Abstract
Objective
The authors studied the dose-dependent effect of topically administered granulocyte-macrophage colony-stimulating factor (GM-CSF) on the connective tissue response using an experimental repair model in surgical patients.
Summary Background Data
GM-CSF is primarily indicated in the treatment of immunosuppressed states. The effect of GM-CSF on the tissue repair response in humans is unclear.
Methods
Expanded polytetrafluoroethylene tubes were implanted subcutaneously and GM-CSF was applied locally at concentrations of 0.1 μg/mL (total dose 0.4 μg), 1.0 μg/mL (4.0 μg), 10 μg/mL (40 μg), or 75 μg/mL (300 μg) in one arm and saline alone (control) in the contralateral arm of 56 surgical patients. The content of collagen and total protein in the tubes was quantified as hydroxyproline and proline by high-performance liquid chromatography 10 days after implantation. Cellularity and the number of procollagen I-positive fibroblasts were determined by histology and immunohistochemistry. The direct effects of GM-CSF on collagen production by and proliferation of wound fibroblasts cultured from granulation tissue were also measured.
Results
Local application of GM-CSF stimulated the inflammatory cell infiltration but reduced the number of fibroblasts in the granulation tissue. GM-CSF treatment suppressed specifically and dose-dependently collagen deposition by up to 81%. A reduced collagen accumulation was also found in the control-treated arm at GM-CSF doses of 4 μg or more, indicating a systemic depressive effect of GM-CSF on tissue repair. The selective downregulation of collagen production by GM-CSF was also found in wound fibroblasts in vitro.
Conclusions
Inhibition of fibrogenesis with GM-CSF intervention may impair tissue repair processes during surgery.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multipotent cytokine with a molecular weight ranging from 15 to 35 Kd. GM-CSF primarily stimulates proliferation and differentiation of hematopoietic progenitor cells in the myeloid and erythroid lineages into neutrophils, eosinophils, and macrophages. Therapeutically, the beneficial effects of GM-CSF are well documented in myelodepressive states associated with radiation therapy, chemotherapy, and bone marrow transplantation. It has also been proposed to use GM-CSF perioperatively to improve immune functions in conjunction with colorectal surgery. 1
Mediators released during the acute inflammatory response coordinate the early connective tissue repair response after injury. The early inflammatory reaction precedes the deposition of granulation tissue composed mainly of macrophages, active fibroblasts, and new vessels in a provisional extracellular matrix. 2 The macrophage plays an essential directory role in tissue repair, demonstrated by the use of antimacrophage serum or radiation-induced monocytopenia in rodents, by releasing a plethora of cytokines. 3–5 The neutrophil granulocyte mainly phagocytoses microorganisms but apart from that is only secondary in tissue repair. 6 GM-CSF regulates several biologic functions of the inflammatory cells and those of macrophages in particular. 7 Apart from modulating the nonspecific inflammatory processes, it has been reported that GM-CSF is chemotactic and mitogenic for fibroblasts and endothelial cells in vitro. 8,9 These effector cells are also stimulated indirectly or synergistically by GM-CSF in an autocrine or paracrine fashion by other cytokines such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α (TNF-α). 7,8,10
There is experimental evidence of a profibrotic effect of GM-CSF in the subcutaneous tissue and skin of rats. GM-CSF delivered continuously from osmotic minipumps implanted subcutaneously elicited a collagenous capsule around the devices and even a stronger one than that of transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF). 11 This effect appeared to be indirectly mediated by the accumulation of macrophages that stimulated α-SM actin synthesis by myofibroblasts. 11,12 In rat skin, GM-CSF delivered continuously over several days by gene transfer induced a dermal inflammatory fibrotic response. 13 A stimulatory effect on fibroplasia and collagen deposition by GM-CSF administered locally was also suggested by the increased mechanical strength of incisional wounds in normal and immunosuppressed rats. In contrast, no effect was found following systemic GM-CSF treatment. 14 Furthermore, granulocyte colony-stimulating factor (G-CSF), which acts mainly on neutrophils, did not enhance wound repair, reemphasizing the crucial role of macrophages in tissue repair. However, human studies on the influence of either endogenous or exogenous cytokines and specifically GM-CSF on tissue fibrosis and repair are scarce. 15
Because the mechanical strength of wounds correlates with the amount of collagen in the early fibroplastic phase, we investigated whether GM-CSF possesses a fibrogenic effect in humans. The deposition of connective tissue with different doses of recombinant human GM-CSF was assessed in a minimally invasive implantable subcutaneous tissue repair model. 16,17
METHODS
The study was performed in accordance with the Helsinki Declaration of 1975 and approved by the Copenhagen Ethics Committee (02–018/95 and 02–012/97) and the Department of Pharmacology, the Danish National Board of Health (5312–35–1995 and 5312–52–1997). The patients were enrolled after written informed consent was obtained.
Patients
Inclusion criteria were planned abdominal or urologic surgery under general anesthesia and age above 18 years. Reasons for exclusion were withdrawal of consent, pregnancy, lactation, allergy to molgramostin, recent medication with immunosuppressive drugs or NSAIDs, diabetes, or chronic renal, hematologic, or pulmonary disease.
