Abstract
Objective
To assess the long-term incidence of venous complications, including portal vein and hepatic vein stenoses, in both whole cadaveric and reduced-size cadaveric and living related liver transplants in a pediatric population, and to assess the therapeutic modalities in the treatment of these lesions.
Summary Background Data
A shortage in appropriate-sized liver grafts for pediatric patients led to the use of segmental liver grafts, which became the predominant graft used in 325 of 600 (54%) transplants at the authors’ institution. To assess the long-term impact of this strategy, the authors examined the incidence of late (>90 days) venous complications and the efficacy of all therapeutic interventions.
Methods
Six hundred pediatric liver transplants were performed in 325 patients, with reduced-size or split (RSS; n = 207), living related (LRD; n = 118), or full-size cadaveric grafts (FS; n = 275) from 1988 to 2000. All transplants identified with late portal vein or vena caval stenoses or thromboses from a cohort of 524 grafts with survival greater than 90 days were reviewed for demographics, symptoms, therapeutic intervention, recurrence, morbidity, and mortality.
Results
Fifty lesions were identified in 49 patients (38 portal vein and 12 hepatic vein–cava stenoses). Sex distribution was similar between portal vein and hepatic vein to cava, as was the mean patient age. Portal vein stenoses occurred in 32 LRD, 3 RSS, and 3 FS, while hepatic vein–cava stenoses occurred in 2 LRD, 8 RSS, and 2 FS. In the 38 portal vein stenoses, 9 had prior perioperative portal vein and/or 5 hepatic artery thrombectomies. Portal vein stenoses were identified after bleeding (17/38), ascites (6/38), increased liver function tests (6/38), splenomegaly (5/38), or screening ultrasound (4/38). Portal vein stenosis was associated most often with cryopreserved vein for portal conduits. Excluding conduits, the incidence of late portal vein complications was reduced to 1%. Lesions became symptomatic at a mean of 50.8 ± 184.2 months posttransplant. All patients underwent venous angioplasty with a 66% (25/38) success rate, while 7 of 25 required further angioplasty and stenting. In the 13 unsuccessful angioplasties, 8 required surgical shunts for complete portal vein thrombosis. Recurrence occurred in 9 patients: all were amenable to stenting. Nine patients (24%) eventually died of sepsis (4) and surgical deaths at shunt or retransplant (5). Hepatic vein–cava stenoses occurred after a mean of 37.2 ± 35.2 months, presenting with ascites (n = 10), increased liver function tests (n = 2), and splenomegaly (n = 2). All patients were diagnosed by venogram and managed by balloon dilatation alone (n = 6) or stented (n = 4), with an 80% (10/12) success, with two late recurrences amenable to repeat angioplasty or stenting. Long-term survival was 80% at 1 year.
Conclusions
The use of segmental grafts without venous conduits is not associated with a significant rate of long-term venous complication. When late venous complications do occur, venous angioplasty and stenting are both a safe and effective management modality. If necessary, venous angioplasty may be repeated with the placement of a stent. When this is required, care must be taken to place the stent in a position where the metallic object will not interfere with future surgical manipulations should retransplantation be necessary.
