Abstract
Objective
To identify the possible reasons of failure of biliary reconstruction in right lobe live donor liver transplantation (LDLT) and to devise the best method of reconstruction and treatment strategy for the complications.
Summary Background Data
Right lobe LDLT was associated with a high biliary complication rate (15–64%) in the reported series. The causes of failure were not completely understood and the best treatment strategy has not been defined.
Methods
From 1996 to 2001, 74 patients received right lobe LDLT. The operative procedures of the first 37 patients were critically reviewed to identify the possible reasons of leakage or stenosis from the anastomosis. The causes included right hepatic duct ischemia, double or triple hepaticojejunostomies, unrecognized branch of right hepatic duct, jejunal opening smaller than the size of right hepatic duct, and ductal plasty without division of newly created septum. The second 37 patients had biliary reconstruction by a modified technique that preserved blood supply to the right hepatic duct and aimed at avoidance of risk factors.
Results
The overall complication rate decreased from 43% in the first 37 patients to 8% in the second 37 patients. There was no leakage from the anastomosis in the second group of patients. Percutaneous transhepatic biliary drainage (PTBD) for the biliary complications resulted in right portal vein and hepatic artery injury in four patients and accounted for mortality in three of them. To avoid complications from PTBD, three patients in the second group developing stenosis of hepaticojejunostomy had repeated hepaticojejunostomy without preoperative PTBD and recovered.
Conclusions
With identification of risk factors and modification of the surgical technique, the complication rate of biliary reconstruction of right lobe LDLT could be reduced. Repeated hepaticojejunostomy without preoperative PTBD is the preferred approach once a complication develops.
Right lobe live donor liver transplantation (LDLT) has been advocated as a means of overcoming the graft shortage in adult recipients, especially in urgent situations, 1 but unlike the left lobe or left lateral segment graft operations, the incidence of biliary tract complications in the early reported series was high, ranging from 15% to 64%. 2–7 In our previous reports, 8,9 we identified possible reasons accounting for the high complication rate and proposed a solution for leak-proof anastomosis. In this report, we describe further refinement in the technique of biliary reconstruction and the management of the patients with complications arising from the reconstruction.
METHODS
From May 1996 to August 2001, 74 patients underwent right lobe LDLT. All except nine patients had a Roux-en-Y hepaticojejunostomy for reconstruction. The other nine patients had a right hepatic duct-to-common hepatic duct anastomosis. The first 37 patients (operated on between May 1996 and May 2000) had reconstruction using methods described previously. 1,8 The latter 37 patients (operated on between June 2000 and August 2001) underwent a procedure that was modified after recognition of possible reasons for anastomosis failure; the procedure is described below.
The donor assessment including hematology, renal and liver biochemistry, ultrasonography, computed tomography, and after confirmation of donation by clinical psychology assessment, hepatic angiography. Preoperative endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography was not performed.
The donor operation started with cholecystectomy and cannulation of the cystic duct. The hilum was explored gently to identify the site of confluence of the left and right hepatic ducts. A large Liga clip (Ethicon Ltd, Edinburgh, UK) was then clipped to the liver capsule about 2 mm to the right of the confluence of the hepatic ducts. This was the proposed site of division of the right hepatic duct. Care was exercised to avoid injury to the hepatic ducts by diathermy and to the right hepatic artery, which might cross in front of the common hepatic duct. The supraduodenal portion of the common bile duct was isolated for about three quarters of its circumference for application of an atraumatic vascular clamp. An operative cholangiogram was performed using undiluted radiographic contrast introduced via the cystic duct cannula under fluoroscopy. Clamping of the common bile duct allowed retrograde filling of contrast into the intrahepatic duct. The sequence of filling of the intrahepatic duct by contrast was closely observed for correct identification of the intrahepatic ducts. The right posterior hepatic duct, which was located at the most dependent part of the liver, was filled first, followed by the right anterior hepatic duct, the left hepatic duct, the left lateral segment hepatic duct, and finally the left medial segment hepatic duct. The distance between the confluence of the hepatic ducts and right hepatic duct branches was noted, and the position of the Liga clip in relation to the right hepatic duct was studied. The x-ray tube was rotated toward the right side of the donor to obtain a right anterior oblique view, by which correct identification of the hepatic ducts of individual segments could be obtained (Figs. 1 and 2).
