Abstract
Objective
To investigate whether liver regeneration is an angiogenesis-associated phenomenon.
Summary Background Data
Angiogenesis is predominantly known for its pivotal role in tumor growth. However, angiogenesis could also play a role in physiologic processes involving tissue repair, such as liver regeneration.
Methods
Mice subjected to 70% partial hepatectomy were treated with human angiostatin (100 mg/kg body weight). Regeneration-induced hepatic angiogenesis was determined by assessing intrahepatic microvascular density using CD31 staining of frozen liver sections. Liver regeneration was evaluated by assessing wet liver weights and BrdU incorporation in DNA at regular intervals after partial hepatectomy. Possible direct effects of angiostatin on hepatocytes were studied by assessment of liver enzymes (ASAT, ALAT, bilirubin, lactate dehydrogenase), MTT assay (cytotoxicity), aminophenol production (metabolic function), and TUNEL (apoptosis).
Results
In a regenerating liver, microvascular density increased by 38%. Angiostatin significantly inhibited this response by 60%. In addition, angiostatin inhibited liver regeneration by 50.4% and 24.9% on postoperative days 7 and 14, respectively. In control mice liver weights regained normalcy in 8 days, whereas those in angiostatin-treated mice normalized after 21 days. In angiostatin-treated mice, the maximal BrdU incorporation was decreased and delayed. Direct adverse effects of angiostatin on cultured and in vivo hepatocytes were not observed. Angiostatin neither induced necrosis on hematoxylin and eosin staining nor affected serum levels of liver enzymes.
Conclusions
Liver regeneration is accompanied by intrahepatic angiogenesis. Antiangiogenic treatment using angiostatin inhibits both phenomena. The authors conclude that liver regeneration is, at least in part, an angiogenesis-dependent phenomenon.
Angiogenesis, defined as the formation of new microvasculature from preexisting blood vessels, has received much attention in recent years since it has been proven to be a pivotal event in tumor growth and metastasis. 1 Inhibition of tumor-associated angiogenesis has, in fact, become one of the most promising developments in cancer treatment; more than 31 angiogenesis inhibitors have been evaluated in clinical trials. 2 However, the role of angiogenesis in physiologic processes, such as wound healing, ovulation, and menstruation, is less well established. In addition, vast differences exist between physiologic and tumor-associated angiogenesis. Tumor-associated angiogenesis is uncontrolled and characterized by a tortuous, dilated, and disorganized microvascular network. 3,4 This is accompanied by vascular leakiness, 5 the influx and presence of inflammatory cells, 6 and the deposition of fibrin. 7 In contrast, resting endothelial cells rarely proliferate under physiologic conditions. Only 0.01% of adult endothelial cells are in the S phase at any given time. 8 This is remarkable since endothelial cells are surrounded by many proangiogenic factors. Furthermore, physiologic angiogenesis is transient, lasting only days before it is turned off abruptly. 9,10
Liver regeneration (LR) is a tissue repair response of the liver following the loss of hepatic tissue. 11 The exact mechanisms underlying LR have not been fully characterized. Nevertheless, studies have shown that after 70% partial hepatectomy (PH) many mitogenic molecules are upregulated, resulting in the regenerative outgrowth of the liver remnant until the full original liver weight has been regained. 12–14 Remarkably, many of the growth factors upregulated in a regenerating liver are known for their angiogenic properties in vivo. For instance, vascular endothelial growth factor (VEGF) is upregulated after PH. 15–17 It is a major proangiogenic factor 18 and is thought to improve sinusoid reconstruction during the LR process. 19 Hepatocyte growth factor levels are dramatically increased following PH, 20,21 but hepatocyte growth factor is also a potent angiogenic factor in vivo and stimulates endothelial cell protease production, motility, proliferation, and differentiation in vitro. 22,23 Although it has been demonstrated that many major proangiogenic factors are upregulated in a regenerating liver, there is no in vivo evidence that intrahepatic angiogenesis occurs during LR.
The present study was undertaken to investigate whether LR is accompanied by angiogenesis, and to test the hypothesis that LR is angiogenesis-dependent. This was done by subjecting mice to potent antiangiogenic treatment using angiostatin during LR. For this purpose we studied LR following 70% PH in non-tumor-bearing mice treated with angiostatin. Angiostatin, a fragment of human plasminogen, is one of the most potent antiangiogenic agents currently known. 24,25 From our studies we conclude that angiogenesis plays an important role in LR.
