Abstract
Objective
To assess a new bloodless technique using radiofrequency energy for segmental liver resection of hepatic tumors.
Summary Background Data
Liver resection remains a formidable surgical procedure; safe performance requires a high level of training and skill. Intraoperative blood loss during liver resection remains a major concern because it is associated with a higher rate of postoperative complications and shorter long-term survival.
Methods
From January 2000 to June 2001, 15 patients with various hepatic tumors were operated on using radiofrequency energy to remove the tumor in its entirety. Radiofrequency energy was applied along the margins of the tumor to create “zones of necrosis” before resection with a scalpel.
Results
No blood transfusions were required. The mean blood loss during resection was 30 ± 10 mL. No mortality or morbidity was observed. The median postoperative stay was 8 days (range 5–9). No liver recurrence was detected in patients undergoing resection with this technique during follow-up periods ranging from 2 to 20 months.
Conclusions
Segmental and wedge liver resection assisted by radiofrequency is safe. This novel technique offers a new method for transfusion-free resection.
With improvements in liver surgery techniques and an increased knowledge of liver anatomy, physiology, and hepatic cell biology, the mortality and morbidity associated with liver surgery have significantly decreased. Nevertheless, liver resection remains a formidable surgical procedure; safe performance requires a high level of training and skill. Intraoperative blood loss remains a major concern for surgeons operating on the liver:1 it is associated with a higher rate of postoperative complications and shorter long-term survival.
Over the years different techniques have been developed to allow safer liver resection. 2–5 Surgeons can decrease intraoperative blood loss by using hypotensive anesthetics, Pringle’s maneuver, or total vascular exclusion. Parenchymal division can be performed with the scalpel, crushing the tissue with the finger or clamps, using ultrasonic dissectors and hydrodissectors, or stapling devices.
In this article we describe a new technique for liver resection using radiofrequency (RF) energy to coagulate the liver resection margins. A 2-cm-wide coagulative necrosis zone is created before division of the parenchyma with a surgical scalpel.
METHODS
Patients
Between January 2000 and June 2001, 15 patients underwent RF-guided liver resection for hepatic tumors at the Hammersmith Hospital, London (Table 1). All patients underwent careful preoperative assessment of their disease, including spiral computed tomography (CT) scanning or magnetic resonance imaging (MRI), and showed no evidence of unresectable extrahepatic disease. Surgical resections ranged from multiple metastasectomies to bisegmentectomies. Only one major resection (i.e., three segments or more according to Couinaud’s classification) was performed. All patients were followed monthly for the first 3 months, then every 6 months. At each follow-up visit, clinical examination was conducted. In addition, a surveillance abdominal CT or MRI was performed at the first and third month, respectively, and every 6 months thereafter. For patients with colorectal cancer, CEA levels were also assessed.
Table 1. CLINICAL DETAILS

Procedure
Under general anesthesia a modified right subcostal incision was made. The peritoneal cavity was examined for evidence of extrahepatic disease. Intra-abdominal adhesions and the falciform ligament were divided. The liver was then mobilized according to the size and site of the lesion to be resected. An intraoperative ultrasonogram was always performed before liver resection to reveal previously undetected lesions.
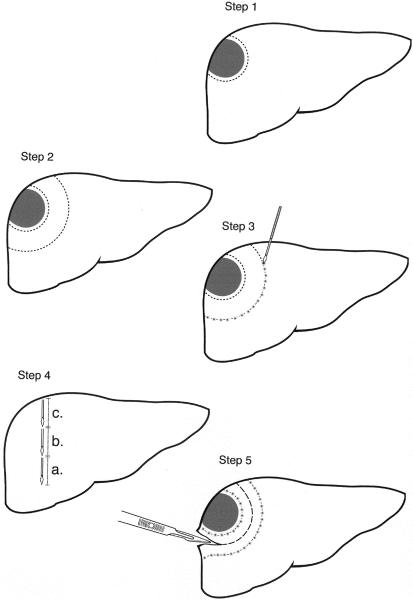
In step 1 (Fig. 1), a first or inner line is made on the liver capsule with argon diathermy to mark the periphery of the tumor, assisted by bimanual palpation and intraoperative ultrasound. It is important to do this first. Because after RF is used, the parenchyma is hardened and it becomes difficult to feel the tumor edge. Also, after RF intraoperative ultrasound fails to visualize the tumor edge due to the increased echogenicity resulting from RF.
Figure 1. The five steps to achieve liver resection using radiofrequency energy.
