Abstract
Objective
To evaluate the applicability of intraoperative parathyroid hormone (quick PTH) assay to monitor parathyroid function and to identify clinically significant hypocalcemia compared with postoperative serum calcium monitoring.
Summary Background Data
Close monitoring of serum calcium levels is a standard of care to identify post-thyroidectomy hypocalcemia due to parathyroid insufficiency.
Methods
Quick PTH assay was performed before and after thyroidectomy for 100 patients at risk of postoperative hypocalcemia and 20 control patients who underwent unilateral lobectomy. Postoperative serum calcium levels were closely monitored.
Results
Control patients had a normal but 38.9 ± 5.9% (mean ± SEM) decline in quick PTH after thyroidectomy. Eleven of 100 at-risk patients (11%) developed postoperative hypocalcemia. Hypocalcemic patients had significantly lower quick PTH values after thyroidectomy compared with that of normocalcemic patients. Serum calcium was significantly lower in hypocalcemic patients the morning after operation but not early after the operation (within 6 hours). A normal or less than 75% decline in quick PTH after thyroidectomy can accurately identify normocalcemic patients during surgery as compared to more than 24 hours by serum calcium monitoring.
Conclusions
The quick PTH assay can monitor parathyroid function during thyroidectomy and identify patients at risk of clinically significant hypocalcemia much earlier than serum calcium monitoring. It may facilitate early discharge and the use of parathyroid autotransplantation during thyroidectomy.
Permanent hypoparathyroidism and recurrent laryngeal nerve damage are two major morbidities of thyroid surgery, accounting for most medical litigation. 1 The nerve stimulator is increasingly used to monitor vocal cord function and to prevent nerve injury during thyroidectomy. 2 In contrast, surgeons have relatively little idea during surgery whether postoperative and permanent hypoparathyroidism will occur. Efforts have been made to identify clinical risk factors for post-thyroidectomy hypocalcemia 3 or for long-term outcome. 4,5 The indications for the operation, the extent of thyroid resection, and the anatomy of the parathyroid glands determine the ability to preserve parathyroid function. Clinically significant hypocalcemia following thyroid surgery is secondary to impairment of parathyroid function in most cases. 4–7
Close monitoring of serum calcium levels is commonly used to identify postoperative hypoparathyroidism, 8,9 but an ideal intraoperative assessment of parathyroid function is lacking. 10,11 An intraoperative parathyroid hormone assay (quick PTH assay) has been increasingly adopted to monitor the success of parathyroid surgery and to facilitate a focused approach during parathyroidectomy. 12–15 It has been suggested that the quick PTH assay might have a value 16 in monitoring the outcome of thyroid surgery with respect to the development of postoperative hypocalcemia. The present study evaluates the ability of the quick PTH assay to monitor parathyroid function and to identify patients at risk for postoperative hypocalcemia.
METHODS
The quick PTH assay was performed in 100 patients undergoing bilateral or completion thyroidectomy who were at risk of developing clinically significant hypocalcemia for various thyroid pathologies. Patients with Graves’ disease were rendered euthyroid by antithyroid medications before thyroidectomy. Twenty patients who underwent unilateral thyroid lobectomy without any risk of developing symptomatic hypocalcemia 3,8,9 were used as control patients. All patients had normal renal function at the time of thyroidectomy.
