Abstract
Objective
To study fibroblasts and mast cells in human peritoneal adhesions and to evaluate whether their interaction plays a role in adhesion development.
Summary Background Data
Myofibroblasts play a critical role in wound repair/fibrosis. Mast cells influence the formation of peritoneal adhesions in a rat model, and they are modulators of fibroblast functions.
Methods
Peritoneal adhesion biopsies were processed for either histology (H&E, toluidine blue) or immunohistochemistry (tryptase, laminin, collagen type IV and VIII, and α-SMA) or grown as explants for obtention of fibroblasts. The effects of mast cell (HMC-1) sonicate and selected mast cell mediators and cytokines on fibroblast proliferation ([3H]thymidine) and collagen synthesis ([3H]proline) and on fibroblast contractile activity (tridimensional collagen lattice) were evaluated. Mast cell mediators influencing fibroblast proliferation were partially characterized by enzymatic susceptibility and FPLC gel filtration column chromatography.
Results
Most of the fibroblasts in peritoneal adhesions were identified as α-SMA-positive myofibroblasts. Mast cell hyperplasia was observed and more than one third of the mast cells were degranulated. Few mast cells showed a faint staining for laminin or collagen type IV and VIII. Mast cell sonicate increased fibroblast proliferation and contractile activity while decreasing collagen synthesis. Mast cell sonicate proliferating activities were found to be proteinase-sensitive with a molecular weight of more than 158 kd, of ∼40 kd, and of less than 10 kd. TGF-β and tryptase enhanced collagen synthesis; TNF-α and chymase decreased it. None of the selected mediators increased fibroblast proliferation.
Conclusions
Myofibroblasts are the main connective tissue cells present in human peritoneal adhesions, and mast cells play a direct role in peritoneal adhesion formation.
Peritoneal adhesions continue to be a significant cause of postlaparotomy complications such as severe mechanical bowel obstruction, female infertility, or pelvic pain. 1 The exact cause of adhesions is still unclear even though mechanical trauma, ischemia, infection, and the presence of foreign bodies have been attributed a role. 2 In fact, all these processes, by inducing a strong inflammatory response and consequent tissue damage, also seem to start tissue repair. It is probably the imbalance between damage and repair that leads to peritoneal adhesion formation. In cutaneous wounds, for example, it is known that both in the inflammatory responses and in the exaggerated healing processes, fibroblasts migrate, proliferate, change the phenotype into myofibroblasts, and produce extracellular matrix. 3 Myofibroblasts, a ubiquitous and unique group of smooth muscle-like fibroblasts, have also been shown to be an integral part of the gastrointestinal system. In addition, myofibroblasts might play a major role in peritoneal adhesion formation because they are the main cells responsible for the production of matrix molecules such as collagen and glycosaminoglycans, and for the contraction of the proliferated granulation tissue to limit the exposed surface area of the wound. 4 In any event, other cells besides myofibroblasts, such as mesenteric stem cells, mesothelial cells, and smooth muscle cells, might also participate in adhesion formation.
Some inflammatory cells, such as mast cells, eosinophils, macrophages, and more recently neutrophils, have been shown to be able to directly influence some of the fibroblast and myofibroblast biochemical-functional properties. 4–6 Among these cells, mast cells, the recognized key cells of allergy, have been implicated in a number of chronic inflammatory diseases such as scleroderma, chronic graft-versus-host disease, idiopathic lung fibrotic disease, and liver cirrhosis. 6,7 In addition, mast cells have been attributed a role in peritoneal adhesions in a rat model in which their inhibition was found to be effective in delaying or attenuating adhesion formation. 8,9 Mast cells, by the activity of specific mediators and of cytokines, can induce proliferation and modulate collagen synthesis in fibroblasts obtained from skin and lung tissues. 10 Additionally, recent observations indicate that mast cells have the ability in normal and pathologic human tissues to directly produce type VIII collagen. 11 Murine mast cell lines and primary mast cells can synthesize basement membrane components such as laminin and collagen type IV. 12 However, studies to assess mast cell activity toward intestinal fibroblasts, and therefore to explain the direct role of mast cells in intestinal fibrosis, are still missing.
In the present study our aim was to clarify the role of mast cells in human peritoneal adhesion formation by evaluating their presence in peritoneal adhesions and by characterizing their interaction with fibroblasts obtained from peritoneal adhesion biopsies.