Innovative surgical techniques have allowed the development of pediatric liver transplantation to treat the ravages of end-stage liver failure in children. 1,2 Since the inception of pediatric liver transplantation, there has been a rapid realization that the supply of appropriate-sized organs is insufficient for demand. This inequity has led to a significant death rate among patients on the waiting list. 3–5 Several reports have indicated that this shortage is especially pronounced in children less than 1 year of age. 6 In an attempt to address this shortage of organs and size discrepancy, the techniques of reduced-size, split, and living related liver transplantation were developed. 1,2 These methods of transplantation bridged the gap in appropriate-sized organs and contributed to a decrease in waiting list mortality. 7,8
The methods of split and living related liver transplantation are technically demanding due to the use of short vascular pedicles, which were complicated by a high incidence of perioperative portal vein and hepatic vein thrombosis during the early experience at the University of Chicago. 9 Analysis of patients who received a living donor graft demonstrated that the most significant contributor to early portal vein thrombosis was the use of venous conduits, and most commonly a cryopreserved venous extension. 9,10 As a result of the high incidence of portal venous complications associated with the use of cryopreserved iliac veins, we eliminated all venous conduits, which decreased the incidence of early portal vein thrombosis. Early living related and reduced-size pediatric grafts were implanted to the right hepatic vein. This position was thought to be preferred as it was believed to minimize kinking. However, early reports demonstrated a high incidence of hepatic vein complications. 11 This complication was markedly reduced by triangulating the vena cava to the juncture of the hepatic veins. 11,12 To eliminate conduits, the graft has been moved to the confluence of the left and middle hepatic veins. This technical alteration was embarked on with concern over increased complications secondary to graft torsion. These concerns were not realized; rather, the early experience with the left and middle hepatic vein anastomosis suggested continuing the triangulation technique and maintaining the short cuffs while imbricating the hepatic veins into the vena cava. 12
With the elimination of venous conduits for portal vein anastomoses, the imbrication of the triangulated caval anastomoses, and the improvement of hepatic artery patency, early graft loss was decreased, thus improving overall graft survival. A large proportion of our pediatric liver transplants have achieved long-term patency, and the long-term implications of these technical modifications must be evaluated. We therefore reviewed all pediatric liver transplants from the advent of segmental transplantation at our institution.
METHODS
The records of all 600 pediatric liver transplants performed in 325 children at the University of Chicago from 1988 to July 2000 were retrospectively reviewed. Records were analyzed for patient demographics including age, sex, primary diagnosis, initial or redo liver transplant, and graft type. Reduced-size and living related liver transplants were placed in a piggyback fashion with either a combined left and middle vein orifice used for the hepatic vein implantation or a caval diamond-shaped incision. In the instance of whole cadaveric grafts, these organs were implanted using a standard orthotopic position with caval replacement. Only in the instance of significant size mismatch between the graft and recipient was the piggyback technique used. Diagnostic methods and presenting symptoms were reviewed, with emphasis placed on the success of all therapeutic interventions and the outcomes of all failed attempts. Criteria used by ultrasound studies to identify patients with portal vein stenosis was established as portal vein diameters of 2.5 mm or less in diameter or an accelerated velocity at the stricture or a poststenotic jet of portal vein flow revealed at Doppler ultrasound. If a portal vein stenosis is suspected secondary to symptoms and ultrasound, the patient was scheduled for an interventional radiologic portal vein evaluation. The portal vein angiogram was performed in the following method.
All procedures were performed with the patient under general anesthesia. The liver was punctured with fluoroscopic or ultrasound guidance using a subxiphoid approach with a 21-gauge needle angled laterally toward the patient’s right side. When a Couinaud segment II or III portal vein branch was identified and entered, the needle was exchanged for a 6 French sheath with a nitinol guidewire. Portal venograms with portal venous pressure measurements were obtained. A 0.035-inch angled hydrophilic guidewire was used to pass the stenotic area. After a 50-U/kg heparin bolus was administered directly into the portal vein, the stenotic area was dilated. Postvenoplasty pressures were measured and a completion venogram was performed. In cases of recurrent stenoses or gradients of 5 mmHg or greater, a 20 × 8-mm metallic stent (Wallstent; Boston Scientific, Watick, MA) was deployed and dilated. Postprocedure the transhepatic tracks were routinely embolized.
If a stricture of the hepatic vein or vena cava was suspected clinically, the patient underwent an inferior vena cava venogram and hepatic vein venogram. If a stenosis was identified, vena cava venoplasty was carried out with a right femoral vein puncture performed under ultrasound guidance. The needle was exchanged for a 6 French sheath combination with a 0.018-inch Ultra Select nitinol guidewire. Pre- and poststenotic vena caval pressures as well as pull-through pressures were measured. A 0.035-inch guidewire was used to traverse the stenotic segment. After administering a heparin bolus directly into the vena cava, the stenotic area was angioplastied. In recurrent stenoses or in cases with persistent gradients of 5 mmHg or greater, an appropriate-sized metallic stent was deployed.
Immediately after either portal vein or vena cava interventions, the patient underwent systemic anticoagulation with heparin sodium for 48 to 72 hours to maintain a partial thromboplastin time of 1.5 times higher than normal levels. Patients were then placed on a single baby aspirin a day for life. All patients except two underwent ultrasound examinations before venography. Postprocedure ultrasounds were performed after 24 hours and before discharge and if symptoms recurred.