The quick PTH assay was a commercially available two-site antibody immunochemiluminometric assay (ICMA) (Nichols Institute Diagnostics QuicK-Pak system, San Juan Capistrano, CA) carried out in the operating room by a trained technician using a portable laboratory set up on a cart. The equipment consisted of a portable luminometer, a modified heated shaker, a small benchtop microcentrifuge, a washing system consisting of two self-refilling syringes, tubing and reagent bottles, and a vacuum pump with a suction catheter and reservoir. In brief, a blood sample (2.5 mL) for measuring quick PTH levels were obtained from a peripheral vein after the induction of anesthesia. Another blood sample was obtained immediately after completion of thyroidectomy (0 minutes) and repeated at 10 minutes from the internal jugular vein. Duplicate samples were spun in a microcentrifuge at 4,000 rpm for 30 seconds. Two hundred microliters of each plasma sample, 100 μL acridinium ester-labeled PTH (1–34) antibody solution, and a PTH (39–84) antibody-coated polystyrene bead tracer antibody were transferred to tubes and incubated at 45°C for 7 minutes (accomplished with a heated shaker at 400 ± 10 rpm). The plasma was aspirated and the beads were washed three times in an automated wash station with 0.5 mL of a wash solution (phosphate-buffered saline with surfactant) diluted with saline (30 mL saline/50 mL standard wash). After the wash solution was aspirated, each bead was transferred to a new test tube for counting in the luminometer. The actual PTH level was determined by computer plotting of measured values against a previously obtained standard curve. The average PTH value from the duplicated samples was expressed in pg/mL. Results were available within 15 to 20 minutes.
The postoperative serum calcium level adjusted to the albumin level was measured within 6 hours after surgery (early postoperative) and on the next morning (within 24 hours) and on subsequent mornings after the operation, until the calcium level was stabilized. Oral calcium supplements (calcium carbonate 2–3 g daily) and/or vitamin D analogue (Rocaltrol 0.25–0.5 μg daily) (Roche Laboratories, Nutley, NJ) were prescribed if patients developed symptomatic hypocalcemia or when the serum calcium level was less than 1.7 mmol/L (normal = 2.10–2.55 mmol/L). Intravenous calcium gluconate (10%) was administered for significant symptoms. Patients were discharged when the serum calcium level was more than 2.0 mmol/L. In addition, PTH was measured on the morning after operation by a standard two-site immunoradiometric (IRMA) assay (Chiron Diagnostics Corp., East Walpole, MA). Peripheral venous samples were collected in glass tubes and immediately spun in a refrigerated centrifuge to separate serum from cells. They were stored at −80°C before the batch assay was performed.
The quick PTH assay and postoperative serum calcium measurements were evaluated and compared among three groups of patients: patients who underwent unilateral lobectomy without any risk of postoperative symptomatic hypocalcemia (group 1); normocalcemic (group 2); and hypocalcemic (group 3) patients after bilateral or completion thyroidectomy. The potential application of the intraoperative quick PTH assay in identifying clinically significant hypocalcemia was evaluated and compared with postoperative serum calcium monitoring. The quick PTH assay after thyroidectomy was correlated with the standard IRMA PTH assay measured the morning after operation.
The Mann-Whitney test was used when comparing continuous variables between different groups; the Wilcoxon rank-sum test was used for comparison of paired samples. P < .05 was regarded as statistically significant.
RESULTS
Unilateral thyroid lobectomy (n = 20; group 1) was performed in 7 men and 13 women (median age 52.5 years; range 21–76 years) with benign uninodular goiters (n = 16) and follicular adenomas (n = 4). Right- and left-sided lobectomies were performed in 8 and 12 patients, respectively. There were 22 men and 78 women who underwent bilateral or completion total thyroidectomy and were at risk of postoperative hypocalcemia; median age was 42 years (range 12–81 years). The operative procedure included total or near-total thyroidectomy in 67, ipsilateral total and contralateral subtotal in 14, completion total (contralateral lobectomy in patients with previous unilateral lobectomy) in 11, and bilateral subtotal thyroidectomy in 8 patients. The indications for operation included nodular goiter in 40 patients, well-differentiated thyroid carcinoma in 33 patients, diffuse toxic goiter in 23 patients, and other in 4 patients. Unilateral and bilateral neck dissections were performed for 11 and 2 patients with thyroid malignancy, respectively. Parathyroid autotransplantation was performed in 39 patients (39%).
None of the patients in Group 1 developed symptomatic hypocalcemia after their operations. Of the 100 patients who underwent bilateral or completion thyroidectomy and who were at risk of postoperative hypocalcemia, 89 (89%) (group 2) were normocalcemic and 11 (11%) (group 3) developed postoperative symptomatic hypocalcemia requiring either calcium supplements only (n = 7) or vitamin D analogue in addition (n = 4) on discharge from the hospital. During follow-up, 10 patients discontinued calcium replacement over a median duration of 2.5 months (range 2–10 months), but one required calcium supplements for more than 12 months after the operation. The incidence of permanent hypocalcemia was 1%.