METHODS
Histology, Immunohistochemistry, and Immunocytochemistry
Twenty specimens were collected at the time of surgery from peritoneal adhesion patients after an informed consent was given according to the Helsinki Declaration. Patients ranged in age from 21 to 84 years. The estimated maturity of the adhesions was calculated from the date of last abdominal or pelvic surgery and ranged from 4 months to 9 years. Specimens were immediately fixed in Carnoy fixative, embedded in paraffin wax, and sequentially sectioned at 3 μm. Sections were routinely stained with hematoxylin-eosin (H&E) for general observation and with acidic toluidine blue (pH 0.5) to identify mast cells. Double immunohistochemical staining was performed by using rabbit antihuman laminin or rabbit antihuman collagen type IV polyclonal antibodies (Biogenex, CA), or rabbit antihuman collagen type VIII polyclonal antibodies (a generous gift from Prof. T. James Nèale, Wellington, New Zealand) and mouse antihuman tryptase monoclonal antibody (Chemicon, Temecula, CA). Sections were predigested at 37°C with 0.1% pepsin (Sigma, St. Louis, MO) in 10 mmol/L HCl for 30 minutes, and subsequently incubated with 3% hydrogen peroxide and goat nonimmune serum for 10 minutes at room temperature. The sections were incubated with either one of the antiextracellular matrix antibodies for 60 minutes. Biotinylated secondary antibody and streptavidin-alkaline phosphatase were applied and NBT/BCIP was used to show the color (dark-purple stain). The sections were then incubated with double staining enhancer for 30 minutes, and subsequently with the antihuman tryptase antibody, the biotinylated secondary antibody, and streptavidin-peroxidase. AEC was used to show the color (intense red) for tryptase. Nonimmune goat serum and all the double staining reagents were bought from Zymed Laboratories Inc. (Histostain-DS system, CA). Tissue sections and cultured fibroblasts (see below) were stained with mouse antihuman α-smooth muscle actin antibody (α-SMA, a kind gift from Prof. Giulio Gabbiani, Dept. of Pathology, CMU, Geneva, Switzerland), followed by Zymed Histostain-Plus staining kit staining. Controls consisted of tissues/cultured fibroblasts incubated with nonrelevant goat serum or phosphate-buffered saline instead of the primary antibodies. Mast cell counts were systematically performed on 10 different areas of the specimen chosen after a qualitative evaluation of the whole section because of the relative higher number of mast cells. Mast cells were counted both in toluidine blue- and tryptase-stained sections at ×200.
Peritoneal Adhesion Fibroblast Obtention and Culture
Peritoneal adhesion biopsies were cut into 1-mm3 pieces, put as explants into multiwell cluster tissue plates (24 wells, NUNC, Nalge Nunc International, Denmark), and cultured in 0.5 mL Dulbecco’s modified Eagle’s medium (DMEM) containing 2 mmol/L glutamine, 100 u/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum (FCS) (supplemented DMEM) (Biologic Industries, Israel) until fibroblast outgrowth. Fibroblasts were then cultured and subcultured after trypsinization (0.5% trypsin-EDTA in balanced salt solution without Ca2+/Mg2+, 5 minutes at 37°C) (Sigma Chemicals, Israel), in supplemented DMEM at 37°C and in a humidified atmosphere of 5% CO2. Fibroblasts were used between the third and eighth passages.
Mast Cell Sonicates and Mast Cell Mediators and Cytokines
The human mast cell line, HMC-1 (a kind gift from Dr. J. Butterfield, Mayo Clinic, Rochester, MN), was cultured as described. 13 HMC-1 sonicates were obtained by bath sonication (1 minute, 4°C, Heat Systems Ultrasonics, 50% duty cycle, output 5). Sonicates were microcentrifuged for 5 minutes and debris-free sonicate supernatants (“sonicate”) were collected into aliquots and stored until use at −80°C.
The following mast cell mediators were used in fibroblast proliferation and collagen production studies: tryptase (6.25–25 nM) and chymase (3.125–12.5 nM) (a kind gift from Dr. Robert Numerof, Arris Pharmaceuticals, San Francisco, CA); histamine (10−4–10−7 M, Sigma, St. Louis, MO); and recombinant human TNF-α (12–120 ng/mL) and ultrapure natural human TGF-β (2–20 ng/mL) (both from Genzyme, MA). In all the experiments involving tryptase, heparin (Sigma, St. Louis, MO) was added to the enzyme at a molar ratio of 1:1 to protect its activity. 14 Therefore, the effect of similar concentrations of heparin on fibroblast proliferation and collagen production was also investigated. In some selected collagen experiments, mast cell sonicate (21 × 104 mast cell sonicate in 105 μL) was preincubated with monoclonal antibodies against recombinant human TNF-α (0.9–9 μg/mL, R&D Systems Inc.) at 37°C for 30 minutes before addition to the fibroblasts. In some experiments, chymase (10 nM) and angiotensin I (10−5–10−7 M, Sigma, MO, USA) were preincubated for 30 minutes to convert angiotensin I to angiotensin II 15 and then were added to the fibroblasts. In these experiments, angiotensin II (10−5–10−7 M, Sigma, MO, USA) was used as control.