METHODS
Experimental Design
For the in vivo experiments, animals were divided in control mice (n = 10), mice subjected to 70% PH (PH group; n = 7), and mice subjected to both 70% PH and angiostatin treatment (PH-AS group; n = 7). The variation in the number of mice in the three groups can be explained by the restricted amount of angiostatin available. In addition, since it is vital that mice of the same age are used for experiments on LR, we were forced to accept the limited supply of mice aged 8 to 10 weeks (24 mice maximum). Intrahepatic vascularization was evaluated by determining the microvascular density using a monoclonal antibody against CD31. LR was evaluated by determining the hepatocyte cell proliferation by nuclear incorporation of bromodeoxyuridine (BrdU) and wet liver weight. For the evaluation of direct effects of angiostatin on hepatocytes, primary isolated murine hepatocytes were subjected to incubation with angiostatin. Apoptosis, synthetic capacity, and detoxifying features of hepatocytes were evaluated.
Animals
Male BALB/c mice were purchased from the General Animal Laboratory, University Medical Center Utrecht (Utrecht, The Netherlands). In all experiments mice were 8 to 10 weeks of age. Animals were maintained under specific pathogen-free conditions, were allowed food and water ad libitum, and were kept on a 12-hour light/dark cycle. Experiments were performed according to the guidelines of the Utrecht Animal Experimental Committee, University Medical Center Utrecht, The Netherlands.
Angiostatin
The antiangiogenic properties and the production of plasminogen-derived human angiostatin have been previously reported. 24,26 Briefly, recovered outdated human plasma was used to elute plasminogen. SDS-polyacrylamide gel electrophoresis of the eluant revealed one band of 92 kd corresponding to plasminogen. The eluant was subjected to proteolytic digestion with porcine pancreatic elastase in a concentration of 0.8 units/mg plasminogen. Then, angiostatin was isolated by applying the solution over a lysine-Sepharose column followed by elution with 0.2 mmol/L ε-ACA. SDS-polyacrylamide gel electrophoresis revealed three distinct bands of approximately 40, 42, and 45 kd, resembling the triplet first described by O’Reilly et al. 27 After freeze-drying, angiostatin was dissolved in phosphate-buffered saline (PBS; 175 mg/mL) and stored at −80°C. The biologic effect of angiostatin was tested using the corneal neovascularization assay in which basic fibroblast growth factor-induced angiogenesis was inhibited almost completely. 24 To examine the effects of angiostatin treatment on regeneration-associated hepatic angiogenesis, BALB/c mice were subjected to PH immediately followed by implantation of subcutaneous pumps containing angiostatin (PH-AS group). Ten minutes before PH, mice received a single bolus injection of angiostatin (2.5 mg/200 μL, given subcutaneously) to ensure therapeutic angiostatin levels during the operation (half-life of human angiostatin in mice is 4–6 hours). 25
Liver Regeneration
LR was induced by subjecting mice to 70% PH as described by Higgins and Anderson. 28 Mice were anesthetized with halothane. After a midline laparotomy, the liver was exposed and the left lateral and caudal lobes were ligated (Vicryl 3-0) and resected, resulting in removal of ±68% of liver volume. Before closure of the abdominal wall in two layers (Vicryl 5-0), 250 μL of 5% glucose solution (at 37°C) was injected into the abdominal cavity.
Cell Proliferation
To determine the proportion of hepatocytes in S phase, a BrdU pulse-labeling technique was used. 29 Two hours before ending the experiment, mice received an intraperitoneal injection with BrdU (150 mg/kg body weight in PBS; Sigma Chemical Co., St. Louis, MO). After the mice were killed, liver lobes were fixed in formaldehyde, 4-μm-thick paraffin sections were deparaffinized, and DNA was denatured with warm HIO4 (60°C; 30 minutes). Slides were washed thoroughly with PBS and then incubated with 5% bovine serum albumin (BSA) in PBS to block nonspecific binding sites of the antibodies. Subsequently, the slides were incubated (60 minutes) with the primary antibody, a monoclonal anti-BrdU antibody (Becton Dickinson, Mountain View, CA), diluted 1:40 in PBS. After washing steps in PBS, slides were incubated for 30 minutes with a secondary nonlabeled polyclonal antimouse IgG (DAKO, ITK Diagnostics BV, Uithoorn, The Netherlands) and diluted 1:80 in PBS, including 1% BSA in a humidified chamber. Furthermore, slides were incubated with 0.45% 10-nm Protein A Gold (PAG; 60 minutes), kindly provided by H. J. G. van de Kant (Department of Cell Biology, University Medical Center Utrecht, The Netherlands). After extensive washing with PBS (30 minutes) and deionized water (30 minutes), the gold particles were silver-enhanced with Aurion R-GENT (30 minutes; Aurion, Wageningen, The Netherlands). Finally, slides were counterstained with Mayer’s hematoxylin and mounted in Pertex mounting medium. A minimum of 1,500 hepatocytes were counted for estimation of the BrdU labeling index, defined as the percentage of BrdU-positive nuclei.