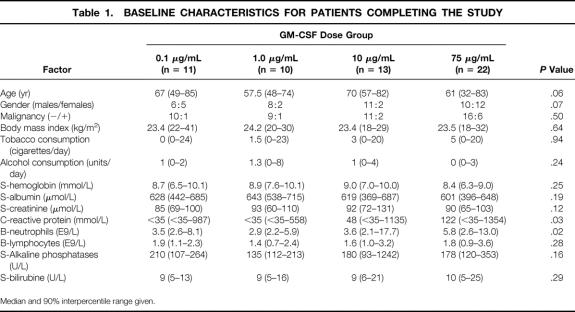
Informed consent was obtained from 59 patients scheduled for general surgery. Two patients, who received 0.1 μg/mL and 75 μg/mL GM-CSF, asked for removal of the implants after 3 and 4 days due to diarrhea or sweating. The third patient, who received 75 μg/mL GM-CSF, died of cardiac shock and anuria 2 days after resection of a partially infarcted jejunum. Thus, 56 patients completed the study. Consecutively, four different dose regimens were carried out: 75 μg/mL (total dose 300 μg), 10 μg/mL (40 μg), 1.0 μg/mL (4.0 μg), and 0.1 μg/mL (0.4 μg). Baseline characteristics for the patients and perioperative data are given in Tables 1 and 2. Patients and assessors were blinded with regard to the treatment code and dose administered.
Table 1. BASELINE CHARACTERISTICS FOR PATIENTS COMPLETING THE STUDY
Median and 90% interpercentile range given.
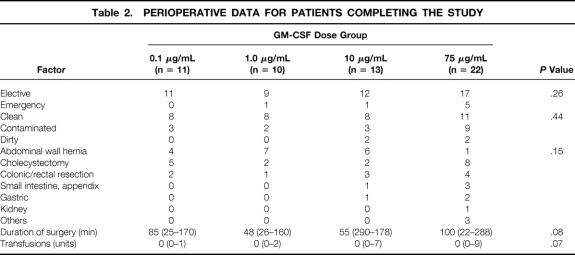
Table 2. PERIOPERATIVE DATA FOR PATIENTS COMPLETING THE STUDY
Implantation Techniques and Administration of GM-CSF
Expanded polytetrafluoroethylene (ePTFE) tubes (International Polymer Engineering, Tempe, AZ; 8 cm in length, 0.12 cm ID, 0.24 cm OD, 90–120 μm pore size) were inserted subcutaneously in the laterodorsal and middle part of the upper left and right arm under general anesthesia at surgery. Two ePTFEs tubes were placed parallel and 4 cm apart in distal-proximal direction in each arm. The technique of insertion using a 0.25-cm cannula has been described previously. 17 Two milliliters of recombinant human GM-CSF (molgramostin, Schering-Plough, Kenilworth, NJ) dissolved in saline or saline alone was continuously and slowly injected through the insertion cannula during the insertion of each ePTFE tube. Each patient thus received 2 × 2 mL of GM-CSF in one arm and 2 × 2 mL saline alone in the contralateral arm. Based on a computerized allocation, the right arm was used for active compound in half of the patients and the left arm for active medication in the other half of the patients.
Preperation of Implants
The implantation sites were examined for the presence of wound secretion, tenderness, or erythema 1 day after insertion. Itching reported by the patient was noted. The ePTFE tubes were removed 10 days after the implantation. Two sections, each 15 to 20 mm of the middle part of each tube, were stored in acetone at 4°C for later analysis of hydroxyproline and proline contents using high-performance liquid chromatography (HPLC).
Histologic and Immunohistochemical Examinations
Histologic and immunohistochemical evaluations were performed on the samples from the 13 patients who received 10 μg/mL GM-CSF. One 0.5-cm transverse section was cut from one ePTFE tube from each arm of the patients and fixed in 10% formalin in phosphate buffer (pH 7.3). The ePTFE samples were embedded in paraffin and 3- to 4-μm-thick sections were cut and stained with hematoxylin-eosin. These sections were evaluated blindly for inflammatory cellularity on a 5-point scale from 0 (no cellularity) to 4 (maximal cellularity). Immunostainings were performed on formalin-fixed paraffin-embedded 3- to 4-μm-thick sections using the labeled streptavidin-biotin method with peroxidase (K5003; Dako, Glostrup, Denmark). Two 3- to 4-μm-thick sections from each ePTFE tube were cut onto capillary glass slides (S2024; Dako) that were deparaffinized in xylene and rehydrated in descending ethanol series (99% to 70%). The sections were incubated in Tris-EGTA, pH 9, at 60°C for 18 hours to retrieve the antigen. Immunostainings were carried out using an automated system (TechMate 500, Dako) at room temperature. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 minutes. To minimize background staining, sections were incubated with 10% bovine serum albumin (A-4503, Sigma, St. Louis, MO) in Tris-buffered saline (pH 7.4). Sections were incubated for 25 minutes with rat antihuman monoclonal antibody against procollagen type I (MAB1912, Chemicon International, Temecula, CA) diluted 1:1,000. The antigen–antibody reaction was visualized using 3-amino-9-ethylcarbazole as chromogen. The slides were counterstained in Mayer’s acid hematoxylin for 2 minutes. No nonspecific staining was seen in control sections treated identically but devoid of the procollagen antibody. The total number of procollagen I-positive cells was counted using a 40× objective, and the average of the readings of the two sections from each ePTFE piece was used for the statistical analysis. Photomicrographs were taken with the Olympus DP11 digital camera system (Olympus, Melville, NY).