Fibroblast Proliferation and Collagen Production
Proliferation of subconfluent fibroblast monolayers was assessed by using the [3H]thymidine-incorporation assay. 16 Fibroblasts from 15 patients were included in this study. Fibroblasts were seeded in 96-well plates (5 × 103/well) in 200 μL of supplemented DMEM/10% FCS overnight. Cells were then washed twice with supplemented DMEM/2% FCS. Mast cell sonicate (0.7–437.5 × 104/mL in 200 μL DMEM/2% FCS) or selected mast cell mediators and cytokines (in 200 μL, see above) were added for 24 hours. [3H]thymidine (NEN Life Science Products, Inc., Boston, MA) was added as a final 24-hour pulse (1 μCi/well), and samples were processed as described previously. 16 Collagen production by confluent fibroblast monolayers was assessed by [3H]proline incorporation into collagenous proteins. Fibroblasts from 11 patients were included in this study. Fibroblasts were seeded in 24-well tissue culture plates (5 × 103/well) in 0.5 mL of supplemented DMEM/10% FCS until confluence. Mast cell sonicate (0.336–210 × 104 cells/mL) or selected mast cell mediators and cytokines (see above) were added in 0.5 mL of DMEM containing 5% FCS, 50 μg/mL β-aminopropionitrile, and 50 μg/mL ascorbic acid, and the cultures were incubated for 24 hours. At that point [3H]proline (10 μCi/well) (NEN Life Science Products) was added for 24 hours, and samples were processed as described previously. 16
Mast Cell Sonicate Proteinase Sensitivity and Fractionation
Proteinase sensitivity of the mast cell sonicate was evaluated by incubating the sonicate with chymotrypsin, proteinase K, papain, or trypsin (Sigma, MO, USA) (0.05 mg/mL, 1 mg proteinase for 4 × 107 cells) for 30 minutes at 37°C. After 30 minutes, phenylmethylsulfonyl fluoride (PMSF, Sigma, MO, USA) was added (1 mmol/L) to neutralize the proteinase remnants before adding the sonicate to the peritoneal adhesion fibroblasts for the proliferation assay. For fractionation, 1.9 mL of concentrated mast cell sonicate (20 × 106/mL) was run on the AKTA Explorer system using a Pharmacia Superdex 75 60 × 1.6-cm gel filtration column pre-equilibrated with phosphate-buffered saline (pH 7.4) at 1 mL/min, and 3-mL fractions were collected. Molecular weight calibration of the Superdex 75 was performed under the same conditions using a gel filtration calibration kit (gamma globulin 158 kd, BSA 67 kd, ovalbumin 43 kd, chymotrypsin A 25 kd, myoglobin 17 kd, cyanocobalamin 1.3 kd) (Pharmacia). The proliferating activity of each eluent was measured using 17.5-μL aliquot.
Tridimensional Collagen Lattice Cultures and Contraction
Three-dimensional collagen lattice cultures were performed as previously described. 17 Fibroblasts from three patients were used in these experiments. Briefly, to type I collagen (rat tail tendon) in 35-mm bacteriological dishes (Corning, NY), fibroblasts (8–11 × 104 per lattice) and mast cell sonicate (103–106 per lattice) were added before fibrillation and lattice formation. Lattice diameter was measured daily by placing the dishes on a graduated ruler placed on a black surface.
Statistical Analysis
Results are presented as mean ± SEM. Mast cell numbers in tissue sections were analyzed using the Student two-tailed t test. Proliferation, collagen production, and collagen lattice contraction by fibroblasts were analyzed by the one-way analysis of variance. P < .05 was considered significant.
RESULTS
Immunohistochemical Evaluation of Peritoneal Adhesions for Mast Cells and Myofibroblasts
Mast cell hyperplasia was demonstrated both in toluidine blue- and antitryptase-stained sections of peritoneal adhesion biopsies. As shown in Figure 1, in toluidine blue-stained sections, several mast cells were identified, and in antitryptase-stained sections, even more mast cells were demonstrated and several partially degranulated mast cells were evident. Mast cell number in toluidine blue-stained sections was 9.88 ± 1.19 (n = 15); in tryptase-stained sections it was 15.73 ± 1.64 (n = 15). No statistically significant difference was found in mast cell numbers in adhesions with an estimated maturity of a few months or a few years. In the tryptase-stained sections, the number of partially degranulated mast cells was evaluated and found to be 37.19% of the total. In H&E-stained sections, monocytes, macrophages, lymphocytes, and neutrophils were also found in some patients, while a few eosinophils were observed occasionally. By double immunohistochemical staining, rarely a few mast cells stained weakly for laminin and collagen type IV and type VIII (not shown).
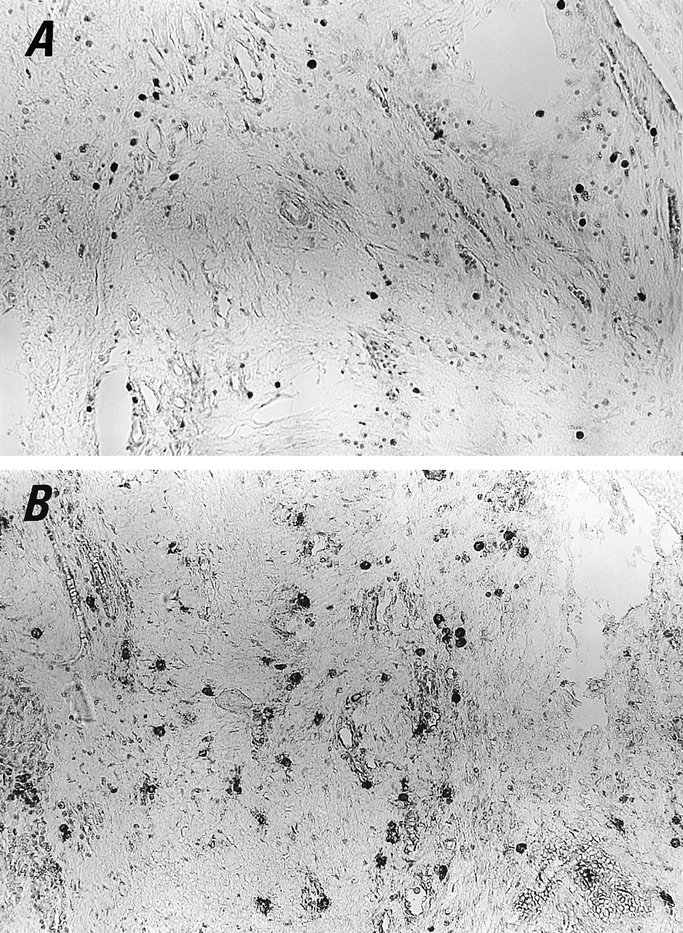
Figure 1. Mast cells are present in human peritoneal adhesion tissue. Biopsies were stained with either acidic toluidine blue (A) or antitryptase antibody (B). A and B were photographed in the same area of a serial section. In B, more mast cells were demonstrated, and several partially degranulated mast cells are evident. (Original magnification ×200)
The immunohistochemical staining for α-SMA used to identify myofibroblasts in the peritoneal adhesion biopsies revealed that the majority of the fibroblasts were strongly positive for α-SMA (Fig. 2). Also, the immunocytochemical staining for α-SMA of fibroblasts that outgrew from peritoneal adhesion biopsies showed that most of the fibroblasts displayed α-SMA protein, indicating their nature as myofibroblasts.