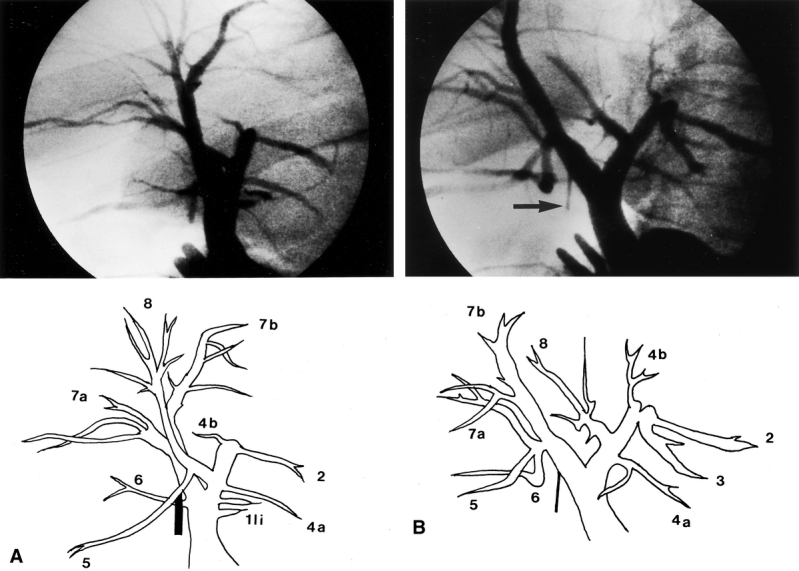
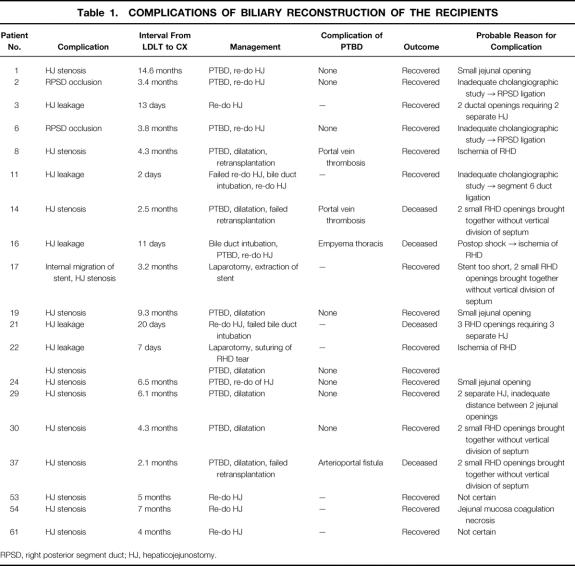
Figure 1. Operative cholangiogram of a 56-year-old donor. (A) This film was taken in an anteroposterior position. Interpretation was difficult because the liver was relatively small and had rotated into the right subphrenic cavity. (B) By rotating the x-ray tube to obtain a right antero-oblique view, two separate right hepatic ducts were clearly seen. The Liga clip (arrow) marks the proposed position of the division of the right anterior hepatic duct.
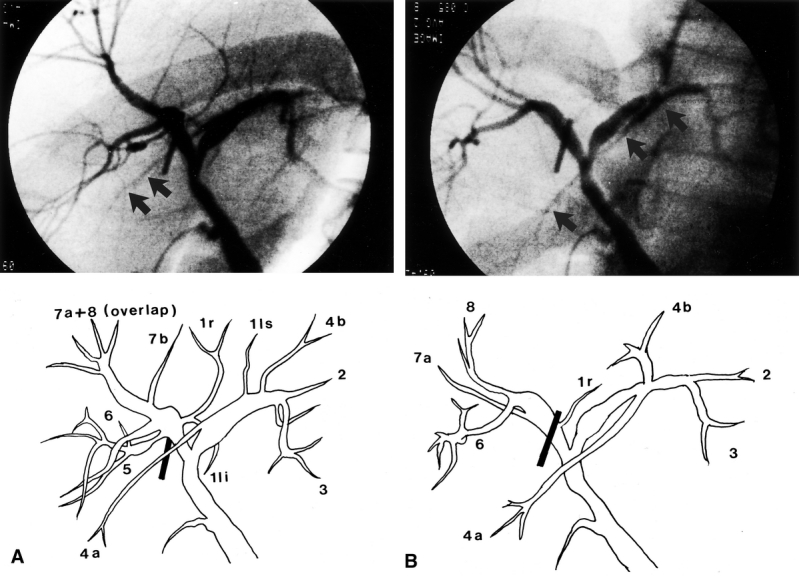
Figure 2. (A) Operative cholangiogram of a donor obtained in the anteroposterior position. A fine hepatic duct (arrows) crossed the right hepatic duct and was suspected to be the segment 6 hepatic duct joining the left hepatic duct. (B) By rotating the x-ray tube, the hepatic duct was seen clearly joining the confluence of segment 2 and 3 ducts and was actually a segment 4a hepatic duct (arrows).
