverse colon infants and children. J Pediatr Surg 1971; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 25.Schiller M, Frye T, Boles T. Evaluation of colonic replacement of the esophagus in children. J Pediatr Surg 1971; 6: 753–760. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers B, Ryckman F, Talbert J. Blunt transmediastinal total esophagectomy with simultaneous substernal colon interposition for esophageal caustic strictures in children. J Pediatr Surg 1981; 16: 184–189. [DOI] [PubMed] [Google Scholar]
- 27.Appignani A, Lauro V, Presipino M, et al. Intestinal bypass of the oesophagus: 117 patients in 28 years. Pediatr Surg Int 2000; 16: 326–328. [DOI] [PubMed] [Google Scholar]
- 28.Khan A, Stiff G, Mohammed A, et al. Esophageal replacement with colon in children. Pediatr Surg Int 1998; 1: 79–83. [DOI] [PubMed] [Google Scholar]
- 29.Stone M, Mahour G, Weitzman J, et al. Esophageal replacement with colon interposition in children. Ann Surg 1986; 203: 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdogan E, Emir H, Eroglu E, et al. Esophageal replacement using the colon: a 15 year review. Pediatr Surg Int 2000; 16: 546–549. [DOI] [PubMed] [Google Scholar]
- 31.Canty T, LoSasso B. One-stage esophagectomy and in situ colon interposition for esophageal replacement in children. J Pediatr Surg 1997; 32: 334–337. [DOI] [PubMed] [Google Scholar]
- 32.Rescorla F, West K, Scherer L III, et al. The complex nature of type A (long-gap) esphageal atresia. Surgery 1994; 116: 658. [PubMed] [Google Scholar]
- 33.Freeman N, Cass D. Colon interposition: A modification of the Waterston technique using the normal esophageal route. J Pediatr Surg 1982; 17: 17–21. [DOI] [PubMed] [Google Scholar]
- 34.Goyal M, Bang D, Cohen L. Adenocarcinoma arising in interposed colon: report of a case. Dis Colon Rectum 2000; 43: 555–558. [DOI] [PubMed] [Google Scholar]
- 35.Anderson K, Randolph J. The gastric tube for esophageal replacement in children. J Thoracic Cardiovasc Surg 1973; 66: 333. [PubMed] [Google Scholar]
- 36.Burrington J, Stephens C. Esophageal replacement with a gastric tube in infants and children. J Pediatr Surg 1968; 3: 246–252. [DOI] [PubMed] [Google Scholar]
- 37.Ein S, Shandling B, Simpson J, et al. Fourteen years of gastric tubes. J Pediatr Surg 1978; 13: 638–642. [DOI] [PubMed] [Google Scholar]
- 38.Ein S. Gastric tubes in children with caustic esophageal injury: A 32-year review. J Pediatr Surg 1998; 33: 1363–1365. [DOI] [PubMed] [Google Scholar]
- 39.Schettini S, Pinus J. Gastric-tube esophagoplasty in children. Pediatr Surg Int 1998; 14: 114–150. [DOI] [PubMed] [Google Scholar]
- 40.Anderson K, Randolph J, Lilly J. Peptic ulcer in children with gastric tube interposition. J Pediatr Surg 1975; 10: 701–707. [DOI] [PubMed] [Google Scholar]
- 41.Anderson K. Replacement of the esophagus. In: Welch K, Randolph JG, Ravitch MM, et al, eds. Pediatric Surgery. Chicago: Yearbook Medical Publishers, 1986: 704–712.
- 42.Valente A, Bereton R, Mackersie A. Esophageal replacement with whole stomach in infants and children. J Pediatr Surg 1987; 22: 913–917. [DOI] [PubMed] [Google Scholar]
- 43.Marujo W, Tannuri U, Maksoud J. Total gastric transposition: An alternative to esophageal replacement in children. J Pediatr Surg 1991; 26: 676–681. [DOI] [PubMed] [Google Scholar]
- 44.Davenport M, Hosie G, Tasker R, et al. Long-term effects of gastric transposition in children: A physiological study. J Pediatr Surg 1996; 31: 558–593. [DOI] [PubMed] [Google Scholar]
- 45.Collard J, Romagnoli R, Otte J, et al. Erythromycin enhances early postoperative contractility of the denervated whole stomach as an esophageal substitute. Ann Surg 1999; 229: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Federici S, Ceccarelli P, Bernardi F, et al. Esophageal leiomyomatosis in children: Report of a case and review of the literature. Eur J Pediatr Surg 1998; 8: 358–363. [DOI] [PubMed] [Google Scholar]
- 47.Lindahl H, Rintala R, Sariola H, et al. Cervical Barrett’s esophagus: a common complication of gastric tube reconstruction. J Pediatr Surg 1990; 25: 446–448. [DOI] [PubMed] [Google Scholar]
- 48.Speckler S. Barrett’s esophagus. N Engl J Med 2002; 346: 836–842. [DOI] [PubMed] [Google Scholar]