Intrahepatic Vascularization
Immunohistochemical staining was performed on acetone-fixed 5-μm cryosections of hepatic tissue. The sections were incubated in PBS containing 1.5% hydrogen peroxide at room temperature for 15 minutes to block endogenous peroxidase activity. The sections were stained with anti-CD31 mAb (MEC 13.3; kindly provided by Dr. Vecchi, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy) for 60 minutes, followed by fixation in phosphate-buffered formaldehyde 3.7% (10 minutes). 30 Sections were incubated for 1 hour with a horseradish peroxidase-coupled rabbit antirat antibody. After three washes, labeling was visualized with 0.03% 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution containing 0.1% hydrogen peroxide for 10 minutes. Sections were counterstained for 45 seconds in Mayer’s hematoxylin and mounted in DPX (Klinipath BV, The Netherlands). The microvascular density (MVD) was determined by analyzing at least four independent microscopic fields per tissue section and at least two sections per liver remnant. Fields containing large macrovascular structures (portal/central venules and hepatic arterioles) were excluded, and only microvessels were counted. The number of microvessels stained for CD31 was counted by two independent observers (T.A.D. and T.M.V.) at a magnification of 250×. MVD was expressed as the mean number of microvessels (± SEM) per high-power field.
Hepatocyte Isolation
Hepatocytes were isolated by a two-step collagenase perfusion of the liver as described by Seglen but modified to compensate for the smaller size of the animals (20% of the original flow rate and 50% reduction in perfusate volume). 31 Mice were anesthetized using a mixture of Hypnorm (0.3 mg/mouse given intraperitoneally; Janssen-Cilag, Brussels, Belgium) and Dormicum (12.5 mg/mouse given intraperitoneally; Roche, Brussels, Belgium). After cannulation of the portal vein the liver was perfused with 200 mL Ca2+- and Mg2+- free Hanks balanced saline solution (37°C; 10 mL/min). After 10 minutes the liver was perfused with the same buffer supplemented with 0.05% collagenase (Boehringer Mannheim Biochemica, Germany) and 2.5 mmol/L CaCl2 for 10 minutes. After the entire liver was resected, the lobes were thoroughly cut in small pieces and diluted in medium (HBBS supplemented with 2.5% BSA, Sigma-Aldrich Chemie A-9647, Zwijndrecht, The Netherlands) and 2.5 mmol/L CaCl2. Dispersed cells were centrifuged (50G∼ 600 rpm) three times for 3 minutes. Cell viability was routinely ∼90%, as determined by trypan blue exclusion. Hepatocytes were plated at a density of 8.3 × 103/cm2 in Williams medium E (Flow Laboratories, Irvine, Scotland) supplemented with 10% heat-inactivated newborn calf serum (Gibco Life Technologies Inc., Rockville, MD), sodium bicarbonate (0.23%; local pharmacy, University Medical Center Utrecht, Utrecht, The Netherlands), L-glutamine (2 mmol/L; BioWhittaker, Verviers, Belgium), gentamycin (50 g/mL; Sigma-Aldrich Chemie G-3632), insulin (5.75 g/mL; Sigma-Aldrich Chemie I-5500), and hydrocortisone (5 g/mL; Sigma-Aldrich Chemie H-4881)). Hepatocytes were incubated at 37°C in an atmosphere of 5% CO2 for 4 hours before they were subjected to experiments.
In Vivo Cytotoxicity of Angiostatin
Three weeks following partial hepatectomy, serum of mice was sampled and analyzed for total bilirubin concentration, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase.
