Abstract
Objective
To review the effect of morbid obesity surgery on type 2 diabetes mellitus, and to analyze data that might explain the mechanisms of action of these surgeries and that could answer the question of whether surgery for morbid obesity can represent a cure for type 2 diabetes in nonobese patients as well.
Summary Background Data
Diabetes mellitus type 2 affects more than 150 million people worldwide. Although the incidence of complications of type 2 diabetes can be reduced with tight control of hyperglycemia, current therapies do not achieve a cure. Some operations for morbid obesity not only induce significant and lasting weight loss but also lead to improvements in or resolution of comorbid disease states, especially type 2 diabetes.
Methods
The authors reviewed data from the literature to address what is known about the effect of surgery for obesity on glucose metabolism and the endocrine changes that follow this surgery.
Results
Series with long-term follow-up show that gastric bypass and biliopancreatic diversion achieve durable normal levels of plasma glucose, plasma insulin, and glycosylated hemoglobin in 80% to 100% of severely obese diabetic patients, usually within days after surgery. Available data show a significant change in the pattern of secretion of gastrointestinal hormones. Case reports have also documented remission of type 2 diabetes in nonmorbidly obese individuals undergoing biliopancreatic diversion for other indications.
Conclusions
Gastric bypass and biliopancreatic diversion seem to achieve control of diabetes as a primary and independent effect, not secondary to the treatment of overweight. Although controlled trials are needed to verify the effectiveness on nonobese individuals, gastric bypass surgery has the potential to change the current concepts of the pathophysiology of type 2 diabetes and, possibly, the management of this disease.
Diabetes mellitus type 2 is an epidemic health problem, affecting more than 150 million people worldwide. This number is expected to double in the first decades of the third millennium. 1 Recently, evidence for reduction of complications of type 2 diabetes with tight control of hyperglycemia has been reported, 2 but current therapies, including diet, exercise, behavior modification, oral hypoglycemic agents, and insulin, rarely return patients to euglycemia. 3
Morbid obesity, in which patients exceed their ideal weight by at least 100 lb or are more than 200% of ideal body weight, is a condition with high mortality and morbidity because of its association with severe comorbid diseases such as hypertension, diabetes, hyperlipidemia, and cardiopulmonary failure. In these patients, surgery represents the most effective therapy in that it achieves significant and durable weight loss as well as resolution or amelioration of comorbidities. 4 Current indications for surgery in morbidly obese patients include body mass index (BMI) greater than 40 or greater than 35 if comorbidities are present. 5
Several operative procedures are performed for treatment of morbid obesity. Roux-en-Y gastric bypass (GBP) is usually done by dividing the stomach with a stapler to create a small gastric pouch, while the jejunum is divided 30 to 50 cm distal to the ligament of Treitz. The distal limb of the jejunum is then anastomosed to the small gastric pouch and a jejunojejunostomy is performed 50 to 150 cm distal from the gastrojejunostomy. Most studies report a weight loss of 60% to 70% of excess body weight. 6,7 In recent series, operative mortality ranges between 0% and 1.5%, 8–10 and the overall incidence of major complications, including anastomotic leaks, pulmonary embolus, and bowel occlusions, is between 0.6%11 and 6%. 12
Biliopancreatic diversion (BPD), introduced by Scopinaro in 1978, includes a gastric resection and diversion of the biliopancreatic juice to the terminal ileum to significantly reduce the absorption of nutrients. 13 In this operation, an enteroentero-anastomosis is performed between the proximal limb of the transected jejunum and ileum, 50 to 100 cm 14 proximally to the ileocecal valve. In a series of 2,241 patients reported by Scopinaro et al, the BPD resulted in a mean permanent reduction of about 75% of the initial excess weight, with an operative mortality of 0.5%. 15
Gastroplasties, which include gastric banding and vertical banded gastroplasty, reduce the volume of the stomach by annular banding or vertical stapling but without bypassing the proximal foregut. Up to 65% of excess weight loss at 5 years has been reported, 16 but there is considerable variation in results among different authors, and a significant number of patients require reoperation for inadequate weight loss. 17