When the quick PTH assay was compared between the three groups, there was no difference in quick PTH values at induction. However, quick PTH values at 0 and 10 minutes after thyroidectomy were significantly lower in group 3 compared with those of group 2 (P < .001) and group 1 (P < .001). There was no difference in the quick PTH values after thyroidectomy between group 1 and group 2 (Fig. 1). In addition, the percentage decline of quick PTH at 0 minutes after thyroidectomy compared with that at induction in group 3 (88.6 ± 4.6%) (mean ± SEM) was significantly greater than those in group 1 (36.5 ± 7.2%) and group 2 (38.1 ± 4.6%) (P < .001). The decline of 96.3 ± 2.2% in group 3 was significantly higher than the decline of 47.5 ± 3.8% in group 2 (P < .001) and that of 38.9 ± 5.9% in group 1 (P < .001) at 10 minutes compared with those at induction. There was no difference in the percentage quick PTH decline after thyroidectomy between groups 1 and 2.
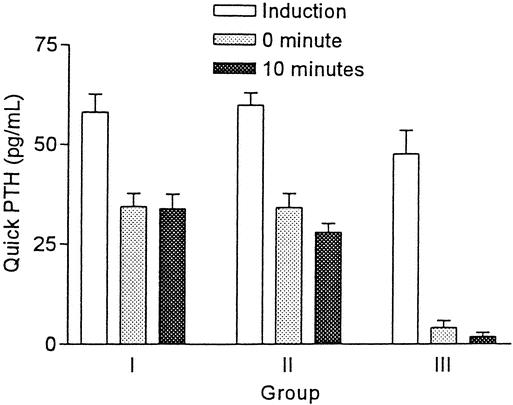
Figure 1. Comparison of quick PTH assay in three groups of patients (mean ± SEM).
The PTH measurement by standard IRMA assay on the morning after operation was significantly lower in group 3 (3.1 ± 1.7 pg/mL) compared with those in group 1 (24.6 ± 2.5 pg/mL) (P < .001) and group 2 (25.0 ± 1.6 pg/mL) (P < .001). There was no difference in early standard PTH values between groups 1 and 2. The correlation between the quick PTH assay after thyroidectomy and the standard early PTH assay by IRMA was determined, and the correlation coefficient was 0.6974 (r2, 0.028), with P < .0001 (Fig. 2).
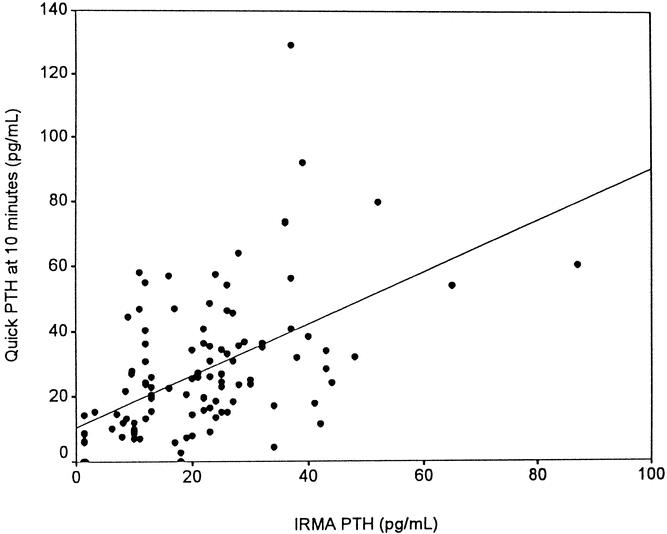
Figure 2. Correlation between quick PTH assay measured at 10 minutes after thyroidectomy and the standard PTH assay by IRMA within 24 hours after thyroidectomy (r2 = 0.028).