Synthetic Capacity of Hepatocytes
To monitor the effects of angiostatin on hepatocellular synthetic capacity, primary hepatocytes were incubated with analin. Analin is metabolized by hepatocytes to 4-aminophenol, which can be measured spectrophotometrically. 32,33 Twenty-four hours after plating, culture medium was refreshed by serum-free medium containing 5 mmol/L analin and different concentrations of angiostatin (0.1, 10, and 1,000 μg/mL). Controls consisted of serum-free medium containing 5 mmol/L analin and PBS. Another 24 hours later, samples were taken from the medium and used for the determination of 4-aminophenol (free and conjugated). 4-Aminophenol was detected according to the method of Evelo et al. 32 The amounts of 4-aminophenol (free and conjugated) were expressed as nmol metabolite formed/106 cells/24 hours.
Determination of Aniline and its Metabolites
For determination of glucuronide conjugate, 4 units of glucuronidase in 600 L of a 200 mmol/L KH2PO4/NaHPO4 buffer (pH 6.2) were added to an equal volume of medium and incubated in a water bath (37°C; 1 hour). Following hydrolysis, protein was precipitated by adding 300 L of aqueous trichloracetic acid solution/1,000 L incubation mixture in Eppendorf vials. After centrifugation, equal volumes of 1 mol/L HCl were added to the supernatants. The acidic solutions were stored overnight at 4°C and the determination of liberated 4-aminophenol was performed the next day. For determination of free 4-aminophenol (i.e., 4-aminophenol not conjugated at C-4) the same procedure was followed, but without addition of glucuronidase.
To hydrolyze the sulfate conjugates, 1 mL of the glucuronidase-treated acidified solution was boiled for 6.5 minutes in a 20-mL glass tube topped with a glass marble. Corrections were made for the amount of free 4-aminophenol lost during acid hydrolysis. The amount of sulfate conjugate liberated in this way was shown to be equal to the amount liberated by enzymic hydrolysis with glucuronidase/ arylsulfatase.
To detect 4-aminophenol, 300 μL phenol reagent (2.5% w/v phenol, 1.25 mol/L NaOH, 1.25 Na2CO3) was added to 750 μL sample, and the absorption was determined using a spectrophotometer.
Hepatocellular Detoxification
To monitor the effects of angiostatin on the detoxification capacity, primary hepatocytes were subjected to incubation with MTT (3-(4, 5–dimethylthiazol)-2,5-diphenyl 2-H-tetraliumbromide; C18H16BrN5S2). MTT is a substrate for mitochondrial succinate dehydrogenase that is catalyzed into formazan. In the presence of a cytoxic agent, the mitochondria are less functional, and this is expressed as less transformation of MTT into formazan. Therefore, the MMT assay is a sensitive assay to detect cytotoxic effects. 34 MTT (1 mg/mL) was added (45 minutes, 37°C) to primary hepatocytes in culture (3 × 105/6-cm well) that had been incubated for 24 hours with angiostatin (0.1–10 mg/mL) or medium (control). After extensive washing in PBS, cell membranes were permeabilized with isopropanol (10 minutes) to extract intracellular MTT; optical density was measured by a multiwell scanning spectrophotometer at 560 nm.
Apoptosis
To determine whether angiostatin induces apoptosis in hepatocytes in vivo, liver slides were subjected to a TUNEL assay. All reagents were part of the In Situ Cell Death Detection Kit-AP (Cat. No 684-809, Roche Diagnostics BV, Almere, The Netherlands). Tissue sections (5 μm) were fixed with formaldehyde in 3.7% PBS for 20 minutes at room temperature. After washing in PBS, sections were incubated in 0.1% Triton X-100 in citrate phosphate buffer (10 minutes) followed by a final wash step in PBS again (10 minutes). Next, sections were incubated in TUNEL reaction mixture (60 minutes at 37°C). After washing in PBS, sections were incubated in TUNEL Converter-AP (30 minutes at 37°C), followed by a PBS wash step. Signal conversion was performed by the TUNEL BM Purple AP substrate. Finally, sections were mounted using DPX mounting medium. A minimum of 1,500 hepatocytes were counted for estimation of the apoptotic index, defined as the percentage of positive cells.
Statistical Analysis
Data were expressed as mean ± SEM unless mentioned otherwise in the text. Comparisons between multiple groups were performed by one-way analysis of variance, followed by the Newman-Keuls protected least significant difference posthoc test. P < .05 was considered statistically significant.