After the cholangiogram, the site of proposed division of the right hepatic duct was determined and marked by diathermy on the inferior surface of the left medial segment near the hilar plate (Fig. 3). This would be the lower end of the line of liver transection.
Figure 3. Line of liver transection at the inferior surface of the liver. The line deviated to the left side of the gallbladder fossa to meet the point of division of the right hepatic duct as determined by operative cholangiography. The asterisk indicates the point where dissection for the right hepatic artery must stop.
On mobilization of the right hepatic artery, dissection into the space between the right hepatic duct and right hepatic artery beyond the proposed point of division of the right hepatic duct was not made in order to protect blood supply to the right hepatic duct arising from the right hepatic artery (see Fig. 3). After mobilization of the right portal and right hepatic veins, parenchymal transection was performed along the Cantlie’s line using an ultrasonic dissector without inflow or outflow vascular occlusion. The middle hepatic vein was included in the graft. At the inferior surface of the liver, the transection plane was deviated to the left side of the gallbladder fossa to meet the point of proposed division site of the right hepatic duct (see Fig. 3). When the liver transection reached the hilar plate, the liver tissue cephalad to the hilar plate was drilled by the ultrasonic dissector down to the caudate process and a right-angle dissecting forceps was passed to encircle the right hilar plate and the right hepatic duct. Another operative cholangiogram was obtained to verify the position of the right hepatic duct division if there was uncertainty. The right hepatic duct and hilar plate were sharply divided without ligating any tissue. The number of right hepatic duct openings was examined. Active arterial bleeding from the hilar plate was controlled using 6-0 prolene sutures (Ethicon Prolene, Johnson & Johnson International, Edinburgh, UK). Tiny caudate lobe hepatic duct openings were also sutured.
At the back table, the number of hepatic duct openings was reexamined. If there were two openings adjacent to each other, they were sutured to form a single hole. The septum between the reconstituted ducts was divided vertically and the gap was sutured using 6-0 PDS (Ethicon PDS II) to create a larger opening (Fig. 4).
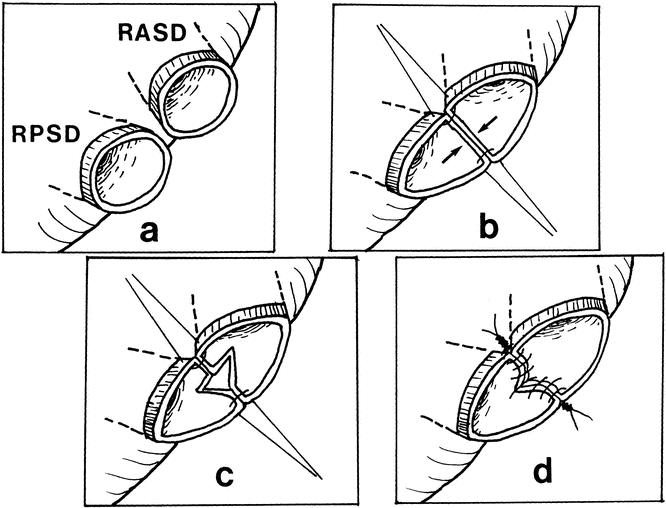
Figure 4. Approximation of two adjacent right hepatic duct orifices to form a single orifice. Simply joining the medial wall creates tension and narrowing of the lumen. The newly created septum should be divided vertically and sutured transversely to create a large opening.
In the recipient, the implantation was performed by methods described previously. 8 The size of the right hepatic duct orifice was measured by a paper ruler placed against it. The Roux jejunal loop was brought through the transverse mesocolon and behind the stomach to reach the right hepatic duct. Using the paper ruler, a jejunal opening of the same size as the right hepatic duct orifice was made. Bleeding from the cut edge of the opening was controlled by diathermy that was set at lower power than usual. In case of two separate right hepatic duct orifices requiring two separate jejunal openings, the distance between the two jejunal openings was at least three times the distance between the two hepatic duct orifices, because the intervening jejunal wall tended to contract after the openings were made, rendering the distance between the two jejunal openings too short for tension-free anastomoses.