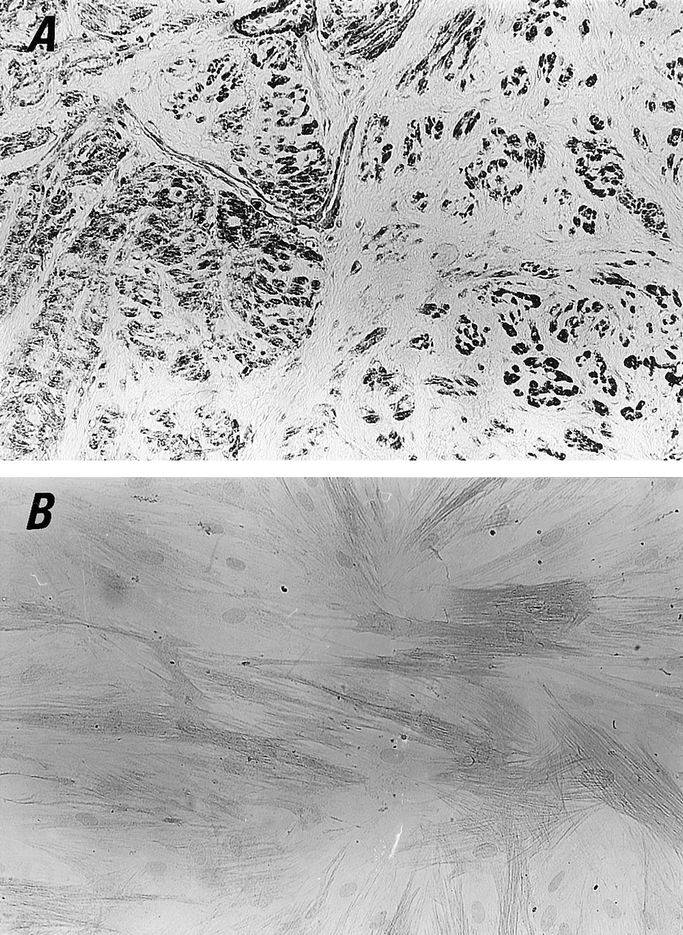
Figure 2. Myofibroblasts in human peritoneal adhesion biopsies and cultured fibroblasts. Immunostaining for the characterization of myofibroblast was performed with antihuman α-SMA antibody (original magnification ×200). (A) Pronounced myofibroblast hyperplasia was observed by the positive staining with α-SMA antibody. (B) Fibroblasts obtained from human peritoneal adhesion explant cultures are mostly myofibroblasts because of their positive staining with α-SMA. Counterstained with hematoxylin.