In Vitro Effects of GM-CSF on Granulation Tissue-Derived Fibroblasts
Fibroblasts were obtained by explanting 10-day-old granulation tissue from two ePTFE tubes implanted in a healthy 30-year-old woman. Freshly withdrawn ePTFE tubes were washed in cold phosphate-buffered saline and minced into 1-mm cubes in thrombin (1.5 NIH units/mL, Sigma) dissolved in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Paisley, UK) containing 25 mmol/L Hepes. Each explant (5–10 explants/dish) was placed on a fibrinogen (3 mg/mL, Sigma, F-8630) drop (5–10 μL) containing the plasminogen activator inhibitor tranexamic acid (Cyclokapron, 50 mg/mL, Pharmacia & Upjohn, Helsingborg, Sweden) in DMEM/Hepes medium on a 60-mm Petri dish. Fibroblast outgrowth was supported by fibroblast growth medium (Clonetics, Walkersville, MD) supplemented with 10% heat-inactivated, mycoplasma-screened fetal bovine serum (FBS, Gibco BRL) at 37°C in a humidified atmosphere of 5% CO2/air. Cells were subcultivated using trypsin/EDTA 18 and used for the assays of their fourth to sixth passage.
The effect of GM-CSF on proteins secreted into the medium by fibroblasts was measured by HPLC. Fibroblasts (50,000/well) were seeded in one 24-well plate in FGM with 10% FBS and grown to confluence for 4 days. The medium was then changed to 0.4 mL fibroblast basal medium (FBM, Clonetics) with 3.125% FBS/well, and 0.1 mL GM-CSF dissolved in FBM/1% bovine serum albumin (BSA) was added to four separate wells per each GM-CSF dose. Control-treated cells received .1 mL FBM/1% BSA alone. L-ascorbic acid in FBM/1% BSA at a final concentration of 50 μg/mL was added to four parallel wells as a positive collagen stimulation control. The quiescent cells were conditioned for 3 days, conditioned medium centrifuged, and the amount of hydroxyproline and proline was determined with HPLC in one 200-μL aliquot per well, as described below.
The mitogenic dose response to human recombinant GM-CSF was determined in confluent and growth-arrested fibroblasts grown in FBM containing 2.0% FBS in 96-well plates. 18 Fibroblasts were incubated without (control) or with GM-CSF (1 ng/mL–100 μg/mL) for 20 hours with the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) present at 10 μmol/L during the last 2 hours of incubation. Human recombinant PDGF-BB (Gibco BRL) at 20 ng/mL was tested simultaneously as a positive mitogenic control. The incorporated BrdU, as a measure of DNA synthesis, was measured colorimetrically with a microtiter plate reader and expressed as optical density. 18
HPLC
The ePTFE tubes were delipidized in acetone/diethyl ether and the length was measured with a caliper ruler. The ePTFE tubes and conditioned media were hydrolyzed in an oxygen-free atmosphere of hydrogen chloride for 24 hours at 114°C. The hydrolysis residues were reconstituted in 0.1 mol/L hydrochloric acid. A fixed amount (45 nmol) of the internal standard L-citrulline (Aldrich, Steinheim, Germany) was added to aliquots of the dissolved hydrolysates, and derivatives of the amino acids were made with phenylisothiocyanate (Pierce, Rockford, IL) using a standard procedure. 19 The amino acid derivatives were redissolved in 5 mmol/L phosphate buffer, pH 7.4, with 5% acetonitrile and analyzed in a HPLC system (Waters, Milford, MA) using a Hypersil BDS C 18 3 μm 150 × 4.6-mm column (Shandon, Runcorn, UK). The derivatized amino acids were eluted with a gradually increasing concentration of acetonitrile in aqueous 0.1 mol/L (final concentration) sodium acetate buffer with 4 mmol/L triethylamine (pH 5.70). Absorbance of the eluate was monitored at 254 nm. A linear calibration curve for each amino acid was constructed from the results on identical analyses of standards of L-4-hydroxyproline (Merck, Darmstadt, Germany) and Amino Acid Standard H (Pierce) representing the range 0.5 to 200 nmol. The amounts of hydroxyproline and proline were calculated from the ratios of areas under the peaks referring to amino acids to the peak from the internal standard. The limit of detection of the methods was 0.4 nmol per sample for hydroxyproline and proline. The repeatability in determination of the percentage of hydroxyproline and proline in pure bovine collagen (Vitrogen 100, Collagen Corporation, Palo Alto, CA) was within 1.8% points.
The deposition of hydroxyproline (nmol/cm) and proline (nmol/cm) in the ePTFE tubes implanted in the upper arm was measured with HPLC. The ratio between hydroxyproline and proline was calculated from an average obtained in each of the two tubes. Thus, a ratio was obtained from the site injected with GM-CSF and saline, respectively. Because the deposition of proline in the model closely reflects the deposition of total protein, 20 the ratio is an indicator of the richness of collagen in the granulation tissue.
Statistics
Univariate data analyses were performed by the Wilcoxon test for paired data, the chi-square test, the Spearman rank correlation test, and the Kruskal-Wallis test. Multivariate testing was done with analysis of variance. P < .05 was considered statistically significant.
The number of patients in each treatment group was calculated from statistical assumptions based on results on hydroxyproline from previous studies: standard deviation = 5.7 nmol/cm, minimal effect difference not to be overlooked = 5.7 nmol/cm, type 1 error = 5%, and type 2 error = 10%. At least 20 patients in each dose group were planned to be recruited, but because a highly significant difference was detected with the first GM-CSF concentration investigated (75 μg/mL), the number of patients to be studied in the following three dose series was reduced to a minimum of 10 patients in each group.
RESULTS
Patients
The four groups with different dose regimens were comparable regarding baseline characteristics, except for significantly higher preoperative levels of circulating neutrophils and C-reactive protein in patients receiving 75 μg/mL GM-CSF (see Tables 1 and 2). There was no trend toward a higher incidence of reported local side effects in the arm with GM-CSF, nor was there any association with the dose of GM-CSF administered.