When the calcium level was compared between the three groups, there was no difference in the preoperative calcium level. Calcium replacement was commenced in group 3 patients 1 to 4 days (median 2 days) after operation. The calcium level was significantly lower in group 3 compared with that in group 2 on the morning after operation (P < .001) but the difference in early postoperative calcium level (within 6 hours) between these two groups did not reach statistical significance (P = .06). However, calcium levels both within 6 hours and on the morning after operation in group 2 patients were significantly lower than those in group 1 (P = .001 and P < .001) (Fig. 3).
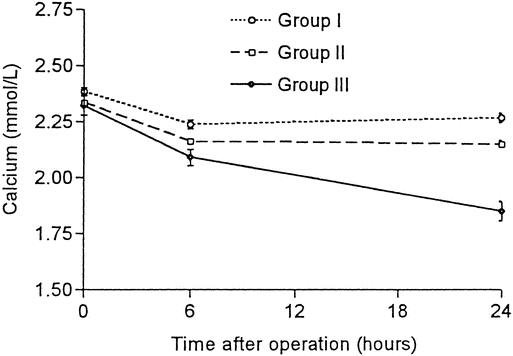
Figure 3. Change in serum calcium levels before (0 hour), immediately after (6 hours), and the morning after thyroidectomy (24 hours) in three groups of patients. All values shown are mean ± SEM.
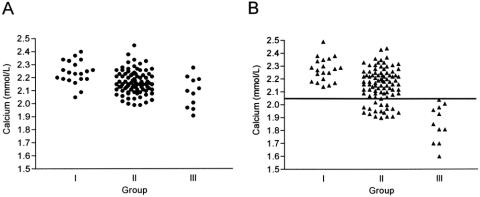
Of the 100 patients at risk of postoperative hypocalcemia, a positive slope, defined as an increase in calcium level during two consecutive measurements within 24 hours after operation (within 6 hours and on the morning after operation), predicted normocalcemia in all patients (n = 46). In contrast, 11 of 54 patients with a negative slope developed postoperative hypocalcemia. The magnitude of a negative slope in hypocalcemic patients was greater than that of normocalcemic patients (10.6% vs. 5.2%;P < .001). In addition, the accuracy of the quick PTH assay in predicting postoperative normocalcemia or hypocalcemia was assessed based on either the absolute value or the decline in quick PTH values after thyroidectomy compared with that at induction. All 73 patients with a normal PTH level after thyroidectomy were subsequently normocalcemic during the postoperative period. In contrast, 11 of 27 patients with a subnormal PTH level developed postoperative hypocalcemia (Fig. 4). Similarly, 64 patients with a less than 75% decline in the quick PTH at 10 minutes after thyroidectomy were normocalcemic, but 11 of 36 patients with a more than 75% decline developed postoperative hypocalcemia (Fig. 5). The sensitivity, specificity, and accuracy of a more than 75% decline in quick PTH at 10 minutes after thyroidectomy in predicting hypocalcemia were 100%, 72%, and 75%, respectively. The early serum calcium was not a good predictor of postoperative normocalcemia until measurement was performed on the morning following thyroidectomy (Fig. 6).
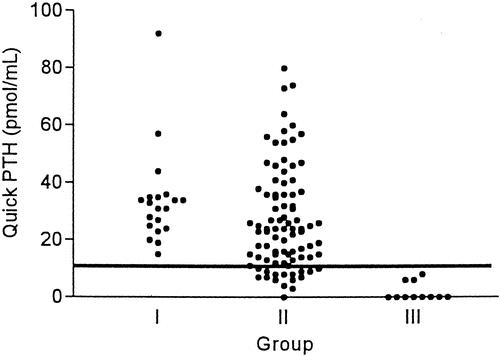
Figure 4. Quick PTH assay at 10 minutes after thyroidectomy in three groups of patients. Reference line represents the low-normal value.
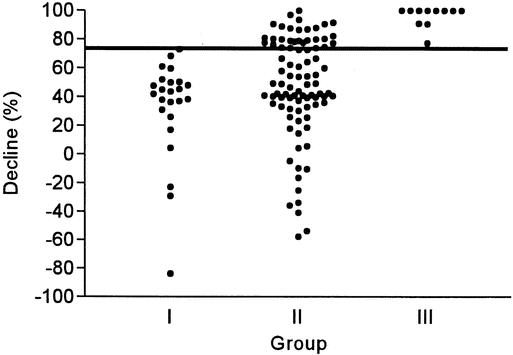
Figure 5. The percentage decline of quick PTH at 10 minutes after thyroidectomy compared with that at induction in three groups of patients. The reference line marks the level at 75% decline.