The bilioenteric anastomosis was performed with the posterior layer completed first. For the corner and penultimate stitches, 6-0 prolene was used and the knots were tied outside the lumen of the anastomosis. If the lumen was small (<5 mm), all the other posterior-row sutures were completed using 6-0 prolene double-needle sutures with the knots outside. Otherwise, absorbable 6-0 PDS sutures were used with the knots placed inside the lumen because it was difficult to obtain close approximation of the ductal and jejunal wall in a large duct with the knots tied outside. The distance between sutures was about 1 mm. After completion of the posterior row, a radiopaque tube of a size just smaller than that of the hepatic duct orifice and with multiple side-holes was inserted into the right hepatic duct until it could not advance further. The tube was cut at about 3 cm from the anastomosis and placed within the lumen of the jejunal loop to serve as an internal stent of the anastomosis. The stent was not anchored but was allowed to migrate distally afterwards. The anterior wall of the anastomosis was completed by using 6-0 prolene sutures with the knots tied outside the lumen of the anastomosis.
Nine patients operated on in 2001 had anastomosis of the right hepatic duct of the right lobe graft to his or her common hepatic duct. The method of reconstruction was similar to hepaticojejunostomy except that all knots were placed outside the lumen. An external stent was used in the first three patients only. Before the anastomosis, choledochoscopy was used to examine the common bile duct in three patients with gallbladder stones and a wide common bile duct. Stones were found in one patient.
The duration of cold ischemia and the interval between completion of the portal vein and hepatic artery anastomoses were noted. After portal vein and hepatic artery anastomoses, the presence of bleeding from the wall of the right hepatic duct orifices and the hilar plate was recorded. The size of right hepatic duct orifices and the method of reconstruction were accurately recorded. When complications developed, the operative cholangiograms of the donors were reexamined for possible reasons of failure.
Continuous variables were expressed as median and range and compared between patients with and without complications by the Mann-Whitney test. The complication rate of the first 37 patients was compared with that of the second 37 patients by the chi-square test.
RESULTS
First 37 Patients
Anastomosis leakage occurred in five patients (patients 3, 11, 16, 21, 22;Table 1, Fig. 5). They were managed by relaparotomy. In patient 22, a tear in the right hepatic duct was found. He recovered after suturing of the tear. For the other four patients, leakage was found in three patients from the hepaticojejunostomy and in one patient from an unrecognized segment 6 duct. Reanastomosis was successful in patient 3 only. The other three patients had intubation of the bile duct, with the tube brought out through the peritoneal cavity and abdominal wall. Patient 21 died of persistent sepsis. Patient 11 had a repeated hepaticojejunostomy 6 months later and recovered. Patient 16 had a repeated hepaticojejunostomy 10 months later; however, he died of empyema thoracis, a complication of percutaneous transhepatic biliary drainage (PTBD), which was inserted for the control of persistent bile leakage before the repeated hepaticojejunostomy.
Table 1. COMPLICATIONS OF BILIARY RECONSTRUCTION OF THE RECIPIENTS
RPSD, right posterior segment duct; HJ, hepaticojejunostomy.
Figure 5. Outcome of the first 37 patients.
Patient 22, who had leakage from a tear of right hepatic duct, and 11 other patients developed acute cholangitis and jaundice 2 to 14.6 months (median 4.3 months) after liver transplantation (see Table 1, Fig. 5). Computed tomography showed migration of the internal stent into the intrahepatic duct in patient 17 and mild dilatation of the biliary tract together with absence of biliary gas in the other patients, suggestive of stenosis of the bilioenteric anastomosis. Patient 17 underwent a repeat laparotomy and exploration of the biliary tract via enterotomy. The stent was removed by a pair of biopsy forceps under fluoroscopic guidance. The other patients had PTBD. The cholangiogram showed stenotic anastomosis in nine patients and complete occlusion of the right posterior segment hepatic duct in two patients. Percutaneous dilatation of the stenotic anastomosis was successful in patients 19, 22, 29, and 30. They were well afterward. Dilatation was not successful in other patients, and PTBD was complicated by portal vein thrombosis in patients 8 and 14 and arterioportal fistula in patient 37. Patient 8 recovered after retransplantation; patients 14 and 37 had failed retransplantation due to severe vascular adhesion. Both patients died of liver failure. The other three patients and the two patients with segregated right posterior segment duct underwent laparotomy and reconstruction of the anastomosis and were well afterward.