In Vivo Effects of GM-CSF on Granulation Tissue
Histology and Immunohistochemistry
The inflammatory cell response was composed predominantly of histiocytes and a few neutrophils. The cellularity, assessed semiquantitatively in hematoxylin-and-eosin-stained sections, was significantly higher in the GM-CSF-treated (10 μg/mL) ePTFE tubes than controls (P < .01) (Figs. 1 and 2). In contrast, the number of fibroblasts, identified with a monoclonal antibody against procollagen type I (Figs. 3 and 4), was significantly (P < .05) decreased in sites treated with 10 μg/mL GM-CSF compared to the contralateral control arm.
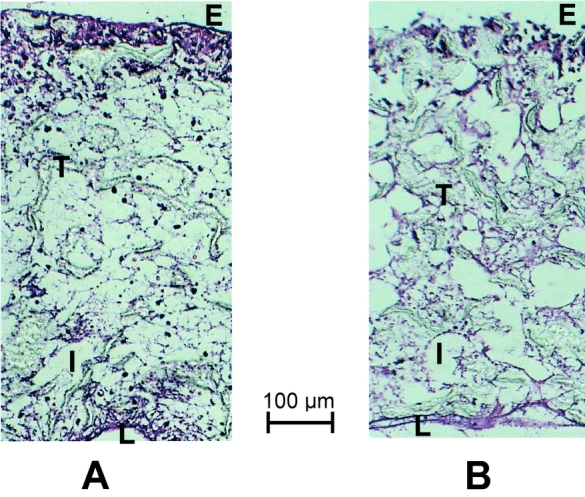
Figure 1. Photomicrographs showing transected ePTFE tube 10 days after implantation. Note the higher cellularity after local treatment with 10 μg/mL GM-CSF (A) compared to control treatment (B). E, external surface of ePTFE tube; T, trabecula of ePTFE material; I, intertrabecular space; L, lumen of ePTFE. Hematoxylin-and-eosin stain. Scale bar = 100 μm.
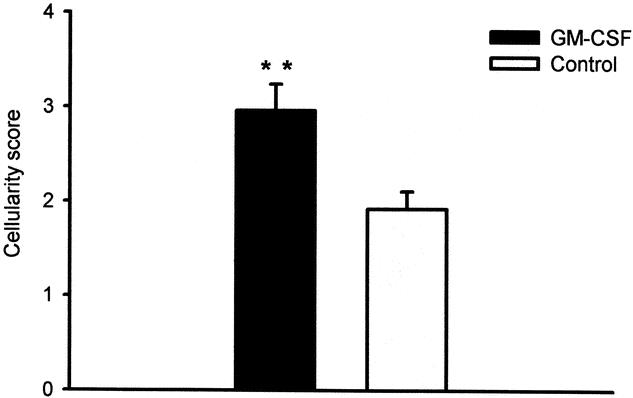
Figure 2. Cellularity in ePTFE tubes 10 days after implantation. Paired comparison between site injected with 10 μg/mL GM-CSF and site injected with vehicle (control) for each patient. Bars represent mean ± SEM cellularity in hematoxylin-and-eosin-stained specimens scored on a scale from 0 to 4. n = 13. **P < .01, Wilcoxon paired sample test.
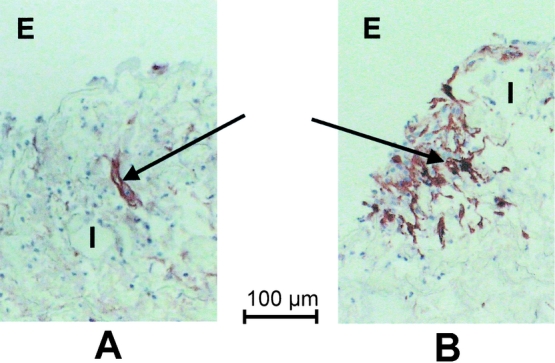
Figure 3. Procollagen type I positive fibroblasts in ePTFE tubes treated with 10 μg/mL GM-CSF (A) and control (B) from the same patient. E, external surface of ePTFE tube, I, intertrabecular space. Arrow indicates immunopositive fibroblast. Scale bar = 100 μm.
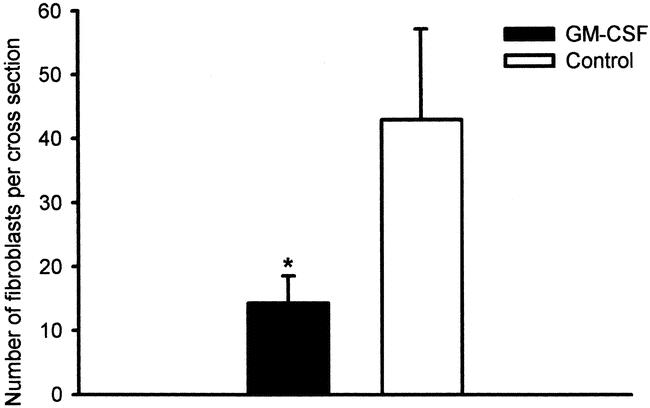
Figure 4. Density of fibroblasts in ePTFE tubes expressed as the number of immunopositive cells for procollagen I. Paired comparison between upper arm treated with GM-CSF 10 μg/mL and upper arm treated with control for each patient. Bars represent mean score ± SEM. n = 10. *P < .05, Wilcoxon paired sample test.