All data are presented as mean ± standard deviation. Comparisons were made between success and failure of radiologic interventions. All statistical analyses were performed using the Student t test, chi-square, or Kaplan-Meier analysis. Statistical significance was denoted by P < .05.
RESULTS
From 1988 to 2000, 600 pediatric liver transplants were performed in 325 children at the University of Chicago. Two hundred seventy-five (45%) whole grafts, 207 (35%) reduced or split grafts, and 118 (20%) living related grafts were required to bridge the shortage of appropriate-sized organs. Five hundred twenty-four grafts were functional at 90 days. Late portal vein or vena cava stenoses were defined as those that occurred after 90 days after transplantation. Thirty-eight portal vein stenoses were identified for an overall incidence of 38/524 (7.4%), while hepatic vein stenoses occurred with an incidence of 12/524 (2.3%). Distribution of late vascular complications within graft types demonstrated an incidence of 2% (5/275) in whole cadaveric grafts and 5% (11/207) in reduced or split grafts. Overall late vascular complications, including portal vein and hepatic vein stenoses, were highest in living related patients, with 28 of 66 (42%) patients affected when reconstructed with venous conduits and 5 of 52 (10%) patients affected when direct vascular anastomoses were performed. The greatest incidence of portal vein stenoses was in living related liver transplants, with a 27% (32/118) incidence, followed by reduced-size or split liver transplants at 1% (3/207) and whole-sized cadaveric grafts at 1% (3/275). Portal vein reconstruction during reduced-size and living related transplantation was used in 66 instances. The overall incidence of long-term portal vein stenosis was significant in this group at 41% (27/66). However, when the method of portal vein anastomosis was analyzed, the use of cryopreserved venous conduits, whether iliac vein or femoral vein, portended a significantly higher long-term stenosis rate compared to native vessel conduits (24/48 [50%] vs. 3/18 [16%], P < .01). Direct anastomosis of the native and donor portal vein had a lower incidence of late venous stenosis of 10% (5/52;P < .05). Overall, the graft types most commonly complicated by late portal vein stenosis were living related grafts with venous conduits (71%), followed by living related grafts (13%), reduced or split grafts (8%), and whole-sized cadaveric liver grafts (8%).
Children with late portal vein stenosis were more commonly boys, with a 1.5:1 male predominance, compared to a more even (1.2:1) distribution of all patients transplanted (Table 2). Patients with portal vein stenoses were younger, with a mean age of 21.7 ± 36.6 months, compared to 51.6 ± 57.6 months in patients without late venous complications (P < .05). The median age in patients with venous complications was also higher at 9.9 months compared to 8.4 months. Presenting symptoms varied, with the most common being variceal bleeding (n = 17 [45%]), ascites (n = 6 [16%]), elevated liver function tests (n = 6 [16%]), splenomegaly (n = 5 [13%]), or diagnosis solely on incidental ultrasonography (n = 4 [11%]). The time to presenting symptoms varied from 110 days to 105 months (mean 50.8 ± 184.2 months).
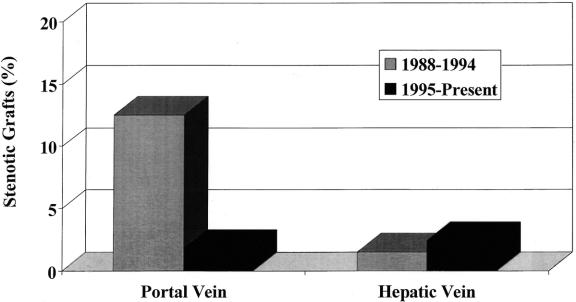
In 36 of 38 patients with portal vein stenosis, the stenosis occurred during their initial transplants for an incidence of 11% (36/325) compared to 0.7% (2/275) after retransplantation (P < .05). With a cumulative incidence of 7.4% (38/524), the lowest occurrence of portal vein stenosis was observed in whole-organ grafts (3/275 [1%]) and reduced grafts (3/207 [1%]). Living related transplants had the highest overall incidence of late venous complications, with 32/118 (27%) (P < .05). When this group was evaluated for type of venous anastomosis performed, those reconstructed with venous conduits had the highest incidence of late stenosis (27/66 [41%]) compared to a direct venous anastomosis (5/52; 10%). When examined by era, there was a significant decrease in late portal vein stenosis when 1988 to 1994 was compared to 1995 to present (33/265 [12.5%] vs. 6/335 [1.8%], P < .01) (Fig. 1). A high incidence of perioperative vascular complications was noted with 11 of 38 (29%) patients reexplored for early portal vein thrombosis (n = 9) or hepatic artery thrombosis (n = 5) (2 patients incurred both complications). Early rejection, defined by a treated rejection with steroid bolus or antibody therapy before 30 days after transplantation, occurred in 3 of 38 (29%), with 5 additional patients experiencing late chronic rejections (5/38 [13%]). These rates are higher than those anticipated in our overall cohort. Six (16%) early biliary complications, defined by bile leak requiring stenting, drainage, or reoperation, were noted in this group. This rate of biliary complications was higher than the 5% rate experienced by the remaining patients.