RESULTS
In normal livers of BALB/c mice (control group sham-operated), 63 ± 5 blood vessels per high-power field were counted (Figs. 1 and 2). In mice subjected to PH, the hepatic MVD increased significantly to 81 ± 5 blood vessels per high-power field (P < .05 vs. control) during the first 3 days following PH. Thereafter, MVD reached a plateau phase of 87 ± 5.7 blood vessels per high-power field (P < .05 vs. control) until the end of the observation period on postoperative day 21. Besides a difference in the number of blood vessels, the histologic appearance of hepatocytes was found to change during LR. This change was characterized by hepatocellular hyperplasia and thickening of the hepatocyte cords (double-cell plates) (Fig. 3).
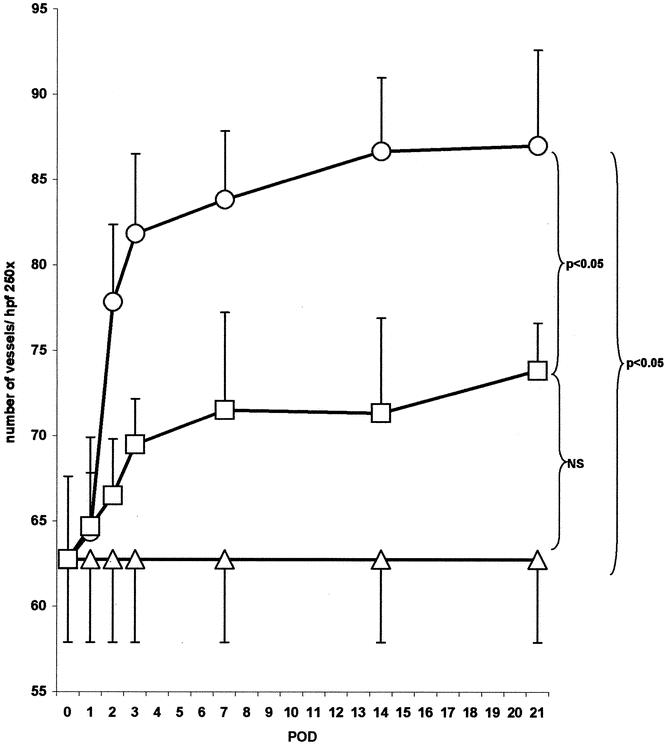
Figure 1. Effects of angiostatin on microvascular density in a regenerating liver. In control mice (ρ) 62.8 ± 4.9 sinusoidal blood vessels per high-power field (hpf) were counted. Following 70% PH (μ = PH-group) microvascular density increased to 87.0 ± 5.6 blood vessels/hpf (P < .05 vs. control; postoperative day 21). In angiostatin-treated mice (ο = AS/PH group) vascular density was reduced to 73.8 ± 2.8 (P = NS from control mice; postoperative day 21).
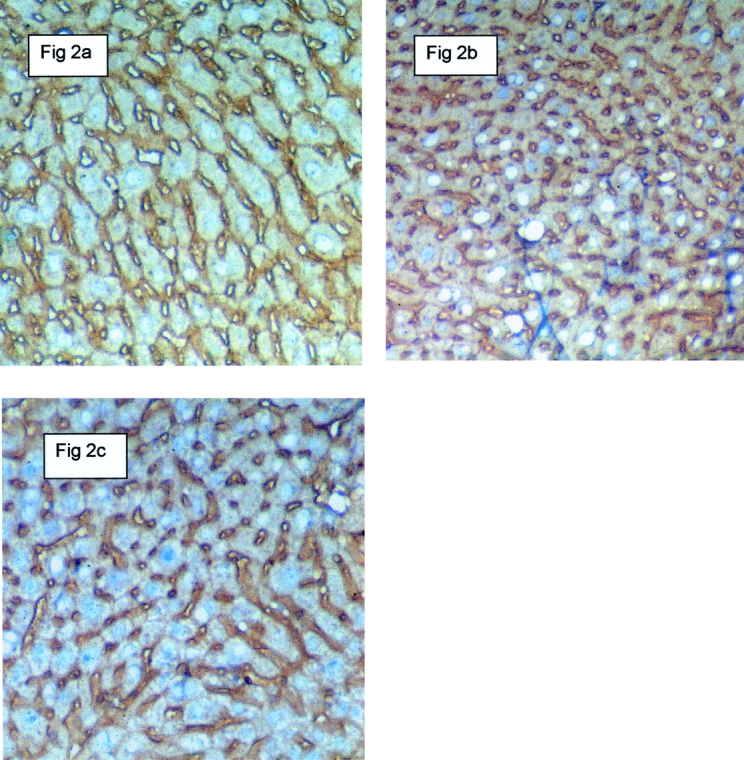
Figure 2. Effects of angiostatin on microvascular density in a regenerating liver 14 days following 70% partial hepatectomy. The identification of sinus endothelial cells was performed by immunostaining with antibodies against CD-31 (PECAM). A, B, and C represent the control group, the partial hepatectomy group, and the partial hepatectomy plus angiostatin group, respectively. Twenty-one days after partial hepatectomy, the microvascular density was increased significantly compared to both angiostatin-treated mice and control, nonhepatectomized mice. Magnification 250×.