Bariatric surgery is now increasingly being performed laparoscopically, resulting in a similar percentage of weight loss with respect to the open series 18,19 and reduced recovery time and perioperative complications. 20
EFFECT OF MORBID OBESITY SURGERY ON TYPE 2 DIABETES
GBP and BPD not only induce significant and durable weight loss but also determine amelioration or resolution of comorbid disease states, especially type 2 diabetes mellitus. 21–23 Sustained normal concentrations of plasma glucose, insulin, and glycosylated hemoglobin have been reported in 80% to 100% of morbidly obese diabetic patients managed surgically by GBP or BPD. 14,15,20,24 Long-term control of glycemia and normal levels of glycosylated hemoglobin have been documented in series with up to 14 years of follow-up. 24 These surgeries seems also to restore insulin sensitivity, 15,25,26 prevent the progression from impaired glucose tolerance to diabetes, 27 and reduce mortality from diabetes mellitus. 28 In the experience of Pories and Albrecht, the mortality risk from diabetes over a 10-year follow-up after gastric bypass was less than that in a cohort of diabetic patients matched for age, weight, and BMI who were not operated on (1.0% vs. 4.5% for every year of follow-up;P < .0003). 29
There is therefore evidence enough to say that obesity surgery is an effective form of therapy for type 2 diabetes, at least in the morbidly obese.
POSSIBLE MECHANISMS OF ACTION
One might think that surgically induced weight loss and decreased food intake are the most reasonable mechanisms for remission of diabetes after surgery for obesity, but most reported series do not support this explanation.
Role of Weight Loss
Most reported series show that return to euglycemia and normal insulin levels occur within days after surgery, long before there is any significant weight loss. 15,24,25 In 1995, Pories et al 24 reported the results of GBP in a series of 608 morbidly obese patients. Preoperatively, 146 patients were diabetic (type 2) and 152 had impaired glucose tolerance. GBP achieved normal levels of plasma glucose, insulin, and glycosylated hemoglobin in 83% of diabetic patients and in 98.7% of patients with impaired glucose tolerance within 4 months after surgery, without the need for any diabetic medication or special diet, and before any weight reduction occurred. In 1998, Scopinaro et al 15 reported normalization of glucose levels in 100% of their morbidly obese patients after BPD with no need for medication and on a totally free diet as early as 1 month after operation, when excess weight was still more than 80%. Hickey et al 25 demonstrated significantly lower levels of fasting plasma glucose, plasma insulin, and serum leptins in a group of patients maintaining stable weight after GBP compared to a group of patients matched in weight, age, and percentage of fat who did not undergo surgery. A clinical case described in detail by Pories and Albrecht 29 is instructive. This woman with a fasting blood glucose of 495 mg/dL despite daily administration of 90 units insulin underwent GBP. On the first day after surgery her blood glucose fell to 281 mg/dL and her insulin requirement was only 8 units. By day 6 she no longer required insulin, and she subsequently remained euglycemic without insulin or other hypoglycemic agents on a regular diet for the following years.
Role of Decreased Food Intake
If decreased food intake explains how the GBP and BPD procedures control diabetes, gastroplasties should be effective too, since these operations significantly lower food intake by reduction of gastric volume. Gastroplasties do indeed improve glucose metabolism, 30,31 but there is no evidence for long-term cure of diabetes in morbidly obese patients. Furthermore, vertical banded gastroplasty results in less reduction of hyperglycaemia and hyperinsulinemia than GBP does. 32 Also, patients undergoing BPD show only temporary food intake limitation; over time, their eating capacity is fully restored or even increased, 15 while blood glucose levels remain under control.
Other Possible Mechanisms Responsible for Diabetes Control
To understand what the mechanism of diabetes control might be, we should look at the anatomical and physiologic alterations that these surgeries cause. GBP and BPD differ in terms of the volume of the gastric remnant and the length of bowel exposed to the mixture of food and biliopancreatic juices. However, these procedures have in common the exclusion of duodenum and at least part of the jejunum from the transit of food. This bypass has two obvious consequences: undigested or incompletely digested food is presented early to the ileum, and the duodenum and jejunum are excluded from the enteroinsular axis. Both mechanisms suggest that the effect of GBP and BPD on diabetes is mediated by a change in the pattern of secretion of gastrointestinal hormones.