Effect of Mast Cell Sonicate and Mast Cell Mediators and Cytokines on Fibroblast Proliferation
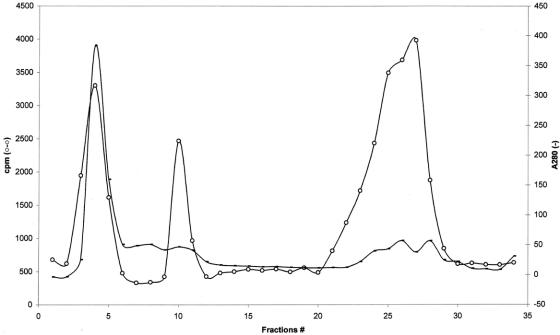
In a preliminary series of experiments we evaluated the effect of different mast cell sonicate concentrations on fibroblast proliferation. As shown in Figure 3, mast cell sonicate induced a concentration-dependent increase in fibroblast proliferation. The highest concentration of mast cell sonicate (437.5 × 104/mL) did not result in a significant increase in proliferation when compared to the next lower mast cell concentration (87.5 × 104/mL), and when tested on some monolayers it was sometimes cytotoxic. Mast cell sonicate significantly increased fibroblast proliferation in all the fibroblast cultures assessed (ie, from the 15 patients). When assessed at 17.5 × 104/mL mast cell concentration, the increase in proliferation was found to be between 1- and 10-fold. To identify the mast cell mediator or mediators that could induce proliferation, selected profibrogenic mast cell mediators and cytokines were added to the fibroblasts. At all the tested concentrations, chymase, histamine, heparin, and TNF-α had no effect, while tryptase decreased proliferation in 1 case out of 8 and TGF-β increased proliferation in 1 case out of 10 (P < .001). In addition, neither chymase together with angiotensin I, or angiotensin II alone affected proliferation (not shown). To partially characterize the mast cell mediator or mediators inducing fibroblast proliferation, we assessed the mast cell sonicate protease sensitivity and performed gel filtration chromatography. Incubation of the mast cell sonicate with chymotrypsin, proteinase K, papain, or trypsin significantly inhibited its proliferating effect (from 32.69% to 88.97%) toward the different patients’ fibroblast monolayers, indicating its protein nature. Chymotrypsin inhibited proliferation by 32.69% to 78.99% (P < .05–0.001, n = 5), proteinase K by 33.13% to 77.64%, papain by 52.69% to 88.97%, and trypsin by 45.09% to 54.56% (P < .001, n = 5). After fractionation on a Superdex 75 column, the proliferating activity of the eluent appeared in three different groups of fractions: fraction I (fractions 3–5, molecular mass > 158 kd), fraction II (fractions 10 and 11, molecular mass ∼40 kd), and fraction III (fractions 22–28, molecular mass < 10 kd) (Fig. 4).
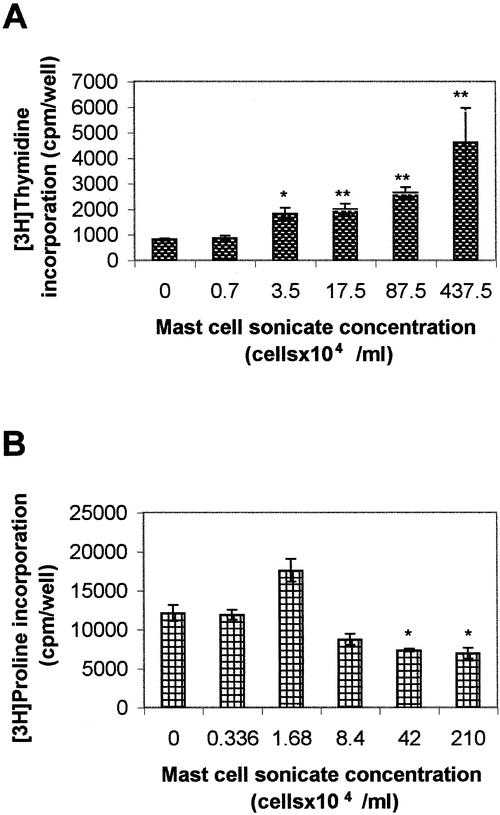
Figure 3. Effects of mast cell sonicate on peritoneal adhesion fibroblast proliferation and collagen synthesis. (A) Subconfluent human peritoneal adhesion fibroblast monolayers were incubated with different concentrations of human mast cell sonicate for 48 hours, and proliferation was evaluated by [3H]thymidine incorporation. Data are the mean ± SEM of three experiments performed in triplicate. (B) Confluent human peritoneal adhesion fibroblast monolayers were incubated with different concentrations of human mast cell sonicate for 48 hours, and collagen production was evaluated by [3H]proline incorporation. Data are the mean ± SEM of three experiments performed in triplicate. *P < .05, **P < .01.
Figure 4. Superdex 75 gel filtration column chromatography of mast cell sonicate. Protein fractionation of mast cell sonicate by a Superdex 75 column was carried out as described in text. –, A280nm value; o-o, proliferating activity.
Effect of Mast Cell Sonicate and Mast Cell Mediators and Cytokines on Fibroblast Collagen Production
In a preliminary series of experiments we evaluated the effect of different mast cell sonicate concentrations on fibroblast collagen production. As shown in Figure 3, mast cell sonicate decreased collagen production at the concentration of 42 × 104 and 210 × 104 mL; at lower concentrations, mast cell sonicate had no effect on collagen synthesis (P > .05). Mast cell sonicate decreased fibroblast collagen production in almost all the 10 different fibroblast cultures assessed. When assessed at 42 × 104/mL mast cell sonicate concentration, the decrease in collagen production was found to be between 1.67% and 86.84%. Both TNF-α and chymase significantly decreased collagen production in almost all the tested fibroblasts, while tryptase increased collagen production in 5 of 7 fibroblast cultures and TGF-β increased collagen production in 6 of 10 fibroblast cultures (Table 1). Histamine, heparin, and angiotensin II alone had no effect on collagen production. Chymase preincubated with angiotensin I decreased collagen production due to chymase’s effect. Anti-TNF-α neutralizing antibodies added to mast cell sonicate had no effect (data not shown).
Table 1. EFFECT OF MAST CELL SONICATE AND MAST CELL MEDIATORS AND CYTOKINES ON HUMAN PERITONEAL ADHESION FIBROBLAST COLLAGEN PRODUCTION
Collagen synthesis was assessed by [3H]proline incorporation. Data are expressed as the percentage of increase (+) or decrease (−) in comparison to cultures incubated with medium alone.