Figure 1. Incidence of long-term portal vein and hepatic vein stenosis divided by era, comparing 1988 to 1994 and 1995 to present.
All patients except two underwent ultrasound before invasive radiologic studies. The overall success rate for interventional radiologic procedures was 25 of 38 (66%). Angioplasty alone was therapeutic in 18/38 (47%), while deployment of endovascular stents was required in 10 patients resulting in a success rate of 70% (7/10) (Fig. 2). Eight patients were noted at the time of radiographic imaging to have complete portal vein thrombosis. Two patients were successfully recanalized by instillation of urokinase and serial angioplasties. This resulted in a 75% (6/8) failure rate when portal vein thrombosis was present. However, excluding portal vein thrombosis, this increased the success rate of percutaneous venous angioplasty and stenting to 77%. The recurrence rate was 36% (9/25), with all occurring in living related grafts at a mean of 6.5 ± 4.8 months. Eight patients presented with associated increases in their liver function tests. Five of the eight (63%) had normalization of their liver tests within 48 hours of their angioplasty. The recurrences were equally divided between the conduit (n = 5) and direct anastomoses (n = 4). All recurrences were amenable to intravascular stenting. Unsuccessful angioplasty patients were medically managed (n = 5) or underwent surgical decompression shunts (n = 8). The majority of patients underwent mesocaval shunts (n = 4), followed by splenorenal (n = 2) and mesorex (n = 2) shunts.
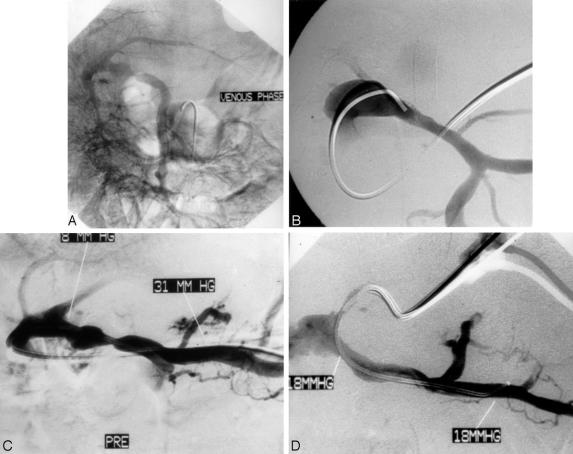
Figure 2. Portal venous stenosis demonstrated by venogram obtained with contrast injection into the extrahepatic portal vein after percutaneous puncture. (A) Fluoroscopic image demonstrating venoplasty of stenotic segment. (B) Site of recurrent stenosis requiring stenting. (C) Placement of endovascular stent at stenotic point (D).