Pories et al provide one possible explanation. 29 Their hypothesis is that GBP (we would include BPD also) excludes the site responsible for the production of the hormone causing type 2 diabetes. In their model, the hyperinsulinemia in type 2 diabetes is the result of an abnormal incretin signal from the gut, while the insulin resistance is a secondary protective phenomenon.
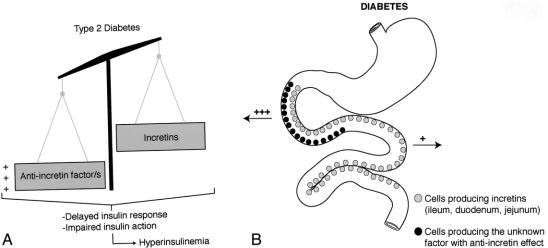
Other explanations are possible. For example, a hormone overproduced in the proximal foregut in diabetic patients might not directly increase the production of insulin, as suggested by Pories et al, but rather counteract the action of insulin, thus inducing insulin resistance and only secondarily hyperinsulinemia. For instance, in susceptible individuals, chronic exaggerated stimulation of the proximal gut with fat and carbohydrates may induce overproduction of an unknown factor that causes impairment of incretin production and/or action, leading to insufficient or untimely production of insulin so that glucose intolerance develops (Figs. 1 and 2). In our opinion, the bypass of the duodenum and jejunum avoids this phenomenon, while the early presentation of undigested or incompletely digested food to the ileum may anticipate the production of hormones such as glucagon-like peptide 1 (GLP1), further improving insulin action (Fig. 3).
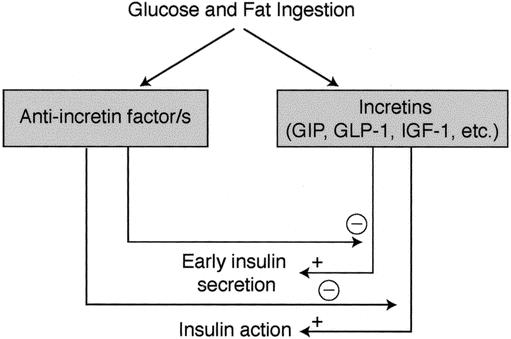
Figure 1. We hypothesize that the complex reaction of the endocrine bowel to meal ingestion in normal subjects includes production of both incretins (which stimulate insulin secretion and action) and other unknown factors that inhibit the effects of incretins as a sort of negative feedback mechanism.
Figure 2. We speculate that type 2 diabetes might be the result of an imbalance in the equilibrium between anti-incretin factors and incretins, which eventually leads to delayed insulin response and impaired insulin action (A). The anti-incretin factors are most likely overproduced in the proximal foregut of diabetics.
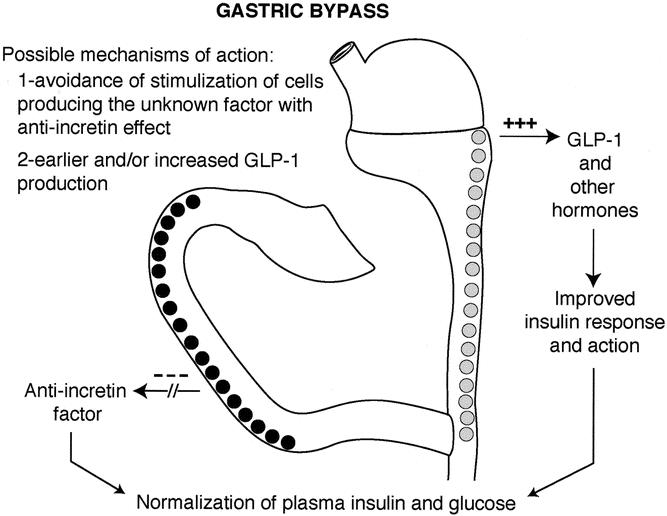
Figure 3. Hypothesis as to the mechanism responsible for the control of diabetes after gastric bypass.