Figure 6. Serum calcium measurements in three groups of patients within 6 hours (A) and 24 hours (B) after thyroidectomy. The reference line marks a serum calcium level of 2.05 mmol/L.
DISCUSSION
The development of a two-site IRMA assay for measurement of intact PTH 12 and the subsequently modified method for rapid intraoperative PTH assessment 15 have revolutionized surgical strategy during parathyroid surgery. The quick PTH assay offers a way to improve the success of parathyroid surgery, to decrease the need for reoperation, and to facilitate a localized exploration. During parathyroid surgery, the failure of the quick PTH assay to decrease by 50% or more after excision of an abnormal gland indicates the presence of additional abnormal glands. The sensitivity of this test in predicting postoperative calcium levels has been found to be 89% to 97%. 13–15
Postoperative hypocalcemia is a concern following bilateral operations on the thyroid gland; it occurs in 1% to 50% of patients after thyroidectomy. 4 Although efforts were made to identify clinical and pathologic risk factors for the development of permanent hypoparathyroidism after thyroidectomy, 3–6 the occurrence of postoperative hypocalcemia remains the single most important factor predicting the occurrence of permanent hypoparathyroidism. 3,4 Close postoperative monitoring of serum calcium levels is a standard of care, and concerns about postoperative hypocalcemia contribute to delays in discharge. 8,9 It has been suggested that a program of routine calcium supplementation could be the future basis for same-day or 1-day admission after extensive thyroid operations. 17 However, the relatively infrequent requirement for calcium supplementation suggests that routine treatment is probably not cost-effective and complicates the detection of true hypocalcemia. 8 Early postoperative calcium monitoring has been used to identify patients at risk of having clinically significant hypocalcemia. 8,9 Marohn and LaCivita described 150 patients undergoing total or near-total thyroidectomy who had their serum calcium level measured 8, 14, and 20 hours after surgery. 8 An upward-sloping curve predicted that postoperative hypocalcemia would not occur. This finding was confirmed by a retrospective study that determined the slope of two consecutive calcium samples obtained within 24 hours after operation. 9 Nevertheless, the inability to demarcate hypocalcemic slopes and to predict hypocalcemia makes clinical application difficult. 9
However, the phenomenon of calcium decline within 24 hours is not a response specific to thyroid surgery. Postoperative evolution of calcium and other electrolytes after operations of the same magnitude and duration performed outside the cervical area, such as herniorrhaphy, strictly parallels that of thyroidectomized patients. 6 In contrast, the PTH level did not fall after such unrelated operations. 6 It has been suggested that rapid PTH ICMA might be of assistance to endocrine surgeons in monitoring parathyroid function after both thyroid and parathyroid surgery. 16 In contrast to parathyroid surgery, the application of this tool during thyroid surgery has not been previously reported. In an earlier study of the application of quick PTH for parathyroidectomy, a 38.5 ± 8.7% (mean ± SE) decline in the PTH level in control patients who underwent unilateral lobectomy was demonstrated, and the authors suggested that such a decline affected the interpretation of hormonal level changes. 18 In our present study, a similar decline in the quick PTH assay was noted, but a consistently normal quick PTH assay in patients who underwent unilateral thyroid lobectomy without any risk of postoperative symptomatic hypocalcemia encouraged us to evaluate the role of the quick PTH assay to monitor parathyroid function during thyroidectomy. For at-risk patients after bilateral operations, the quick PTH assay correlated closely with early postoperative standard PTH measurement by IRMA, and both measurements correlated significantly with the development of symptomatic hypocalcemia. Similarly to parathyroid surgery, 19 plasma calcium levels the evening after an operation are of little value in predicting the onset of subsequent hypocalcemia or symptoms because of its slow decline. 18 Normocalcemic patients who underwent bilateral operations had a significantly lower calcium level compared with those who underwent unilateral lobectomy. However, the quick PTH value after thyroidectomy or the degree of decline in quick PTH compared with that at induction was comparable between these two groups, but both were significantly lower than that of hypocalcemic patients. This may reflect the nonspecificity of postoperative calcium measurements compared with the quick PTH assay or consecutive calcium monitoring in identifying clinically significant hypocalcemia. Apart from PTH, other factors may determine the occurrence of postoperative hypocalcemia, since not all patients with a subnormal or more than 75% decline in the quick PTH assay developed this phenomenon in the postoperative period.