*P < .05.
**P < .01.
***P < .001.
Effect of Mast Cell Sonicate on Tridimensional Collagen Lattice Contraction
A tridimensional collagen lattice was used as an in vitro model for the study of the fibroblast contractile property. Addition of increasing concentrations of mast cell sonicate (103–106 per lattice) to peritoneal adhesion fibroblasts embedded in a collagen lattice significantly enhanced their contraction (1.07- to 1.31-fold, P < .05). This effect was particularly evident and significant on days 2 to 4 and at the concentration of 105 to 106 mast cells per lattice (Fig. 5).
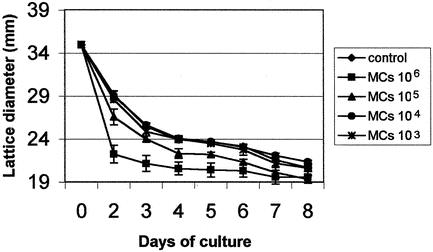
Figure 5. Mast cells increase collagen lattice contraction by peritoneal adhesion fibroblasts. Human peritoneal adhesion fibroblasts were seeded in a collagen lattice in the presence of different concentrations of mast cell sonicate, and the lattice diameter was measured for up to 8 days. Control: lattices incubated with medium alone. Data are the mean ± SEM of three experiments performed in triplicate.
DISCUSSION
In this paper we have shown that the majority of fibroblasts present in human peritoneal adhesions are myofibroblasts, and that human mast cells have the capacity to directly influence the proliferation, collagen production, and contractile activity of these myofibroblasts.
Myofibroblasts are ubiquitous cells found in many locations in the human body. They are also an integral part of the human gastrointestinal system, where they represent the interstitial cells of Cajal and the subepithelial intestinal myofibroblasts. 4,18 They are smooth muscle-like fibroblasts that express some cytoskeletal proteins, such as α-SMA, and play a central role in wound healing by the synthesis of the extracellular matrix and finally by contracting the wound. 4 Myofibroblasts also play a role in the inflammatory stage that proceeds tissue repair by the production of chemokines, cytokines, and arachidonic acid metabolites. 4 In the present work, we have shown for the first time that the majority of fibroblasts that build up human peritoneal adhesions are indeed myofibroblasts because of their positive staining with α-SMA. 4
Mast cells, the critical effector cells of allergic inflammation, also have a central role in tissue reparative reactions and natural immunity. 7,19 Mast cells are normal resident cells of the gastrointestinal tract, where they appear to represent a host defense system that evolved as part of the protective mechanisms. 7 Indeed, mast cells are capable of reacting to a variety of noxious physical, biologic, and chemical stimuli and are usually degranulated when an inflammatory or fibrotic process takes place. 5,7 In a rat peritoneal adhesion model, mast cells have been shown to play an important role. In fact, the administration of the mast cell stabilizers nedocromil or disodium cromoglycate can delay or attenuate adhesion formation. 9 In a study that we recently carried out in a rat model of peritoneal adhesions induced by small intestinal scraping, we showed that postoperative peritoneal adhesion formation correlates with mast cell changes in numbers and activation state. 20 The number of toluidine blue-stainable mast cells was found to increase significantly in the adhesion tissues immediately after the surgical procedure. Thereafter, when adhesions were built of fibroblasts and collagen fibers, it decreased and remained stable. In the present study, we observed that mast cells are a prominent component of the human peritoneal adhesions both by toluidine blue and tryptase staining in both adhesions with a younger and an older estimated period of maturity. The numerical values obtained by the immunohistochemical technique were higher than those obtained by staining mast cell proteoglycans with toluidine blue. Staining of mast cells with toluidine blue seems to be dependent on a sufficient number of intact mast cell granules, while the more sensitive immunohistochemical technique is able to detect partially degranulated mast cells, which still contain enough tryptase. 21 We have indeed found that more than one third of mast cells in peritoneal adhesion tissues stained by antitryptase antibodies were partially degranulated. When the biopsies were stained for laminin and collagen type IV and VIII, only a few mast cells showed a faint staining. This is in accordance with what was described by Rüger et al., who found that mast cells from tissues of the human respiratory tract and of Crohn’s disease mucosa stained infrequently for type VIII collagen. 11 In addition, to the best of our knowledge, only rodent mast cells have been shown to secrete collagen type IV and laminin. 12 Our histochemical observation would therefore indicate that mast cells influence peritoneal adhesion formation via degranulation and mediator release that can in turn influence fibroblasts, and not directly by producing connective tissue products.