EFFECT OF MORBID OBESITY SURGERY ON GASTROINTESTINAL HORMONES
The hypothesis that the effect of GBP and BPD on diabetes is mediated by a change in the pattern of secretion of gastrointestinal hormones is supported by other evidence. GBP increases the enteroglucagon 32,33 and gastroinhibitory peptide 34 response to oral glucose, increases insulin-like growth factor 1 levels (IGF-1), 35 and lowers plasma leptin levels in diabetic and nondiabetic morbidly obese patients. 25 Postprandial reduction of pancreatic polypeptide has also been documented. 33 Biliopancreatic diversion reduced leptin levels before weight loss occurred 36 and increased the enteroglucagon response to glucose test. 37 Decreased plasma lipid levels have also been reported after biliopancreatic diversion. 38 High levels of plasma glucagon-like peptide 1 have been reported after jejunoileal bypass, 39 and this is thought to play a role in the mechanism of diabetes control after bariatric surgeries. 40 Certainly, the lack of knowledge about the exact pathophysiologic mechanisms that lead to diabetes does not allow us to ascertain which of these changes can explain the “antidiabetic” effect of GBP and BPD.
CURRENT THEORIES ABOUT THE PATHOGENESIS OF TYPE 2 DIABETES
The most recent theories portray type 2 diabetes mellitus as a heterogeneous disorder. In addition to insulin resistance, clinical studies in humans and animal data have documented a variety of defects in β-cell function, 41 and most researchers agree that both insulin secretion impairment and insulin resistance contribute to the fully established disease. 42 GBP and BPD restore insulin sensitivity, but the possibility of an additional incretin-mediated effect on insulin secretion cannot be ruled out.
Though insulin is the chief acute physiologic stimulus of glucose disposal, other stimuli can also activate glucose uptake and control glycemia. 43 In vivo administration of IGF1 has a potent hypoglycemic effect and has been proven to effectively lower blood glucose concentrations in subjects with type 1 or type 2 diabetes. 44,45 Decreased levels of IGF-1 have also been documented in patients with type 2 diabetes mellitus. 46 Poulos at al 35 demonstrated that GBP significantly increases IGF-1 levels only in morbidly obese patients with diabetes and not in nondiabetic subjects.
Recent data indicate that leptin may directly affect glucose and fat metabolism. 47 Administration of leptin to normal, genetically obese, or diabetic rodents improved sensitivity to insulin and reduced hyperinsulinemia before any changes in food intake or body weight occurred. 48,49 Leptin-induced increase in fatty acid oxidation could also improve glucose uptake 50 and influence insulin sensitivity indirectly through the brain and sympathetic nervous system 49,51 or by changes in the concentration of serum fatty acids and glucose flux in the liver. 51 In the light of these effects, it is of extreme interest that leptin levels decrease rapidly after GBP and BPD without correlation with postoperative BMI. 25,36 This observation suggests that body fat composition is not the only factor that regulates leptin levels. It might be speculated that an unknown factor, produced in the duodenum or jejunum in response to food stimulation, is responsible for a sort of “leptin resistance” and compensatory increased plasma levels of leptins, which is a common finding in obese patients. 47 Accordingly, when the duodenum and jejunum are bypassed, as after GBP and BPD, the cause of leptin resistance is abolished or greatly reduced, leptin resistance is resolved, and plasma leptin levels decrease. The effect of GBP and BPD on leptin may therefore in part explain their efficacy in treatment of both obesity and diabetes.
SURGERY AS A CURE FOR TYPE 2 DIABETES?
The evidence of this extraordinary control of diabetes by obesity surgery stimulates another intriguing question: since GBP and BPD seem to achieve control of diabetes as a primary, specific, and independent effect rather than secondary to the treatment of overweight, would these operations also be effective in moderately obese or in nonobese diabetics?