Hydroxyproline and Proline HPLC Measurements
A highly significant (P < .00001) suppression of collagen deposition, measured as the ratio of hydroxyproline to proline, was found following GM-CSF treatment compared to control treatment (Fig. 5). Collagen deposition in GM-CSF-treated sites correlated with that of control-treated sites (r = 0.47, P < .001). An inverse correlation between collagen deposition in both GM-CSF-treated sites (r = −0.43, P = .001) and saline-treated sites (r = −0.48, P < .001) was found with the dose of GM-CSF, suggesting a systemic depressing effect of GM-CSF on collagen accumulation in the subcutaneous ePTFE tubes.
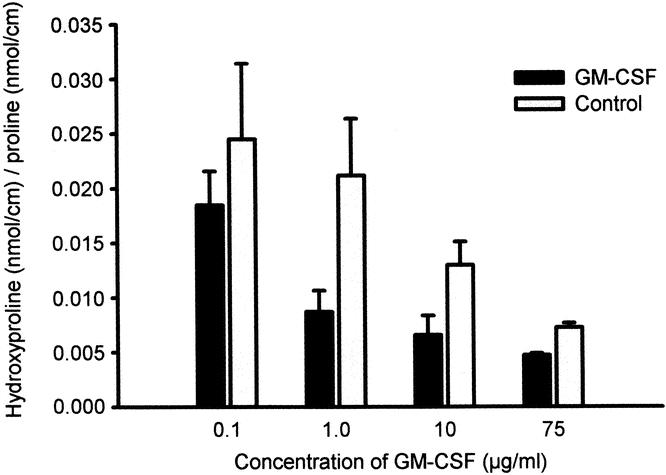
Figure 5. Deposition of collagen, expressed as the ratio between hydroxyproline and proline, in ePTFE 10 days after implantation in vivo. In each patient two ePTFE tubes were injected with GM-CSF in one upper arm and two ePTFE tubes injected with saline in the contralateral upper arm as control. Collagen deposition was impeded in the arm injected with GM-CSF (P < .001) and with higher doses of GM-CSF (P < .005, analysis of variance).
None of the baseline parameters listed in Tables 1 and 2 correlated with collagen deposited at the GM-CSF site, whereas the blood concentrations of neutrophils (r = −0.30, P < .05) and daily consumption of cigarettes (r = −0.28, P < .05) were associated with low levels of collagen at the control site. In a multivariate analysis model, the use of GM-CSF (P < .00001) and increasing doses of GM-CSF (P < .005) were the only parameters that showed a significant and independent association with collagen deposition, both resulting in up to 81% suppression of collagen.
In Vitro Effects of GM-CSF on Granulation Tissue-Derived Fibroblasts
To study the direct action of GM-CSF on fibroblast activity, fibroblasts derived from granulation tissue obtained from nontreated ePTFE tubes implanted for 10 days were treated with GM-CSF in vitro. As found in vivo, GM-CSF appeared to selectively downregulate collagen production in cultured fibroblasts (Fig. 6). Ascorbic acid, on the other hand, specifically enhanced collagen production about threefold compared with control-treated fibroblasts.
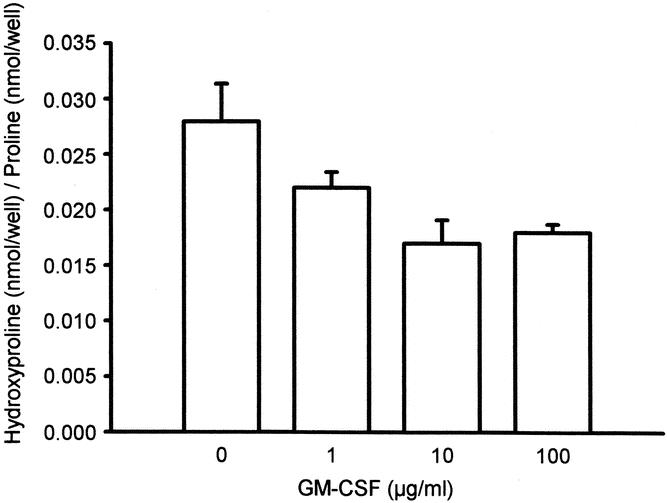
Figure 6. Depressive effect of GM-CSF on collagen production by wound-derived fibroblasts in vitro. Mean ± SEM. P < .05, analysis of variance.
DNA synthesis of quiescent wound fibroblasts was not influenced by the addition of GM-CSF at any concentration tested (Fig. 7). In contrast, PDGF-BB elicited a more than sixfold increase in DNA synthesis over control-treated fibroblasts.
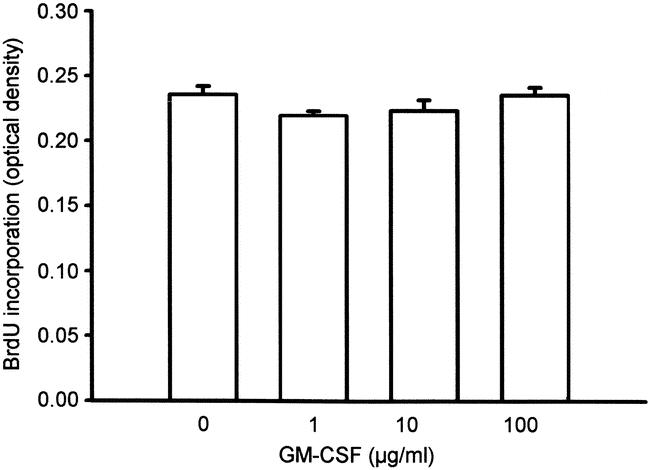
Figure 7. GM-CSF had no significant effect (analysis of variance) on DNA synthesis, measured as incorporation of the thymidine analogue bromodeoxyuridine (BrdU) by wound-derived fibroblasts in vitro. Mean ± SEM.