We next evaluated the effects of mast cells and mast cell products on fibroblast properties directly involved in fibrotic tissue formation such as proliferation, collagen synthesis, and contractile activity. Addition of mast cell sonicate to peritoneal adhesion fibroblast monolayers increased fibroblast proliferation. Tryptase, chymase, histamine, heparin, TNF-α, and TGF-β have been shown to influence lung or skin fibroblast proliferation, 6,22 and histamine has been reported to induce 3T3 fibroblast proliferation. 23 In addition, receptors for TNF-α, TGF-β RI and RII, c-kit, angiotensin II, and histamine have been shown to be expressed on myofibroblasts. 4 However, these mediators had almost no significant effect on peritoneal adhesion fibroblast proliferation. By proteinase sensitivity and FPLC gel filtration column chromatography, we can conclude that the proliferative activity of the mast cell sonicate belongs to protein(s) fractions of a molecular weight of more than 158 kd, ∼40 kd, and less than 10 kd. The more-than-158-kd fractions might represent a conjugated protein with a very high molecular weight such as proteoglycans, the ∼40-kd fractions may be some cytokines, and the less-than-10-kd fractions may be some small molecules such as histamine and chemokines. It is therefore conceivable that the mast cell proliferative factor or factors act in synergy when together in the sonicate but have no significant activity on peritoneal adhesion fibroblasts when added as single, purified mediators. When assessed on collagen production, mast cell sonicate was found to decrease rather than to increase this fibrosis hallmark. Interestingly, although HMC-1 sonicate and rat peritoneal mast cells increased collagen production in human skin fibroblasts, and a similar effect was observed by the addition of eosinophils, both mast cells and eosinophils decreased collagen synthesis in lung fibroblasts. 10,16 This would indicate an heterogeneity in fibroblast responses rather than in fibroblast activators. Tryptase has been found to enhance lung fibroblast proliferation and collagen type I production, and chymase can directly convert type I procollagen to collagen fibrils. 24–26 Both are able to activate indirectly or directly interstitial procollagenase. 27,28 Chymase is a potent activator of angiotensin I and can convert angiotensin I to angiotensin II, a promotor of myofibroblast activation. 15,29 In addition, TNF-α and TGF-β were found to be fibrogenic factors for human fibroblasts. 22 HMC-1 contains mainly tryptase, produces matrix metalloproteinase 9 (MMP9), and constitutively expresses mRNA for TNF-α and TGF-β. 30,31 In our studies, the addition of TGF-β and tryptase to the peritoneal adhesion fibroblasts significantly increased collagen production, TNF-α and chymase decreased it, and angiotensin II had no effect. On the other hand, TNF-α and TGF-β have been reported to induce intestinal myofibroblast activation or proliferation on myofibroblast derived from different fibrotic tissues. 4,18 These contradictory results may be due to differences in receptor expression or affinity on myofibroblasts outgrown from peritoneal adhesions or other intestinal fibrotic conditions. We can learn from these experiments that the complex interactions of the various mast cell mediators provide the final response of the fibroblasts to the mast cells, and this is probably the most representative of an in vivo situation in which mast cells are activated to release their mediators together.
The contraction of proliferated granulation tissue in wound repair is a physiologic reaction that limits the exposed surface area and therefore facilitates the wound repair process. In this study, we found that the mast cell sonicate significantly enhanced the contraction of a three-dimensional collagen lattice in which peritoneal adhesion fibroblasts were embedded. Interestingly, these data are similar to those reported by Berton et al. for human dermal fibroblasts. 17
In fibrotic disorders in which mast cells’ presence has been described, their hyperplasia usually appears before a dense fibrotic tissue is established. Thereafter, there is a decrease in stainable mast cells, and then their number remains constant. 32–34 This would point to a clear involvement of mast cells not only at the onset of the process but also during later stages.
In this study we have shown that mast cells are a constant feature of human peritoneal adhesions and that they influence myofibroblast proliferation, collagen production, and their contraction, indicating a role for mast cells both at the onset of fibrosis and at later stages. These observations are also in line with the fact that mast cells are long-living cells that have the property to be repeatedly activated and to resynthesize their mediators. 35
Even though we cannot exclude that other cell types can also contribute to adhesion formation, our studies indicate that mast cells are an important regulator of peritoneal adhesion formation through their activities on myofibroblasts and therefore are feasible targets for an immunopharmacological intervention in the prophylaxis and treatment of postoperative peritoneal adhesions.
Acknowledgments
The authors thank Mr. M. Lebendiker (Life Science Institute, Hebrew University of Jerusalem) for his kind help in fractionating mast cell sonicate.
Footnotes
Supported in part by grants from the Ministry of Science and Culture of the State of Niedersachsen, Germany, and the Aimwell Charitable Trust (F.L.-S.).
Correspondence: Francesca Levi-Schaffer, PhD, Department of Pharmacology, School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem, POB 12065, Jerusalem 91120, Israel.
E-mail: fls@cc.huji.ac.il
Accepted for publication November 29, 2001.