The operative records and cholangiograms were reviewed to identify the probable causes of failure of biliary reconstruction (see Table 1). Unrecognized branch of the right hepatic duct at donor operations, double or triple hepaticojejunostomies (Table 2), a jejunal opening smaller than the right hepatic duct orifice, ductal plasty without division of the septum between the ducts, short internal stent, and ischemia of the right hepatic duct (based on the findings of absent bleeding from the ductal wall or hilar plate after portal vein or hepatic artery anastomosis) were probably the major reasons. The size of the right hepatic duct orifice (median 6.0 mm in patients with complications vs. 5.5 mm in patients without complications), cold ischemic duration (median 117 minutes in patients with complications vs. 118 minutes in patients without complications), and interval between completion of portal vein and hepatic artery anastomoses (median 94.5 minutes in patients with complications vs. 109.5 minutes in patients without complications) were not important contributory factors to complications.
Table 2. INCIDENCE OF COMPLICATIONS IN RELATION TO THE NUMBER OF HEPATICOJEJUNOSTOMY
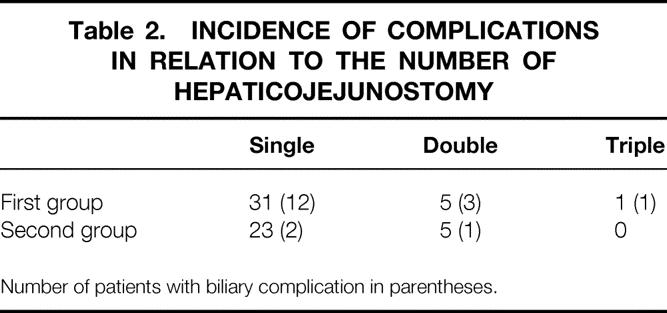
Number of patients with biliary complication in parentheses.
Second 37 Patients
Patients 53, 54, and 61 presented with acute cholangitis and their computed tomography scans suggested stenosis of the hepaticojejunostomy 5, 7, and 4 months after transplantation, respectively (see Table 1). Acute cholangitis was initially controlled by antibiotics alone and the patients underwent repeat laparotomy and reconstruction of the hepaticojejunostomy without any preoperative PTBD. At laparotomy, the bilioenteric anastomosis was taken down and the site of the stenotic hepatic duct was located by intraoperative ultrasonography, with the ultrasonic probe placed over the liver hilum. The tissue overlying the dilated hepatic duct was removed by an ultrasonic dissector. A wide bilioenteric anastomosis was reconstructed according to the method described previously. The patients were well after the operations.
The operative records were reviewed to identify the reasons for stenosis of the hepaticojejunostomy. For patient 54, necrosis of the jejunal mucosa induced by diathermy for hemostasis might be responsible, but the reasons for patients 53 and 61 were uncertain.
Overall Results
The complication rate of the biliary reconstruction for the entire group was 26%. The complication appeared 2 days to 14.6 months after liver transplantation (median 3.4 months). The complication rate decreased from 43% (16/37) in the first 37 patients to 8% in the second 37 patients (3/37) (P < .001). The leakage rate from the anastomosis was 7%. There was no leakage in the second 37 patients. The anastomotic stenosis rate was 28%. The stenosis rate was much reduced in the second 37 patients (12/37 [32%] vs. 3/37 [8%], P = .009).
DISCUSSION
The success of the bilioenteric anastomosis in the second half of this series was probably attributable to the modifications in the surgical technique. They included preservation of blood supply to the right hepatic duct by avoiding dissection into the space between the right hepatic artery and the right hepatic duct, precise location of division of the right hepatic duct by operative cholangiography to avoid two or three duct openings, preservation of the hepatic parenchyma overlying the right hepatic duct to preserve blood supply and venous drainage, ductal plasty to create a single large ductal opening, and creation of a jejunal opening of the same size as the right hepatic duct. These modifications were made basing on analysis of failed cases.