In 1997, Mingrone et al 52 reported a case of a young diabetic woman of normal weight who underwent BPD for chylomicronemia and whose plasma insulin and blood glucose levels were normalized within 3 months, even though she gained weight due to an unrestricted diet rich in sugar and lipids. Noya et al 53 reported remission of type 2 diabetes in 9 of 10 moderately obese (mean BMI 33.2) diabetic patients undergoing BPD.
Although some suggested that the etiology of type 2 diabetes mellitus might be different in obese patients because of the greater insulin resistance with respect to non-obese diabetic patients, 54 this finding was not confirmed by most studies. 55,56 It has been reported that the degree of insulin resistance is correlated with the degree of obesity only up to a BMI of about 30, after which there is little further change. 57 These observations suggest that GBP and BPD might achieve control of plasma glucose levels and insulin abnormalities at least in moderately obese patients (BMI > 30). Since more than 60% of patients with type 2 diabetes have a BMI above 28, 58 the potential is huge.
If morbid obesity surgery could become a specific treatment for type 2 diabetes, which operation should be performed? We believe that because of its low complication rate and lack of important late metabolic sequelae, GBP is more suitable than BPD for nonmorbidly obese diabetic patients. The fact that GPB is performed laparoscopically more often and in more centers is an additional advantage.
SUMMARY AND CONCLUSIONS
GBP and BPD seem to be an effective form of therapy for type 2 diabetes, at least in morbidly obese patients. Significant changes in gastrointestinal hormones have been documented, but no clear explanation has been given yet about the mechanism of action of this surgery. The bypass of the duodenum and proximal jejunum, a common feature of both GBP and BPD, may contrasts a hormonal or neural signaling originating from the gut in response to the passage of food and responsible for the impaired action and/or secretion of insulin that characterizes type 2 diabetes. Case reports and observations on the timing of the restoration of glucose metabolism after surgery suggest that the control of diabetes occurs as a primary, specific, and independent effect of this surgery, not secondary to the treatment of overweight.
Controlled trials in centers with a wide experience of GBP surgery are needed to verify the possibility of a surgical cure specific for type 2 diabetes; however, surgeries for obesity seem to have a potential for changing the current concepts of the pathophysiology of type 2 diabetes and, possibly, the management of this disease.
Acknowledgment
The authors thank Mrs. Nancy Heim for the illustrations in this article.
Footnotes
Correspondence: Francesco Rubino, MD, IRCAD-European Institute of Telesurgery, 1 Place de l’Hopital BP426, 67091 Strasbourg Cedex, France.
E-mail: f.rubino@lycos.com
Accepted for publication January 18, 2002.
References
- 1.Venkat Narayan KM, Gregg EW, Fagot-Campagna A, et al. Diabetes: a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract 2000; 50 (Suppl 2): S77–84. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPD 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 3.Detournay B, Cros S, Charbonnel B, et al. Managing type 2 diabetes in France: the ECODIA survey. Diabetes Metab 2000; 26: 363–369. [PubMed] [Google Scholar]
- 4.Brolin RE. Update, NIH consensus conference, Gastrointestinal surgery for severe obesity. Nutrition 1996; 12: 403–404. [DOI] [PubMed] [Google Scholar]
- 5.Gastrointestinal surgery for severe obesity. NIH consensus development conference, March 25–7, 1991. Nutrition 1996; 12:397–404. [PubMed]
- 6.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr 1992; 55 (2 Suppl):560S–566S. [DOI] [PubMed] [Google Scholar]
- 7.Benotti PN, Forse RA. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg 1995; 169: 361–367. [DOI] [PubMed] [Google Scholar]