In step 2, a second or outer line, again using argon diathermy, is made on the liver capsule 2 cm outside (away from) the inner line to mark the site where the probe is positioned to achieve coagulative necrosis.
In step 3, coagulative necrosis is produced along a line that follows the second or outer line using the cooled-tip RF probe and a 500-kHz RF Generator (Model RFG-3D, Radionics Europe, N.V., Wettdren, Belgium), which produces 100 W of power and allows measurements of the generator output, tissue impedance, and electrode tip temperature. The probe contains a 3-cm exposed electrode, a thermocouple on the tip to monitor temperature and impedance, and two coaxial cannulae through which chilled saline is circulated during RF energy application to prevent tissue boiling and cavitation immediately adjacent to the needle.
The number of probe applications required to obtain a zone of necrosis is related to the depth of the liver parenchyma to be resected. For example, to obtain a zone of necrosis in a core of tissue with a 1-cm radius and 3 cm in depth, each application of RF energy will need to be applied for about 60 seconds. Thus, for a core of tissue 12 cm in depth, four applications will be needed in vertical succession (step 4).
Application of the RF energy should begin with the area deepest and farthest from the upper surface of the liver. The surgeon should check that each probe is correctly positioned with ultrasound. The preferred technique is to have the tip of the probe pierce the liver capsule of the inferior surface of the liver and to feel it with the middle finger of the left hand while holding the probe with the right hand. The areas of coagulative necrosis can be monitored using intraoperative ultrasound to show the change in tissue impedance and the formation of microbubbles in the tissue.
Once the deepest 3 cm of tissue is coagulated, the probe is withdrawn by 3 cm to coagulate the next cylinder of tissue, and so on until the upper surface of the liver is reached. Each application requires about 60 seconds of RF energy. For example, a cylinder of tissue 12 cm in depth will require four applications, each application coagulating 3 cm of tissue, and will take about 4 minutes to produce. Once an area is coagulated, the probe is withdrawn completely and placed 1 to 2 cm away from the previous application. This allows complete coagulation of a band of parenchyma extending along the second line. The points of entry of each probe should be kept close to each other (i.e., 1 cm) to achieve some overlap of the areas to be coagulated to ensure that the coagulation has been complete. Just before each probe removal, the saline infusion is stopped to increase the temperature close to the electrode. This results in coagulation of the needle tract during withdrawal and reduces the possibility of bleeding from the probe tract and liver capsule. Pringle’s maneuver is not needed.
In step 5, the liver parenchyma is divided using the scalpel. The plane of division should be situated midway between the first and second line so as to leave a 1-cm resection margin away from the tumor and leave in situ 1 cm of burned coagulated surface.
Coagulative necrosis from inside the resection margin could be applied to stop any potential point of bleeding and to increase the safety margin, particularly if the resection is being done to remove cancerous tissue. A drain is placed at the site of resection. The abdomen is subsequently closed in layers.
In all patients, biochemical liver function tests were monitored before and after resection within the first 24 hours (Table 2). All values expressed as means ± SD. A paired t test was used to compare liver function tests before and after resection. P < .05 was considered significant.
Table 2. CHANGES OF BIOCHEMICAL LIVER FUNCTION BEFORE AND AFTER LIVER RESECTION
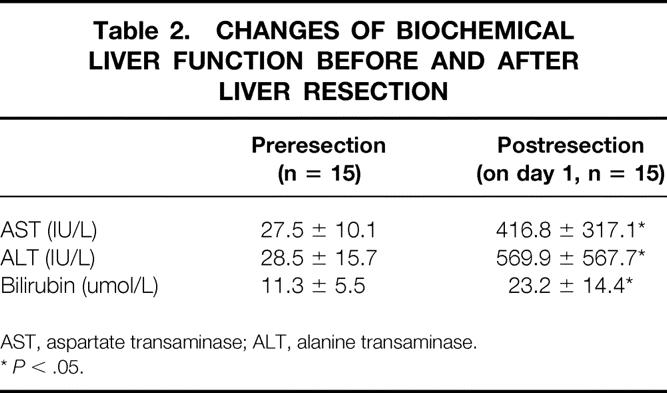
AST, aspartate transaminase; ALT, alanine transaminase.
*P < .05.