In our early series, a jejunal opening a size smaller than the hepatic duct opening was routinely made because we feared that the jejunal opening could become excessively large for the right hepatic duct orifice after manipulation. This has turned out to be invalid. With a small jejunal opening, stretching of the opening led to ischemia and failure. We therefore consider accurate measurement of the ductal opening and creation of a jejunal opening the same size as the hepatic duct opening mandatory in a hepaticojejunostomy. Double or triple hepaticojejunostomies are an obvious risk factor for biliary complications. 5 Hence, operative cholangiography was used to ensure precise localization and division to obtain a single right hepatic duct orifice. Despite the effort, double right hepatic ducts are encountered in up to 34% of normal persons. 10 Approximation of two adjacent ductal openings into one orifice simply by suturing their medial walls was found to be inappropriate because this maneuver actually reduced the lumen size and created tension on the lateral wall. To create a large opening without tension, the newly created septum should be divided vertically and sutured transversely (see Fig. 4).
Right hepatic duct hepaticojejunostomy is a standard operative procedure. However, unlike other patients, the ductal opening in right lobe LDLT is usually small and blood supply to the right hepatic duct of the right lobe graft might be compromised as a result of detachment of the liver from the diaphragm and cold or warm ischemic insult. The blood supply to the right hepatic duct is from the arterial arcade which runs in the wall of the duct and is derived from the left and right hepatic arteries and gastroduodenal artery. 11–13 After division of the right hepatic duct, the arcade is interrupted and blood supply to the right hepatic duct is from the right hepatic artery, the hilar plate, and the caudate lobe. 11 Therefore, the right hepatic artery dissection must not be carried beyond the point of the right hepatic duct division, and the hilar plate must be preserved. Without dissection into the space between the right hepatic artery and right hepatic duct, the length of the right hepatic artery that can be harvested might be short. However, in our experience, the length of the right hepatic artery is usually sufficient for microvascular anastomosis. The confluence of the left and right hepatic ducts is to the left of the gallbladder fossa, so it is necessary to deviate the parenchymal transection line to the left side of the gallbladder fossa, whether the middle hepatic vein is preserved with the graft or not. By so doing, the upper part of the right hepatic duct is not denuded and venous drainage of right hepatic duct is preserved. 14
The role of stenting of the bilioenteric anastomosis is controversial. 4,5,15,16 For a large ductal opening, a stent may not be necessary. For a small opening, a stent can prevent occlusion of the anastomosis by edema in the early stage of healing and catching of the posterior wall by the anterior row of sutures. We prefer to use a stent that is as large as the ductal opening and push the tube all the way into the duct until it cannot advance further to prevent migration of the tube into the intrahepatic duct in the postoperative period. Whether an external stent is superior to an internal stent is controversial. An external stent allows observation of the bile color and quantity, allowing assessment of graft function. However, the procedure requires tunnel formation in the jejunum and fixation of the jejunum to the anterior abdominal wall, which could be cumbersome in the presence of an edematous jejunum, a phenomenon that usually occurs after portal vein clamping. To avoid this hazard, we prefer to use an internal stent.
The management of leakage of the bilioenteric anastomosis in LDLT is difficult. Few reports have been found in the literature on this subject. 5,17,18 Reanastomosis is performed by most surgeons before infection is beyond control. However, at the time of reexploration, the ductal tissue might be unfavorable for anastomosis because of edema and deprivation of blood supply. Intubation was successful in two of our three patients, but it might not be able to control leakage completely as the duct orifice was not totally occluded and the tube might slip out. In retrospect, transhepatic tube drainage and suturing of the orifice may be a better choice. It was not performed in our patients as we were concerned about injury to the right hepatic artery and portal vein at that time. Fortunately, with modification of the technique, leakage from the hepaticojejunostomy could be eliminated.
Unlike other patients with biliary obstruction, the intrahepatic ducts of the right lobe graft recipients did not become dilated as much in the presence of anastomotic obstruction. The exact reason is not known. Probably, warm ischemic injury to the ductal wall occurred during implantation before the portal vein and hepatic artery were reconstructed. In the absence of gross ductal dilation, PTBD was difficult and resulted in injury to the right portal vein and hepatic artery in four patients and mortality in three of them. To prevent further mortality from PTBD, we decided on laparotomy without any preoperative biliary drainage. With the help of intraoperative ultrasonography, the duct could be easily identified at the liver hilum and exposed for reconstruction.