- 8.Smith SC, Goodman GN, Edwards CB. Roux-en-Y gastric bypass. A 7-year retrospective review of 3,855 patients. Obes Surg 1995; 5: 314–318. [DOI] [PubMed] [Google Scholar]
- 9.Fobi MA, Lee H, Holness R, et al. Gastric bypass operation for obesity. World J Surg 1998; 22: 925–935. [DOI] [PubMed] [Google Scholar]
- 10.MacLean LD, Rhode BM, Sampalis J, et al. Results of the surgical treatment of obesity. Am J Surg 1993; 165: 155–160. [DOI] [PubMed] [Google Scholar]
- 11.Linner JH. Comparative effectiveness of gastric bypass and gastroplasty: a clinical study. Arch Surg 1982; 117: 695–700. [DOI] [PubMed] [Google Scholar]
- 12.Griffen WO, Bivins BA, Bell RM, et al. Gastric bypass for morbid obesity. World J Surg 1981; 5: 817–822. [DOI] [PubMed] [Google Scholar]
- 13.Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg 1979; 66: 618–620. [DOI] [PubMed] [Google Scholar]
- 14.Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg 1998; 22: 947–954. [DOI] [PubMed] [Google Scholar]
- 15.Scopinaro N, Adami GF, Marinari GM, et al. Biliopancreatic diversion. World J Surg 1998; 22: 936–946. [DOI] [PubMed] [Google Scholar]
- 16.Deitel M. Overview of operations for morbid obesity. World J Surg 1998; 22: 913–918. [DOI] [PubMed] [Google Scholar]
- 17.Belachew M, Legrand M, Vincent V, et al. Laparoscopic adjustable gastric banding. World J Surg 1998; 22: 955–963. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen NT, Ho HS, Palmer LS, et al. A comparison study of laparoscopic versus open gastric bypass for morbid obesity. J Am Coll Surg 2000; 191: 149–155. [DOI] [PubMed] [Google Scholar]
- 19.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y: 500 patients: technique and results, with 3–60 month follow-up. Obes Surg 2000; 10: 233–239. [DOI] [PubMed] [Google Scholar]
- 20.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg 2000; 232: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley EF, Benotti PN, Borlase BC, et al. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg 1992; 163: 294. [DOI] [PubMed] [Google Scholar]
- 22.Smith S, Edwards CB, Goodman GN. Changes in diabetic management after Roux-en-Y gastric bypass. Obes Surg 1996; 6: 345–348. [DOI] [PubMed] [Google Scholar]
- 23.Cowan GS, Buffington CK. Significant changes in blood pressure, glucose, and lipids with gastric bypass surgery. World J Surg 1998; 22: 987–992. [DOI] [PubMed] [Google Scholar]
- 24.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey MS, Pories WJ, MacDonald KG, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg 1998; 227: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castagneto M, De Gaetano A, Mingrone G, et al. Normalization of insulin sensitivity in the obese patient after stable weight reduction with biliopancreatic diversion. Obes Surg 1994; 4: 161–168. [DOI] [PubMed] [Google Scholar]
- 27.Pories WJ, MacDonald KG, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 1992; 55 (2 Suppl):582S–585S. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald KG, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997; 1: 213–220. [DOI] [PubMed] [Google Scholar]
- 29.Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg 2001; 25: 527–531. [DOI] [PubMed] [Google Scholar]
- 30.Bourdages H, Goldenberg F, Nguyen P, et al. Improvement in obesity-associated medical conditions following vertical banded gastroplasty and gastrointestinal bypass. Obes Surg 1994; 4: 227–231. [DOI] [PubMed] [Google Scholar]
- 31.Neve HJ, Soulsby CT, Whitely GS, et al. Resolution of diabetes following vertical gastroplasty in morbidly obese patients. Obes Surg 1993; 3: 75–78. [DOI] [PubMed] [Google Scholar]
- 32.Kellum JM, Kuemmerle JF, O’Dorisio RM, et al. GI hormone response to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990; 211: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meryn S, Stein D, Straus EW. Pancreatic polypeptide, pancreatic glucagon and enteroglucagon in morbid obesity and following gastric bypass operation. Int J Obes 1986; 10: 37–42. [PubMed] [Google Scholar]
- 34.Halverson JD, Kramer J, Cave A, et al. Altered glucose tolerance, insulin response, and insulin sensitivity after massive weight reduction subsequent to gastric bypass. Surgery 1982; 92: 235–240. [PubMed] [Google Scholar]