In contrast to postoperative calcium measurements, in our study, the quick PTH assay during thyroidectomy was both sensitive and specific in identifying normocalcemic patients. Patients can be discharged home early without the need for serum calcium monitoring. Furthermore, the quick PTH assay can identify patients at risk of developing clinically significant hypocalcemia. Serum calcium levels should be closely monitored and replacement therapy with calcium supplements can be administered early to avoid symptomatic hypocalcemia and to facilitate early discharge. 16,17 Both the quick PTH assay and consecutive early calcium measurements are accurate in predicting postoperative normocalcemia. However, the quick PTH assay is available within 15 to 20 minutes after operation, but serum calcium monitoring requires at least 24 hours, as well as an additional 4-hour turnover time, before the results are available. By facilitating early hospital discharge and reducing the need for additional serum calcium testing, the quick PTH assay may avoid the additional costs involved in serum calcium monitoring.
One potential additional application of this tool is to select patients for parathyroid autotransplantation during thyroidectomy. 20 Apart from reimplanting inadvertently removed or devascularized parathyroid glands routinely, determination of parathyroid viability based on their color changes may not be reliable. 21 Induced capsular bleeding by incisional biopsy is commonly performed 11,21,22 but is subject to observers’ variation, and inadvertent damage to the parathyroid gland may occur. Assessment of individual parathyroid gland viability based on sophisticated Doppler flowmetry has been reported, 23 but the results are difficult to apply in daily clinical use. Routine autotransplantation of at least one parathyroid gland has been advocated as a procedure to prevent permanent hypoparathyroidism 11,20 but results in an increased risk of postoperative hypocalcemia. 20 The use of the quick PTH assay provides a way to assess overall parathyroid function during thyroidectomy 16 and to assist in selecting patients requiring parathyroid autotransplantation. 20 Parathyroid reimplantation for questionably viable glands should be performed when parathyroid insufficiency is confirmed during an operation. The surgeon can avoid unnecessary dissection to search for parathyroid glands in the presence of normal parathyroid function.
Our preliminary result shows that the quick PTH assay can be applied to monitor parathyroid function during thyroidectomy. It is a sensitive tool to confirm postoperative normocalcemia and to identify patients at risk of developing postoperative hypoparathyroidism. It allows intraoperative monitoring of parathyroid function and promotes early discharge. By predicting the postoperative outcome during operation, it may also assist the surgeon in considering the use of parathyroid autotransplantation to prevent permanent hypoparathyroidism.
Acknowledgment
The authors thank Miss W. M. Mak, Miss Vickie Lam, Miss Clara Chong, and Miss Pauline Leung for performing the quick PTH assay.
Footnotes
Correspondence: Chung Yau Lo, MS, FRCS (Edin), Division of Endocrine Surgery, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Pokfulam Road, Hong Kong, China.
E-mail: cylo@hkucc.hku.hk
Accepted for publication March 7, 2002.