Instead of hepaticojejunostomy, an alternative in biliary reconstruction for right lobe LDLT is right hepatic duct-to-common hepatic duct anastomosis. 4,7,19 This method is applicable when the recipient’s common hepatic duct is preserved for a long length and blood supply is intact. It may also be applicable to a graft with two adjacent ductal openings that were brought together by ductal plasty. Stenting may not be required, but it is important to exclude common bile duct stones by choledochoscopy before anastomosis. The immediate result appears satisfactory and is an attractive alternative. However, it might not be applicable to the situation when the two right hepatic ductal openings are far apart, unless the recipient’s own left and right hepatic ducts are dissected for long lengths at the time of hepatectomy. It is not unlikely that duct-to-duct anastomosis will become the preferred method of biliary reconstruction in the future. Nevertheless, the principle of right hepatic duct preparation remains the same as in hepaticojejunostomy.
The deficiency of this study was that the duration of follow-up of the second group of patients might not be sufficiently long. However, since the majority of complications occurred within 4 months after transplantation, we believed that most of the early problems of biliary reconstruction in right lobe LDLT had been resolved.
In conclusion, with attention and modification of the surgical technique, the complication rate from biliary reconstruction was markedly reduced, but not to 0% yet. Further study is needed to define causes of failure apart from those we could identify. Once a complication develops, aggressive reoperation without preoperative PTBD is the preferred approach.
Footnotes
Supported by the University Research Committee grant and Distinguished Research Achievement Award of the University of Hong Kong.
Correspondence: Sheung-Tat Fan, MS, MD, FRCS (Glasg & Edin), FACS, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong.
E-mail: hrmsfst@hkucc.hku.hk
Accepted for publication January 7, 2002.
References
- 1.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe graft. Ann Surg 1997; 226: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inomata Y, Uemoto S, Asonuma K, et al. Right lobe graft in living donor liver transplantation. Transplantation 2000; 69: 258–264. [DOI] [PubMed] [Google Scholar]
- 3.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl 2000; 6: 296–301. [DOI] [PubMed] [Google Scholar]
- 4.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg 2001; 234: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa G, Malagó; M, Valentín-Gamazo C, et al. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transplant 2000; 6: 710–714. [DOI] [PubMed] [Google Scholar]
- 6.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transplant 2001; 7: 680–686. [DOI] [PubMed] [Google Scholar]
- 7.Grewal HP, Shokouh-Amiri MH, Vera S, et al. Surgical technique for right lobe adult living donor liver transplantation without venovenous bypass or portocaval shunting and with duct-to-duct biliary reconstruction. Ann Surg 2001; 233: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg 2000; 231: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan ST. Adult-to-adult living liver transplantation. Adv Surg 2001; 35: 187–202. [PubMed] [Google Scholar]
- 10.Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999; 16: 459–467. [DOI] [PubMed] [Google Scholar]
- 11.Vellar ID. The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust NZ J Surg 1999; 69: 816–820. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg 1998; 85: 202–207. [DOI] [PubMed] [Google Scholar]
- 13.Imamura H, Makuuchi M, Sakamoto Y, et al. Anatomical keys and pitfalls in living donor liver transplantation. J Hepatobiliary Pancreat Surg 2000; 7: 380–394. [DOI] [PubMed] [Google Scholar]
- 14.Vellar ID. Preliminary study of the anatomy of the venous drainage of the intrahepatic and extrahepatic bile ducts and its relevance to the practice of hepatobiliary surgery. Aust NZ J Surg 2001; 71: 418–422. [DOI] [PubMed] [Google Scholar]
- 15.DiFronzo LA, Egrari S, O’Connell TX. Safety and durability of single-layer, stentless, biliary-enteric anastomosis. Am Surg 1998; 64: 917–920. [PubMed] [Google Scholar]
- 16.Lillemoe KD, Melton GB, Cameron JL, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg 2000; 232: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng YF, Chen YS, Huang TL, et al. Biliary complications in living related liver transplantation. Chang Gung Med J 2001; 24: 174–180. [PubMed] [Google Scholar]
- 18.Egawa H, Inomata Y, Uemoto S, et al. Biliary anastomotic complications in 400 living related liver transplantations. World J Surg 2001; 25: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 19.Shokouh-Amiri MH, Grewal HP, Vera SR, et al. Duct-to-duct biliary reconstruction in right lobe adult living donor liver transplantation. J Am Coll Surg 2001; 192: 798–803. [DOI] [PubMed] [Google Scholar]