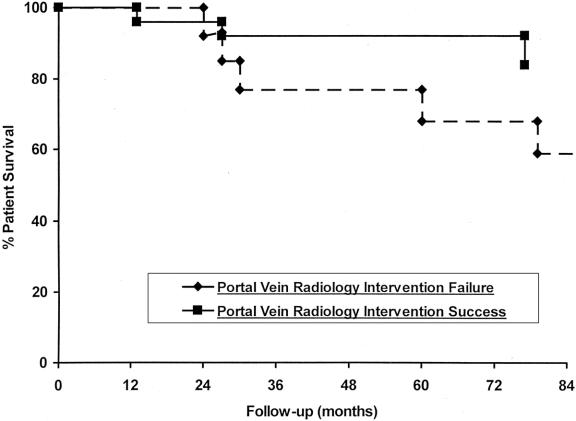
The overall mortality for this group was 24% (9/38), with an overall mortality of 13% (3/24) for those with successful angioplasties and 46% (6/13) for those with failed angioplasties. Successful angioplasty improved survival over patients who failed to respond to radiologic modalities (Fig. 3). After successful angioplasty, two patients eventually developed recurrent end-stage liver disease from chronic rejection and died after retransplant; the other developed cytomegalovirus hepatitis and died pretransplant. The third patient died of PTLD with an overwhelming pulmonary viral infection. The highest mortality was incurred in the unsuccessful angioplasty group, where four patients died after shunt surgery, one of PTLD, and the last from graft-versus-host disease. Retransplantation was required in eight patients overall, with the majority for chronic (5/6) and acute (1/6) rejection. One patient developed hepatic artery thrombosis after an unsuccessful angioplasty attempt; an additional patient had a failed mesocaval shunt that required retransplantation.
Figure 3. Patient survival graph with respect to successful and unsuccessful balloon angioplasty or stenting for late portal vein stenoses.
Hepatic vein stenosis occurred at a lower frequency, with 12 identified cases. The majority of patients were boys, with a 2:1 ratio; mean age was 25.0 ± 34.8 months (median 9.9 months) (see Table 2). Patients with hepatic vein stenosis were younger (median age 9.9 years) versus patients without hepatic vein stenosis (mean age 51.4 ± 57.2 months, median 15.8 months;P < .05). Mean time to presentation of symptoms was 37.2 ± 35.2 months (range 2–120 months). The highest incidence of this type of stenosis was in the reduced-size or split group (4% [8/207]), followed by living related grafts (2% [2/118]) and whole-sized grafts (1% [2/275]). The overall incidence of this complication was low at 12/600 (2%). When examined by era, 1988 to 1994 and 1995 to present, the incidence of this complication was unchanged (4/265 [1.5%] vs. 8/335 [2.4%], P = .4) (see Fig. 1). The most common presenting symptom was ascites (n = 10), followed by rising liver function tests (n = 2) and new-onset splenomegaly (n = 2). There was an even division between occurrence in primary transplants (n = 6) and retransplants (n = 6). The highest incidence was in patients requiring three or more transplants (4/80 [5%]) compared to a single transplant (6/325 [1.8%]) or two transplants (2/275 [0.7%]) (P < .05). Early vascular complications were also more common in patients with late hepatic vein stenoses (4/12 [33%]). Early rejection or biliary leaks were not present in this group.
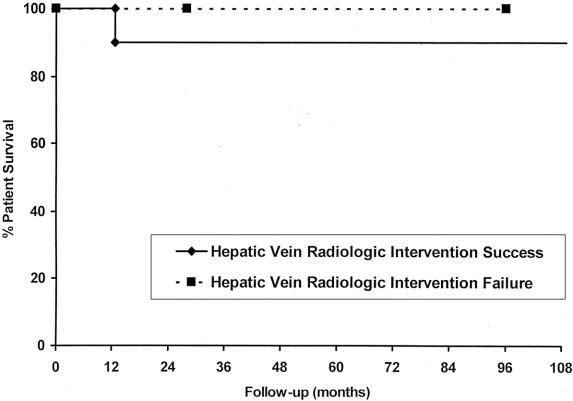
The overall success rate for radiologic interventions was 83% (10/12), with balloon angioplasty alone successful in 6/8 (75%) and stenting successful in 7/10 (70%) (Fig. 4). Recurrence occurred in a single patient after balloon angioplasty alone at 17 months and was managed with stenting. Two patents died after angioplasty, including the one patient who had the recurrence while on the waiting list for retransplantation. One patient died of multiorgan sepsis and the other of uncontrolled variceal bleeding. Three patients were retransplanted, two for chronic rejection and the other for biliary complications. The two patients with angioplasty failures were successfully managed medically, with a 100% survival in this small group. The overall survival in the successful angioplasty group was 75% at 11 years but was not statistically different from the treatment failure group (Fig. 5).