- 49.Gross R, Firestone N. Colonic reconstruction of the esophagus in infants and children. Surgery 1967; 61: 955. [PubMed] [Google Scholar]
- 50.Carneiro P, Doig C. Colon interposition for wide gap oesophageal atresia. East Africa Med J 1993; 70: 682. [PubMed] [Google Scholar]
- 51.Raffensperger J. Intestinal bypass of the esophagus. J Pediatr Surg 1996; 31: 38. [DOI] [PubMed] [Google Scholar]
- 52.Otherson H, Clatworthy H. Functional evaluation of esophageal replacement in children. J Thorac Cardiovasc Surg 1967; 53: 55. [PubMed] [Google Scholar]
- 53.Soave F. Intrathoracic transposition of the transverse colon in complicated esophageal atresia. Proc Pediatr Surg 1972; 4: 91. [PubMed] [Google Scholar]
- 54.Martin L. The use of colon for esophageal replacement in children. Aust NZ J Surg 1972; 42: 160. [DOI] [PubMed] [Google Scholar]
- 55.German J, Waterston D. Colon interposition for replacement of the esophagus in children. J Pediatr Surg 1976; 11: 227. [DOI] [PubMed] [Google Scholar]
- 56.Campbell J. Esophageal replacement in infants and children by colon interposition. Am J Surg 1982; 144: 29. [DOI] [PubMed] [Google Scholar]
- 57.Hendren W, Hendren W. Colon interposition for esophagus in children. J Pediatr Surg 1985; 20: 829. [DOI] [PubMed] [Google Scholar]
- 58.Rode H. Colonic esophageal replacement in children: functional results. Z Kindershir 1986; 41: 201. [DOI] [PubMed] [Google Scholar]
- 59.West K, Vane D, Grosfeld J. Esophageal replacement in children: experience with thirty-one cases. Surgery 1996; 100: 751. [PubMed] [Google Scholar]
- 60.Ahmed A, Spitz L. The outcome of colonic replacement of the esophagus in children. Prog Pediatr Surg 1996; 19: 37. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell I. Colon interposition in children. Br J Surg 1989; 76: 681. [DOI] [PubMed] [Google Scholar]
- 62.Lindahl H, Louhimo I, Virkola K. Colon interposition or gastric tube? Follow-up study of colon-esophagus and gastric tube-esophagus patients. J Pediatr Surg 1983; 18: 58–63. [DOI] [PubMed] [Google Scholar]
- 63.Dickson J. Esophageal substitution with colon: the Waterston operation. Pediatr Surg Int 1996; 11: 224–226. [DOI] [PubMed] [Google Scholar]
- 64.Ure B, Slany E, Eypasch E, et al. Long-term functional results and quality of life after colon interposition for long-gap oesophageal atresia. Eur J Pediatr Surg 1994; 5: 206–210. [DOI] [PubMed] [Google Scholar]
- 65.Bax N, Rovekamp M, Pull ter Gunne A, et al. Early one-stage orthotopic jejunal pedicle-graft interposition in long-gap esophageal atresia. Pediatr Surg Int 1994; 9: 483–485. [Google Scholar]
- 66.Spicer R, Cusick E. Oesophageal substitution by jejunal free graft: follow-up data and an evaluation. Pediatr Surg Int 1996; 11: 227–229. [DOI] [PubMed] [Google Scholar]
- 67.Ring W, Varco R, L’Heureux P, et al. Esophageal replacement with jejunum in children: An 18- to 33-year follow-up. J Thoracic Cardiovasc Surg 1982; 83: 918–927. [PubMed] [Google Scholar]
- 68.Jezioro Z, Kus H. Experiences with the retrosternal esophageal replacement employing jejunum or ileum. J Pediatr Surg 1958; 44: 275. [PubMed] [Google Scholar]
- 69.Saeki M. Long-term result of jejunal replacement of the esophagus. J Pediatr Surg 1988; 23: 483. [DOI] [PubMed] [Google Scholar]
- 70.Cusick E, Batchelor A, Spicer R. Development of a technique for jejunal interposition in long-gap esophageal atresia. J Pediatr Surg 1993; 28: 990. [DOI] [PubMed] [Google Scholar]
- 71.Cohen D, Middleton A, Fletcher J. Gastric tube esophagoplasty. J Ped Surg 1974; 9: 451–460. [DOI] [PubMed] [Google Scholar]
- 72.Anderson K, Randolph J. Gastric tube interposition: a satisfactory alternative to the colon for esophageal replacement in children. Ann Thorac Surg 1978; 25: 521. [DOI] [PubMed] [Google Scholar]
- 73.Goon H, Cohen D, Middleton A. Gastric tube oesophagoplasty–a long-term assessment. Z Kindershir 1985; 40: 21. [DOI] [PubMed] [Google Scholar]
- 74.Ein S. Twenty-one year experience with the pediatric gastric tube. J Pediatr Surg 1987; 22: 77. [DOI] [PubMed] [Google Scholar]
- 75.Spitz L. Gastric transposition for esophageal substitution in children. J Pediatr Surg 1992; 27: 252. [DOI] [PubMed] [Google Scholar]