RESULTS
All the procedures were completed as planned. The median operative time was 205 minutes (range 95–300), with a median resection time of 45 minutes (range 30–80). None of the patients required blood transfusion during or after surgery. There was no operative mortality or morbidity. The length of postoperative stay was 5 to 9 days (see Table 1). There was a significant change in the biochemical liver function after resection compared with that preoperatively, reflecting the effect of liver resection on liver function (see Table 2).
Following discharge, all patients were followed up regularly. Although the follow-up period was short, ranging from 2 to 20 months, there was no detected liver recurrence in patients undergoing resection with this technique on either clinical examination or imaging studies.
DISCUSSION
We have described an innovative technique using RF energy for bloodless liver resection without the use of sutures, surgical knots, clips, or glue. When this procedure is used carefully, the surgeon can perform a liver resection with less than 30 ± 10 mL blood loss, and practically zero blood loss on most occasions. It is easy to teach, and surgeons with a good knowledge of liver anatomy can apply it to segmental resections. The technique has not yet been used for a major liver resection, but we believe it is possible after ligation of major blood vessels at the liver hilum. In this small experience, bile leak was not encountered. This might be because following coagulative necrosis the cut surface of the liver parenchyma is homogeneous and no individual bile duct structure or blood vessel can be identified.
The technique reduces the anesthetic time, operative time, and amount of blood loss. These are significant improvements for both the patient and the surgeon. Liver resection becomes a less risky surgical procedure; it eliminates the need for intensive care unit facilities; and less postoperative mortality and morbidity is encountered because of the smaller surgical insult to the patient.
We have developed this technique at a time when liver resection has become unpopular because of competitive therapeutic modalities based on novel engineering technologies to achieve local tumor control and improved chemotherapeutic regimens. As it is a simple technique to teach, it may encourage surgeons to perform more liver resections and popularize liver surgery as a “safe” therapeutic modality in the management of liver tumors. This technique represents a considerable step in making laparoscopic liver resection safer and more feasible for many liver surgeons. We believe that further technical advances will increase the safety and speed of the technique when used for liver resection.
The concept of using heat to cause coagulative necrosis is not new; many authors have published manuscripts reporting series of RF tumor ablation for malignant tumors. 6–9 The innovative step with this technique is that coagulation of normal liver parenchyma is very fast, which is in contrast to coagulation of liver tumor tissue. Typically, achieving coagulative necrosis in tumor tissue takes about 20 minutes for one probe application; it takes only 40 seconds to coagulate the same amount of normal liver tissue.
There are two obvious limitations to the technique. The first is that RF energy cannot be applied near the hilus or the vena cava for fear of damaging these structures. The second is that it sacrifices parenchymal tissue that is usually spared using other resectional techniques. We can be criticized for using it only in “easy” cases. We expect to find further limitations and to observe additional complications in the use of this technique with more time and experience in a larger number of patients.
RF probably is not the most appropriate energy model to use for this technique. We are assessing other energy modalities and different device applications more suitable for the resection of liver parenchyma.
Footnotes
Correspondence: Nagy Habib, ChM, FRCS, Head of Liver Surgery Section, Department of Surgical Oncology and Technology, Imperial College Faculty of Medicine, Hammersmith Hospital Campus, Du Cane Road, London W12 0NN, United Kingdom.
E-mail: nagy.habib@ic.ac.uk
Accepted for publication December 4, 2001.
References
- 1.Bismuth H. Major hepatic resection under total vascular exclusion. Ann Surg 1989; 210: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuzzo G, Guiliante F, Giovianni I, et al. Hepatic resections in normothermic ischemia. Surgery 1996; 120: 852–858. [DOI] [PubMed] [Google Scholar]
- 3.Tranberg KG, Rigotti P, Brackett KA, et al. Liver resection. A comparison using Nd-YAG laser, an ultrasonic surgical aspirator, or blunt dissection. Am J Surg 1986; 151: 368–373. [DOI] [PubMed] [Google Scholar]
- 4.Hansen PD, Isla AM, Habib NA. Liver resection using total vascular exclusion, scalpel division of the parenchyma and a simple compression technique for haemostasis and biliary control. J Gastrointest Surg 1999; 3: 537–542. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Ikai I, Kume M, et al. New technique for hepatic parenchymal resection using a Cavitron ultrasonic surgical aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg 1999; 23: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 6.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999; 230: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao LR, Hansen PD, Havlik R, et al. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg 1999; 177: 303–306. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A, Braclen J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy 1999; 31: 318–321. [DOI] [PubMed] [Google Scholar]
- 9.Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. Radiology 2000; 217: 633–646. [DOI] [PubMed] [Google Scholar]