DISCUSSION
GM-CSF is a multipotent cytokine that regulates biologic activities of several hematopoietic and nonhematopoietic cells participating in tissue repair processes. In the present study, the effect of GM-CSF was studied in a subcutaneous tissue repair model (ePTFE tubes), which measures the connective tissue response in an acute type of injury. We found a dose-dependent and specific inhibition of collagen deposition in the ePTFE tubes after a single local application of human recombinant GM-CSF in humans.
The tissue repair response was assessed indirectly as the deposition of collagen measured as hydroxyproline. Hydroxyproline is almost uniquely present in mature collagen, whereas proline is an accurate measure of the deposition of total protein. 20 Therefore, the ratio between accumulated hydroxyproline and proline in the ePTFE tubes represents the amount of collagen relative to the total amount of proteins.
The amount of collagen deposited subcutaneously correlates with the tensile strength of wounds healing by primary intention in rats. 21 However, there is conflicting evidence on the role of GM-CSF in the healing of primary closed wounds. Jyung et al. 14 reported a 36% increase in the breaking strength of incisional wounds in rats 14 days after topical, but not after systemic, administration of GM-CSF. Robson et al. 22 found a positive effect of local GM-CSF on the contraction and closure of experimental acute and contaminated wounds, possibly by the induction of myofibroblasts. 23 However, no effect of GM-CSF on the breaking strength of these wounds was found. 22 Recently, systemic erythropoietin, but not GM-CSF, was shown to increase the tensile strength of anastomoses in rat colon. 24 The addition of GM-CSF actually counteracted the beneficial effect of erythropoietin.
The mechanism by which GM-CSF exerted its suppressive effect on collagen deposition is not clear. It is conceivable that the lowered number of fibroblasts observed in GM-CSF-treated sites would result in less collagen deposition. Fibroblasts have cell surface receptors with affinity to GM-CSF. 25–27 This finding indicates that GM-CSF may influence fibroblastic functions directly in a tissue repair reaction. Fibroblasts, isolated and cultured from granulation tissue of nontreated ePTFE tubes, showed no mitogenic response to GM-CSF, confirming earlier results with human dermal fibroblasts and endometrial stromal cells. 28,29 On the other hand, GM-CSF specifically downregulated collagen accumulation by the wound fibroblasts. It is not clear whether the reduced collagen deposition was a result of impaired synthesis or enhanced breakdown of collagen, or both. In contrast to cytokines like IL-1 and TNF-α, GM-CSF had no effect on the synthesis of collagen or other matrix molecules by human dermal fibroblasts. 30 Matrix metalloproteinases (MMPs) are capable of degrading extracellular matrix components, including interstitial collagens. The actions of GM-CSF alone or in combination with other cytokines on MMP activity are complex. In murine peritoneal macrophages, GM-CSF stimulated the production of macrophage elastase (MMP-12). Although MMP-12 preferentially degrades elastin, it also degrades other extracellular matrix components. 31 GM-CSF, TNF-α, or IL1-β, when added individually, are capable of stimulating human monocytes to produce MMP-9 and its inhibitor TIMP-1. 32
The reduced collagen deposition might also have been secondary to the observed increased and prolonged mononuclear inflammatory infiltration at day 10 after GM-CSF treatment. Major surgery does not appear to be associated with increased systemic levels of GM-CSF. 33,34 Thus, the detrimental effects of GM-CSF, either locally or at distant repair sites, were unlikely attributed to a general elevation of GM-CSF. On the other hand, the timing and type of cytokine or growth factor administered appear to be crucial for enhancement of wound repair. In an interesting experimental study in rats, proinflammatory GM-CSF was injected into nontraumatized tissue 5 days preoperatively. 35 The priming with GM-CSF of the wound sites was followed by an intralesional injection with the profibrotic TGF-β2 at the time of wounding. This sequential treatment regimen resulted in a substantially increased breaking strength compared to administration of the factors alone. Activation of inflammation by GM-CSF prior to rather than at wounding may therefore accelerate wound healing when combined with an appropriate growth factor. 35
The effect of GM-CSF on inflammation and fibrosis varies with the organ or tissue studied. In an experimental model of established lung fibrosis in mice, systemic intraperitoneal administration of GM-CSF inhibited the development of bleomycin-induced pulmonary inflammation and fibrosis as measured by the total hydroxyproline content of the lungs. Analogously, simultaneous systemic administration of an anti-GM-CSF antibody elevated the deposition of lung collagen by twofold. 36 In contrast, in normal mouse lung transfected with the GM-CSF gene, a typical fibrotic response developed, possibly via upregulation of TGF-β. 13 In skin, GM-CSF delivered continuously by transfection with the GM-CSF gene elicited a dermal inflammatory reaction and eventually fibrosis. 13 We found an impaired fibrotic response in the subcutaneous tissue in humans treated locally with GM-CSF.