References
- 1.Ready AR, Barnes AD. Complications of thyroidectomy. Br J Surg 1994; 81: 1555–1556. [DOI] [PubMed] [Google Scholar]
- 2.Brennan J, Moore EJ, Shuler KJ. Prospective analysis of the efficacy of continuous intraoperative nerve monitoring during thyroidectomy, parathyroidectomy, and parotidectomy. Otolaryngol Head Neck Surg 2001; 124: 537–543. [DOI] [PubMed] [Google Scholar]
- 3.McHenry CR, Speroff T, Wentworth D, et al. Risk factors for postthyroidectomy hypocalcemia. Surgery 1994; 116: 641–648. [PubMed] [Google Scholar]
- 4.Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: Incidence and prediction of outcome. World J Surg 1998; 22: 718–724. [DOI] [PubMed] [Google Scholar]
- 5.Lo CY, Lam KY. Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery 1998; 124: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 6.Demeester-Mirkine N, Hooghe L, Van Geertruyden J, et al. Hypocalcemia after thyroidectomy. Arch Surg 1992; 127: 854–858. [DOI] [PubMed] [Google Scholar]
- 7.Wingert DJ, Friesen SR, Iliopoulos JI, et al. Post-thyroidectomy hypocalcemia: Incidence and risk factors. Am J Surg 1986; 152: 606–610. [DOI] [PubMed] [Google Scholar]
- 8.Marohn MR, LaCivita KA. Evaluation of total/near-total thyroidectomy in a short stay hospitalisation: safe and cost-effective. Surgery 1995; 118: 943–947. [DOI] [PubMed] [Google Scholar]
- 9.Adams J, Andersen P, Everts E, et al. Early postoperative calcium levels as predictor of hypocalcemia. Laryngoscope 1998; 108: 1829–1831. [DOI] [PubMed] [Google Scholar]
- 10.Anders S, Johansson K, Smeds S. In situ preservation of the parathyroid glands during operations on the thyroid. Eur J Surg 1997; 163: 33–37. [PubMed] [Google Scholar]
- 11.Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust NZ J Surg 1999; 69: 794–799. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum SR, Thompson AR, Hutcheson BA. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery 1988; 104: 1121–1127. [PubMed] [Google Scholar]
- 13.Irvin GL III, Derio GT III. A new, practical intraoperative hormone assay. Am J Surg 1994; 168: 466–468. [DOI] [PubMed] [Google Scholar]
- 14.Gordon LL, Snyder WH III, Wians F Jr, et al. The validity of quick intraoperative parathyroid hormone assay: an evaluation of seventy-two patients based on gross morphologic criteria. Surgery 1999; 126: 1030–1050. [DOI] [PubMed] [Google Scholar]
- 15.Gardner SC, Leight GS Jr. Initial experience with intraoperative PTH determinations in the surgical management of 100 consecutive cases of primary hyperparathyroidism. Surgery 1999; 126: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 16.Kao PC, van Heerden JA, Taylor RL. Intraoperative monitoring of parathyroid procedures by a 15-minute parathyroid hormone immunochemiluminometric assay. Mayo Clin Proc 1994; 69: 532–536. [DOI] [PubMed] [Google Scholar]
- 17.Moore Jr. Oral calcium supplements to enhance early hospital discharge after bilateral surgical treatment of the thyroid gland or exploration of the parathyroid glands. J Am Coll Surg 1994; 178: 11–16. [PubMed] [Google Scholar]
- 18.McHenry CR, Pollard A, Walfish PG, et al. Intraoperative parathormone level measurement in the management of hyperparathyroidism. Surgery 1990; 108: 801–808. [PubMed] [Google Scholar]
- 19.Wong WK, Wong NACS, Farndon JR. Early postoperative plasma calcium concentration as a predictor of need of calcium supplement after parathyroidectomy. Br J Surg 1996; 83: 532–534. [DOI] [PubMed] [Google Scholar]
- 20.Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery 2001; 129: 318–323. [DOI] [PubMed] [Google Scholar]
- 21.Kuhel WI, Carew JF. Parathyroid biopsy to facilitate the preservation of functional parathyroid tissue during thyroidectomy. Head Neck 1999; 21: 442–446. [DOI] [PubMed] [Google Scholar]
- 22.Katz AD. Parathyroid autotransplantation in patients with parathyroid disease and total thyroidectomy. Am J Surg 1981; 142: 490–493. [DOI] [PubMed] [Google Scholar]
- 23.Johansson K, Ander S, Lennquist S, et al. Human parathyroid blood supply determined by laser-Doppler flowmetry. World J Surg 1994; 18: 417–420. [DOI] [PubMed] [Google Scholar]