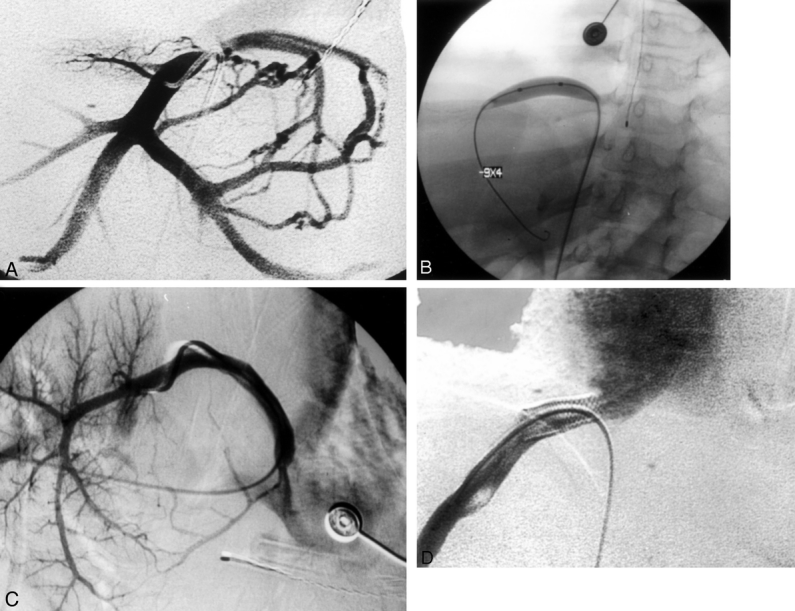
Figure 4. Hepatic vein stenosis demonstrated by venogram obtained with contrast injection into the inferior vena cava after a percutaneous femoral puncture. (A) Fluoroscopic image demonstrating venoplasty of stenotic segment. (B) Site of recurrent stenosis requiring stenting. (C) Placement of endovascular stent at stenotic point (D).
Figure 5. Patient survival graph with respect to successful and unsuccessful balloon angioplasty or stenting for late hepatic vein stenoses.
DISCUSSION
Reduced-size, split, and living related liver transplantation has bridged the gap of appropriate-sized organs for patients on the waiting list. These innovative techniques have diminished the mortality on the waiting list; indeed, it has dramatically declined at our center. 1,7,8 Early reports from our group and others indicated that there is a high incidence of portal vein vascular complications when venous conduits are used to reconstruct the donor and recipient portal vein. 9,10 The reported incidence of early portal vein thrombosis varies between 0% to 30%, depending on the series selected. 9,13–16 Several factors have been implicated, including portal vein size, type of graft used, and positional factors. Despite several reports on the early incidence of portal vein stenosis, little has been reported on both the late incidence and impact of portal vein stenosis.
Late portal vein and hepatic vein stenoses commonly present with varied clinical scenarios, including new-onset ascites, variceal bleeding, splenomegaly, increased liver function tests, lower extremity edema, or renal insufficiency. 9 The diagnosis of portal vein stenosis or thrombosis can be suggested by abdominal ultrasound, but the specificity of this test is limited. Confirmation and therapeutic intervention for all venous stenoses are done by invasive angiography, thus making interventional radiology essential. 17,18 However, in our experience the presence of symptoms in conjunction with angiographic evidence of major retroperitoneal collaterals may be more indicative of a clinically significant stenosis than a measurable angiographic gradient. 18 Thus, a majority of portal vein and hepatic vein lesions have been managed by balloon angiography and metallic stenting for recurrent lesions. 19–21
Our series indicates that the use of living related grafts with portal vein conduits carries a higher incidence of portal vein complications. In our report of early portal vein thrombosis and stenosis, we recommended that vascular conduits should be avoided and the technique of direct portal vein reconstruction using the right and left branch patch of the donor be adopted. Since the change in surgical technique, our incidence of late portal vein stenosis has dropped precipitously. We must recognize, however, that with the evolution and improvement of both surgical techniques and immunosuppression, the overall incidence of these complications has decreased. Thus, the decrease in complications in our series reflects both a clinical practice change as well as the evolution of our program. It was this need to eliminate the portal venous conduit that led to a change in the hepatic venous anastomosis. The early description of the living donor operation involved the right hepatic vein orifice. To bring the graft’s neo-hilum closer to the native portal vein, the graft must be situated on the confluence of the left and middle hepatic veins. Using this area still allows for triangulation technique of the anastomosis.