- 35.Poulos JE, Leggett-Frazier N, Khazanie P, et al. Circulating insulin-like growth factor I concentrations in clinically severe obese patients with and without NIDDM in response to weight loss. Horm Metab Res 1994; 26: 478–480. [DOI] [PubMed] [Google Scholar]
- 36.de Marinis L, Mancini A, Valle D, et al. Plasma leptin levels after biliopancreatic diversion: dissociation with body mass index. J Clin Endocrinol Metab 1999; 84: 2386–2389. [DOI] [PubMed] [Google Scholar]
- 37.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. [PubMed]
- 38.Mingrone G, DeGaetano A, Greco AV, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia 1997; 40: 599–605. [DOI] [PubMed] [Google Scholar]
- 39.Naslund E, Backman L, Holst JJ, et al. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 1998; 8: 253–260. [DOI] [PubMed] [Google Scholar]
- 40.Mason EE. Ileal transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg 1999; 9: 223–228. [DOI] [PubMed] [Google Scholar]
- 41.Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest 2000; 106: 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahler RJ, Adler ML. Clinical review 102: Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment. J Clin Endocrinol Metab 1999; 84: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med 1999; 341 (4): 248–257. [DOI] [PubMed] [Google Scholar]
- 44.Le Roith D. Insulin-like growth factors. N Engl J Med 1997; 336 (9): 633–640. [DOI] [PubMed] [Google Scholar]
- 45.Simpson HL, Umpleby AM, Russell-Jones DL. Insulin-like growth factor-I and diabetes. A review. Growth Horm IGF Res 1998; 8: 83–95. [DOI] [PubMed] [Google Scholar]
- 46.Yde H. The growth hormone dependent sulfation factor in serum from patients with various types of diabetes. Acta Med Scand 1969; 186: 293–297. [DOI] [PubMed] [Google Scholar]
- 47.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med 1999; 20:130:671–680. [DOI] [PubMed]
- 48.Sivitz WI, Walsh SA, Morgan DA, et al. Effects of leptin on insulin sensitivity in normal rats. Endocrinology 1997; 138: 3395–3401. [DOI] [PubMed] [Google Scholar]
- 49.Kamohara S, Burcelin R, Halaas JL, et al. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 1997; 389: 374–377. [DOI] [PubMed] [Google Scholar]
- 50.Muoio DM, Dohm GL, Fiedorek FT, et al. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 1997; 46: 1360–1363. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Karkanias GB, Morales JC, et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem 1998; 20:273:31160–31167. [DOI] [PubMed]
- 52.Mingrone G, De Gaetano A, Greco AV, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia 1997; 40: 599–605. [DOI] [PubMed] [Google Scholar]
- 53.Noya G, Cossu ML, Coppola M, et al. Biliopancreatic diversion preserving the stomach and pylorus in the treatment of hypercholesterolemia and diabetes type II: results in the first 10 cases. Obes Surg 1998; 8: 67–72. [DOI] [PubMed] [Google Scholar]
- 54.Arner P, Pollare T, Lithell H. Different aetiologies of type 2 (non-insulin-dependent) diabetes mellitus in obese and non-obese subjects. Diabetologia 1991; 34: 483–487. [DOI] [PubMed] [Google Scholar]
- 55.Kolterman OG, Gray RS, Griffin J, et al. Receptor and post-receptor defect tribute to the insuline resistance in non-insulin dependent diabetes mellitus. J Clin Invest 1981; 68: 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolinder J, Lithell H, Skarfors E, et al. Effects of obesity, hyperinsulinemia and glucose intolerance on insulin action in adipose tissue of sixty-year-old men. Diabetes 1986; 35: 282–290. [DOI] [PubMed] [Google Scholar]
- 57.Elton CW, Tapscott EB, Pories WJ, et al. Effect of moderate obesity on glucose transport in human muscle. Horm Metab Res 1994; 26: 181–183. [DOI] [PubMed] [Google Scholar]
- 58.Melton LJ III, Palumbo PJ, Dwyer MS, et al. Impact of changes in diagnostic criteria on the apparent history of diabetes mellitus. Am J Epidemiol 1983; 117: 559–565. [DOI] [PubMed] [Google Scholar]