A within-subject placebo-controlled trial in humans reported that GM-CSF administered locally increased the local levels of IL-8. 15 However, GM-CSF did not influence the wound fluid levels of the profibrotic polypeptide growth factors TGF-β, PDGF, or fibroblast growth factor, nor the angiogenic vascular endothelial growth factor or epidermal growth factor. 2,15 Taken together, these findings suggest that GM-CSF primarily altered the proinflammatory cytokine network and not the growth factor profile in a tissue repair reaction in humans. IL-8, a potent chemokine, might have caused the increased inflammatory cell infiltrate induced by GM-CSF giving rise to the changed cytokine profiles. No effect of GM-CSF was found on epithelialization of acute wounds. 15 This contradicts the report of da Costa et al., 37 who found accelerated healing in patients with chronic venous leg ulcers compared to placebo after local application of GM-CSF in doses from 200 to 400 μg. Epithelialization was not assessed with our wound model.
Furthermore, GM-CSF exerted a systemic effect at doses exceeding 4 μg subcutaneously. The physiologic concentration of GM-CSF in blood is about 20 pg/mL. 38 In this study, GM-CSF was administered at concentrations from 0.1 to 75 μg/mL, representing values 5,000 to 4 million times higher, although the local endogenous GM-CSF levels rise about 100-fold at an inflammatory site to 1,000 to 3,000 pg/mL. 15,33,39 In the study by Ure et al., 15 intradermal injections in the thigh of healthy volunteers with 20 μg GM-CSF 1 and 4 days after wounding resulted in elevated GM-CSF levels not only in the treated leg but also (albeit less so) in the wound on the contralateral leg not injected with GM-CSF. This finding supports our biochemical data of reduced collagen deposition also in the control ePTFE tubes as a distant effect of GM-CSF.
In conclusion, topical application of GM-CSF to the subcutaneous layer of humans impeded, dose-dependently and specifically, collagen deposition in an experimental wound. In vitro studies on cultured fibroblasts from our wound model confirmed these findings and showed no mitogenic effect of GM-CSF. Local treatment of wounds with GM-CSF in doses exceeding 4 μg may result in systemic effects. Although there are no direct associations between measurements of collagen deposition obtained in minimal experimental and surgical incisional wounds, 20 this study does not support a therapeutic role for single application of GM-CSF for healing of uncomplicated surgical wounds.
Acknowledgments
Dr. Henrik Klem Thomsen supervised the immunohistochemical work. The skilled work of laboratory technicians Rikke Roel and Annie Høj is appreciated. The authors thank Dr. Michael Crawford for revision of the manuscript and Schering-Plough A/S, Denmark, for supplying GM-CSF.
Footnotes
Supported in part by Schering-Plough A/S and the A. & J. Louis-Hansens Foundation, Denmark.
Correspondence: Lars Nannestad Jorgensen, MD, Dept. K, Surgical Gastroenterology, Bispebjerg University Hospital, DK-2400 Copenhagen NV, Denmark.
E-mail: lnj@dadlnet.dk
Accepted for publication March 21, 2002.
References
- 1.Mels AK, Statius Muller MG, van Leeuwen PA, et al. Immune-stimulating effects of low-dose perioperative recombinant granulocyte-macrophage colony-stimulating factor in patients operated on for primary colorectal carcinoma. Br J Surg 2001; 88: 539–544. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs EJ, DiPietro LA. Fibrogenic cytokines and connective tissue production. FASEB J 1994; 8: 854–861. [DOI] [PubMed] [Google Scholar]
- 3.Rappolee DA, Werb Z. Macrophage-derived growth factors. Curr Top Microbiol Immunol 1992; 181: 87–140. [DOI] [PubMed] [Google Scholar]
- 4.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock 1995; 4: 233–240. [PubMed] [Google Scholar]
- 5.Cromack DT, Porras-Reyes B, Purdy JA, et al. Acceleration of tissue repair by transforming growth factor beta 1: identification of in vivo mechanism of action with radiotherapy-induced specific healing deficits. Surgery 1993; 113: 36–42. [PubMed] [Google Scholar]
- 6.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest 1972; 51: 2009–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones TC. The effects of rhGM-CSF on macrophage function. Eur J Cancer 1993; 29A: S10–13. [DOI] [PubMed] [Google Scholar]
- 8.Bussolino F, Wang JM, Defilippi P, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989; 337: 471–473. [DOI] [PubMed] [Google Scholar]
- 9.Vaillant P, Muller V, Martinet Y, et al. Human granulocyte- and granulocyte-macrophage-colony stimulating factors are chemotactic and “competence” growth factors for human mesenchymal cells. Biochem Biophys Res Commun 1993; 192: 879–885. [DOI] [PubMed] [Google Scholar]
- 10.Hancock GE, Kaplan G, Cohn ZA. Keratinocyte growth regulation by the products of immune cells. J Exp Med 1988; 168: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubbia-Brandt L, Sappino AP, Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol 1991; 60: 73–82. [DOI] [PubMed] [Google Scholar]
- 12.Vyalov S, Desmouliere A, Gabbiani G. GM-CSF-induced granulation tissue formation: relationships between macrophage and myofibroblast accumulation. Virchows Arch B Cell Pathol 1993; 63: 231–239. [DOI] [PubMed] [Google Scholar]
- 13.Xing Z, Gauldie J, Tremblay GM, et al. Intradermal transgenic expression of granulocyte-macrophage colony- stimulating factor induces neutrophilia, epidermal hyperplasia, Langerhans’ cell/macrophage accumulation, and dermal fibrosis. Lab Invest 1997; 77: 615–622. [PubMed] [Google Scholar]
- 14.Jyung RW, Wu L, Pierce GF, et al. Granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor: differential action on incisional wound healing. Surgery 1994; 115: 325–334. [PubMed] [Google Scholar]
- 15.Ure I, Partsch B, Wolff K, et al. Granulocyte/macrophage colony-stimulating factor increases wound-fluid interleukin 8 in normal subjects but does not accelerate wound healing. Br J Dermatol 1998; 138: 277–282. [DOI] [PubMed] [Google Scholar]
- 16.Goodson WH, Hunt TK. Development of a new miniature method for the study of wound healing in human subjects. J Surg Res 1982; 33: 394–401. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen LN, Kallehave F, Karlsmark T, et al. Evaluation of the wound healing potential in human beings from the subcutaneous insertion of expanded polytetrafluoroethylene tubes. A methodologic study. Wound Rep Reg 1994; 2: 20–30. [DOI] [PubMed] [Google Scholar]
- 18.Ågren MS, Steenfos HH, Dabelsteen S, et al. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Invest Dermatol 1999; 112: 463–469. [DOI] [PubMed] [Google Scholar]
- 19.Bidlingmeyer BA, Tarvin TL, Cohen SA. Amino acid analysis of submicrogram hydrolyzate samples. In: Walsh KA, ed. Methods in Protein Sequence Analysis. Clifton, NJ: Humana Press, 1987: 229–245.