Both late portal vein and hepatic vein stenoses appear to occur more commonly in male patients who were significantly younger and smaller than patients not noted to have late venous stenoses. Also, our patients with late portal venous stenoses had a higher incidence of early vascular complications, including hepatic artery thrombosis and portal vein thrombosis, within the first several postoperative days. A higher incidence of early graft rejection, requiring either steroid bolus therapy or antibody preparations, and a later incidence of chronic rejection were observed in patients with portal vein stenosis. A higher incidence of early biliary complications necessitating either radiographic or surgical intervention was also associated with long-term portal vein stenosis. The presentation of portal vein stenosis varies in symptoms; the most common is variceal bleeding, followed by recurrent ascites, rising liver function tests, and splenomegaly. Presentation of symptoms can be variable, from 15 weeks to years, with a mean of 50 months. Few screening ultrasounds detected portal vein stenoses in asymptomatic patients. However, almost all patients uniformly underwent ultrasounds before percutaneous venography. The technique of portal vein venoplasty used to repair venous stenoses in pediatric patients was first described by Raby et al. in 1991. 19 This technique has been established as the treatment of choice for posttransplantation portal venous stenosis. A recent report by Funaki et al. describes the long-term patency of portal vein angioplasty and stenting. 18 This study also noted portal vein lesions were elastic when repeat dilation was attempted, necessitating metallic stent deployment.
Concerns have surfaced over the placement of metallic stents at a proximal portal vein lesion: it is believed that such positioning may interfere with repeat transplantation if it becomes necessary. This has fortunately not been borne out. The stents can be excised at the time of retransplantation or left in situ, with the new anastomosis performed at the level of the superior mesenteric vein. Six patients required retransplantation after successful angioplasty, but all were for rejection and not vascular complications. Two additional patients required retransplantation after failed angioplasty for vascular complications. Patients who fail to respond to balloon angioplasty or venous stenting subsequently do worse, with a lower overall survival that approaches statistical significance. Hepatic vein stenoses, like portal vein stenoses, predominately present in boys of younger age and lower weights, at a mean time of presentation of 37 months. All patients with hepatic vein–caval stenosis were diagnosed by the presentation of systemic symptoms. The most common of these were ascites, splenomegaly, and rising liver function tests. In terms of graft type, reduced-size and split livers are most susceptible to hepatic vein stenosis. As one might predict, the performance of multiple transplants (>2) and the presence of perioperative vascular complications, including portal vein and hepatic artery thromboses, increase the risk of hepatic vein stenosis. Angioplasty in combination with stenting for elastic lesions carries an excellent success rate. The patency does not appear to have any impact on graft or patient survival. The presence of abnormal liver function tests at the time of the lesion recognition did not appear to alter the success of the radiographic intervention or the overall graft survival. In the majority of cases, these liver function abnormalities returned to baseline and were a reflection of the stenosis. These data have shaped a philosophy in our group to aggressively investigate and treat liver function abnormalities, especially those associated with portal or hepatic vein stenosis, rather than approach these complications with retransplantation.
With these data, we have modified our practice of pediatric liver transplantation by eliminating vascular conduits to the portal vein anastomosis and imbricating the donor hepatic vein to the middle and left hepatic rather than the right vein. The presence of symptoms alerts us to potential pathology that requires interventional venography. Angioplasty of both portal vein and hepatic vein lesions has a high success rate, and all recurrent lesions appear to be amenable to repeat angioplasty or radiographic intervention. These data also confirm that the use of venous conduits and perioperative vascular thromboses correlate with a higher incidence of late venous complications. With the elimination of these venous conduits, the incidence of portal vein stenosis has decreased. Overall, the incidence of all venous complications is low, with portal vein stenosis occurring in 6% of patients and hepatic vein stenosis occurring in 2% of patients. This study also confirms the safety and efficacy of venous angioplasty as a treatment modality that, if successful, results in a trend toward improved survival.