References
- 1.Scott-Coombes DM, Vipond MN, Thompson JN. General surgeons’ attitudes to the treatment and prevention of abdominal adhesions. Ann R Coll Surg Engl 1993; 75: 123–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson J. Pathogenesis and prevention of adhesion formation. Dig Surg 1998; 15: 153–157. [DOI] [PubMed] [Google Scholar]
- 3.Clark RAF. Regulation of fibroplasias in cutaneous wound repair. Am J Med Sci 1993; 306: 42–48. [DOI] [PubMed] [Google Scholar]
- 4.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999; 277: C1–C19. [DOI] [PubMed] [Google Scholar]
- 5.Levi-Schaffer F. Mast cell/fibroblast interactions in health and disease. Chem Immunol 1995; 61: 161–185. [PubMed] [Google Scholar]
- 6.Levi-Schaffer F, Weg VB. Mast cells, eosinophils and fibrosis. Clin Exp Allergy 1997; 27 (Suppl 1): 64–70. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997; 77: 1033–1079. [DOI] [PubMed] [Google Scholar]
- 8.Langer JC, Liebman SM, Monk PK, et al. Mast cell mediators and peritoneal adhesion formation in the rat. J Surg Res 1995; 59: 344–348. [DOI] [PubMed] [Google Scholar]
- 9.Liebman SM, Langer JC, Marshall JS, et al. Role of mast cells in peritoneal adhesion formation. Am J Surg 1993; 165: 127–130. [DOI] [PubMed] [Google Scholar]
- 10.Smith S, Levi-Schaffer F. Mast cell-eosinophil-fibroblast crosstalk in allergic inflammation. In: Robinson DS, ed. Immunological Mechanisms in Asthma and Allergic Diseases. London: Karger, 2000. [DOI] [PubMed]
- 11.Rüger B, Dunbar PR, Hasan Q, et al. Human mast cells produce type VIII collagen in vivo. Int J Exp Pathol 1994; 75: 397–404. [PMC free article] [PubMed] [Google Scholar]
- 12.Tompson HL, Burbelo PD, Gabriel G, et al. Murine mast cells synthesize basement membrane components. A potential role in early fibrosis. J Clin Invest 1991; 87: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson G, Blom T, Kusche GM, et al. Phenotypic characterization of the human mast cell line HMC-1. Scand J Immunol 1994; 39: 489–498. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LB, Bradford TR. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem 1986; 261: 7372–7379. [PubMed] [Google Scholar]
- 15.Reilly CF, Tewksbury DA, Schechter NM, et al. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem 1982; 257: 8619–8622. [PubMed] [Google Scholar]
- 16.Levi-Schaffer F, Garbuzenko E, Rubin A, et al. Human eosinophils regulate human lung and skin-derived fibroblast properties in vitro: a role for transforming growth factor β (TGF-β). Proc Natl Acad Sci USA 1999; 96: 9660–9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berton A, Levi-Schaffer F, Emonard H, et al. Activation of fibroblasts in collagen lattices by mast cell extract: a model of fibrosis. Clin Exp Allergy 1999; 30: 485–492. [DOI] [PubMed] [Google Scholar]
- 18.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999; 277: C183–C201. [DOI] [PubMed] [Google Scholar]
- 19.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol 2000; 12: 624–631. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Pappo O, Garbozenko E, et al. Mast cell dynamics and involvement in the development of peritoneal adhesions in the rat. Life Sci 2002; 70: 951–967. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff SC, Wedemeyer J, Herrmann A, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology 1996; 28: 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs EJ, PiPietro LA. Fibrogenic cytokines and connective tissue production. FASEB J 1994; 8: 854–861. [DOI] [PubMed] [Google Scholar]
- 23.Kupietzky A, Levi-Schaffer F. The role of mast cell-derived histamine in the closure of an in vitro wound. Inflamm Res 1996; 45: 176–180. [DOI] [PubMed] [Google Scholar]
- 24.Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest 1991; 88: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber BL, Kew RR, Jelaska A, et al. Human mast cells activate fibroblasts. Tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol 1997; 158: 2310–2317. [PubMed] [Google Scholar]
- 26.Kofford MW, Schwartz LB, Schechter NM, et al. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem 1997; 272: 7127–7131. [DOI] [PubMed] [Google Scholar]
- 27.Gruber BL, Schwartz LB, Ramamurthy NS, et al. Activation of latent rheumatoid synovial collagenase by human mast cell tryptase. J Immunol 1988; 140: 3936–3942. [PubMed] [Google Scholar]
- 28.Saarinen J, Kalkkinen N, Welgus HG, et al. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem 1994; 269: 18134–18140. [PubMed] [Google Scholar]
- 29.Campbell SE, Janicki JS, Weber KT. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J Mol Cell Cardiol 1995; 27: 1545–1560. [DOI] [PubMed] [Google Scholar]
- 30.Kanbe N, Tanaka A, Kanbe M, et al. Human mast cells produce matrix metalloproteinase 9. Eur J Immunol 1999; 29: 2645–2649. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson G, Svensson V, Nilsson K. Constitutive and inducible cytokine mRNA in the human mast cell line HMC-1. Scand J Immunol 1995; 42: 76–81. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins RA, Claman HN, Clark RA, et al. Increased dermal mast cell populations in progressive systemic sclerosis: a link in chronic fibrosis? Ann Intern Med 1985; 102: 182–186. [DOI] [PubMed] [Google Scholar]
- 33.Claman HN, Jaffe BD. Chronic graft-vs-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol 1985; 94: 73–84. [DOI] [PubMed] [Google Scholar]
- 34.Persinger MA, Lepage P, Sinard JP, et al. Mast cell numbers in incisional wounds in rat skin as a function of distance, time and treatment. Br J Dermatol 1983; 108: 179–187. [DOI] [PubMed] [Google Scholar]
- 35.Levi-Schaffer F, Gare M, Shalit M. Unresponsiveness of rat peritoneal mast cells to immunologic reactivation. J Immunol 1990; 145: 3418–3424. [PubMed] [Google Scholar]