- 20.Jorgensen LN, Sorensen LT, Kallehave F, et al. Increased collagen deposition in an uncomplicated surgical wound compared to a minimal subcutaneous test wound. Wound Rep Reg 2001; 9: 194–199. [DOI] [PubMed] [Google Scholar]
- 21.Wicke C, Halliday BJ, Scheuenstuhl H, et al. Examination of expanded polytetrafluoroethylene wound healing models. Wound Rep Reg 1995; 3: 284–291. [DOI] [PubMed] [Google Scholar]
- 22.Robson MC, Kucukcelebi A, Carp SS, et al. Effects of granulocyte-macrophage colony-stimulating factor on wound contraction. Eur J Clin Microbiol Infect Dis 1994; 13: 41–46. [DOI] [PubMed] [Google Scholar]
- 23.Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract 1994; 190: 851–853. [DOI] [PubMed] [Google Scholar]
- 24.Fatouros MS, Vekinis G, Bourantas KL, et al. Influence of growth factors erythropoietin and granulocyte macrophage colony-stimulating factor on mechanical strength and healing of colonic anastomoses in rats. Eur J Surg 1999; 165: 986–992. [DOI] [PubMed] [Google Scholar]
- 25.Postiglione L, Montagnani S, Riccio A, et al. Expression of GM-CSF receptor and “in vitro” effects of GM-CSF on human fibroblasts. Life Sci 1998; 63: 327–336. [DOI] [PubMed] [Google Scholar]
- 26.Eder M, Griffin JD, Ernst TJ. The human granulocyte-macrophage colony-stimulating factor receptor is capable of initiating signal transduction in NIH3T3 cells. EMBO J 1993; 12: 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chegini N, Tang XM, Dou Q. The expression, activity and regulation of granulocyte macrophage-colony stimulating factor in human endometrial epithelial and stromal cells. Mol Hum Reprod 1999; 5: 459–466. [DOI] [PubMed] [Google Scholar]
- 28.Birkland TP, Cheavens MD, Pincus SH. Human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Arch Dermatol Res 1994; 286: 312–318. [DOI] [PubMed] [Google Scholar]
- 29.Chegini N, Tang XM, Ma C. Regulation of transforming growth factor-beta1 expression by granulocyte macrophage-colony-stimulating factor in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab 1999; 84: 4138–4143. [DOI] [PubMed] [Google Scholar]
- 30.Duncan MR, Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol 1989; 92: 699–706. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, Dong Z, Fidler IJ. Differential regulation of metalloelastase activity in murine peritoneal macrophages by granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor. J Immunol 1996; 157: 5104–5111. [PubMed] [Google Scholar]
- 32.Zhang Y, McCluskey K, Fujii K, et al. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol 1998; 161: 3071–3076. [PubMed] [Google Scholar]
- 33.Weissflog D, Kroegel C, Luttmann W, et al. Leukocyte infiltration and secretion of cytokines in pleural drainage fluid after thoracic surgery: impaired cytokine response in malignancy and postoperative complications. Chest 1999; 115: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 34.Denizot Y, Karoutsos S, Nathan N. Differential alterations in plasma colony-stimulating factor concentrations after coronary artery bypass graft surgery with extracorporeal circulation. Cytokine 2001; 13: 314–316. [DOI] [PubMed] [Google Scholar]
- 35.Smith PD, Kuhn MA, Franz MG, et al. Initiating the inflammatory phase of incisional healing prior to tissue injury. J Surg Res 2000; 92: 11–17. [DOI] [PubMed] [Google Scholar]
- 36.Piguet PF, Grau GE, de Kossodo S. Role of granulocyte-macrophage colony-stimulating factor in pulmonary fibrosis induced in mice by bleomycin. Exp Lung Res 1993; 19: 579–587. [DOI] [PubMed] [Google Scholar]
- 37.da Costa RM, Ribeiro JF, Aniceto C, et al. Randomized, double-blind, placebo-controlled. Wound Rep Reg 1999; 7: 17–25. [DOI] [PubMed] [Google Scholar]
- 38.Mortensen BT, Schifter S, Pedersen LB, et al. Development and application of a sensitive radioimmunoassay for human granulocyte-macrophage colony-stimulating factor able to measure normal concentrations in blood. Exp Hematol 1993; 21: 1366–1370. [PubMed] [Google Scholar]
- 39.Harris IR, Yee KC, Walters CE, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol 1995; 4: 342–349. [DOI] [PubMed] [Google Scholar]