Footnotes
Correspondence: Michael J. Millis, MD, Section of Transplantation, Department of Surgery, The University of Chicago, Pritzker School of Medicine, 5841 S Maryland Avenue, Chicago, Illinois 60637.
E-mail: joseph.buell@uc.edu
Accepted for publication February 18, 2002.
References
- 1.Whitington PF, Alonso EM, Piper JB. Pediatric liver transplantation. Semin Liver Dis 1994; 14: 303. [DOI] [PubMed] [Google Scholar]
- 2.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitington PF, Balisteri WF. Liver transplantation in pediatrics: indications, contraindications, and pre-transplant management. J Pediatr 1991; 118: 169. [DOI] [PubMed] [Google Scholar]
- 4.Busuttil RW, Seu P, Millis JM, et al. Liver transplantation in children. Ann Surg 1991; 213: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lilly JR, Hall RJ. Liver transplantation and Kasi operation in the first year of life: therapeutic dilemma in biliary atresia. J Pediatr 1987; 110: 561. [DOI] [PubMed] [Google Scholar]
- 6.Vital statistics of the US 1982. Mortality (parts A and B). Hyattsville, MD: United States Bureau of Vital Statistics, 1986.
- 7.Emond JC, Whitington PF, Thistlethwaite JR, et al. Reduced-size liver transplantation: use in the management of children with chronic liver disease. Hepatology 1989; 10: 867. [DOI] [PubMed] [Google Scholar]
- 8.Emond JC, Heffron TG, Kortz EO, et al. Improved results of living related liver transplantation (LRT) with routine application in pediatric program. Transplantation 1993; 55: 835. [DOI] [PubMed] [Google Scholar]
- 9.Millis JM, Seaman DS, Piper JB, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation 1996; 62: 748. [DOI] [PubMed] [Google Scholar]
- 10.Shah RM, Faggioli GL, Mangione S, et al. Early results with cryopreserved saphenous vein allografts for infrainguinal bypass. J Vasc Surg 1993; 18: 965. [DOI] [PubMed] [Google Scholar]
- 11.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors: surgical techniques and results. Ann Surg 1991; 214: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emond JC, Heffron TG, Whitington PF, et al. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet 1993; 176: 11. [PubMed] [Google Scholar]
- 13.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced size liver transplants as split grafts, auxiliary orthotopic grafts and living related segmental transplants. Ann Surg 1990; 212: 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langnas AN, Marujo W, Stratta RJ, et al. Vascular complications after orthotopic liver transplantation. Am J Surg 1991; 161: 76. [DOI] [PubMed] [Google Scholar]
- 15.Kalayoglu M, D’Alessandro AM, Knechtle SJ, et al. Long-term results of liver transplantation for biliary atresia. Surgery 1993; 114: 711. [PubMed] [Google Scholar]
- 16.Wood RP, Katz SM, Ozaki CF, et al. Development of the living related donor liver transplant at the Texas Medical Center: initial results and surgical complications. Transplant Proc 1993; 25 (supp 3):50. [PubMed] [Google Scholar]
- 17.Funaki B, Rosenblum JD, Leef JA, et al. Portal vein stenosis in children with segmental liver transplants: treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol 1995; 165: 161. [DOI] [PubMed] [Google Scholar]
- 18.Funaki B, Rosenblum JD, Leef JA, et al. Percutaneous treatment of portal vein stenosis in children and adolescents with segmental hepatic transplants: Long-term results. Radiology 2000; 215: 147. [DOI] [PubMed] [Google Scholar]
- 19.Raby N, Karani J, Thomas S, et al. Stenosis of vascular anastomosis after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol 1991; 157: 167. [DOI] [PubMed] [Google Scholar]
- 20.Rollins NK, Sheffield EG, Andrews WS. Portal vein stenosis complicating liver transplantation in children: percutaneous transhepatic angioplasty. Radiology 1992; 182: 731. [DOI] [PubMed] [Google Scholar]
- 21.Funaki B, Rosenblaum JD, Leef JA, et al. Angioplasty treatment of portal vein stenosis in children with segmental transplants: mid-term results. AJR Am J Roentgenol 1997; 169: 551. [DOI] [PubMed] [Google Scholar]