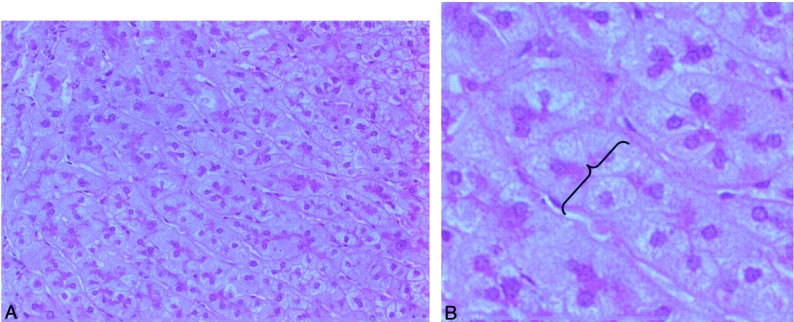
Figure 3. Histologic appearance of regenerating liver 7 days following 70% partial hepatectomy. (A) In angiostatin-treated mice no necrosis was observed after partial hepatectomy (hematoxylin and eosin, magnification 100×). (B) Hyperplasia (increase in the number of hepatocytes) and thickening of the hepatocyte cords (double-cell plates) as pointed out by the accolade (hematoxylin and eosin, magnification 400×).
In angiostatin-treated animals MVD decreased significantly by 64% (P < .05 vs. PH group) to a level that was not significantly different from non-PH controls. Angiostatin affected not only the vascular density after PH but also the kinetics of liver regeneration. In control mice wet liver weight returned to the normal range within 8 days after PH. In contrast, in angiostatin-treated mice, wet liver weight normalized only after 21 days. As judged by wet liver weight, angiostatin inhibited LR by 51% and 26% on postoperative days 7 and 14, respectively (Fig. 4). The observed delay in LR was accompanied by a decrease in the maximal BrdU incorporation from 26% in the control group to 13.3% in the angiostatin group. In addition, the BrdU peak shifted from 48 hours to 96 hours after hepatectomy (Fig. 5).
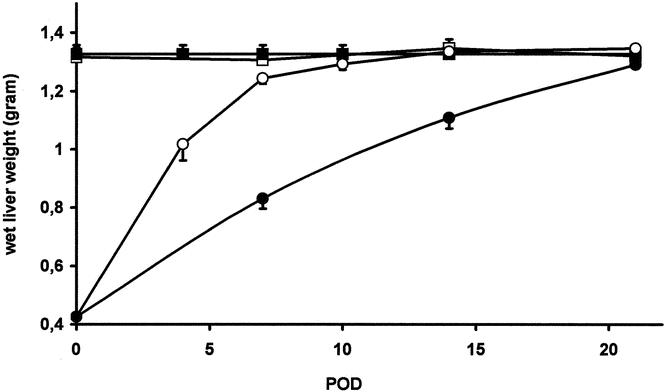
Figure 4. Effects of angiostatin on wet liver weight in resting and regenerating liver. In normal mice (< = control) wet liver weight was 1.326 ± 0.031 g. Angiostatin treatment did not affect wet liver weight in normal mice (ο = AS group): 1.323 ± 0.015 g (P = NS vs. control). Following 70% partial hepatectomy in nontreated mice (μ = PH group), the liver remnant returned to normal (original) weight in 8 days. In angiostatin-treated mice (• = AS/PH-group) this took approximately 3 weeks.
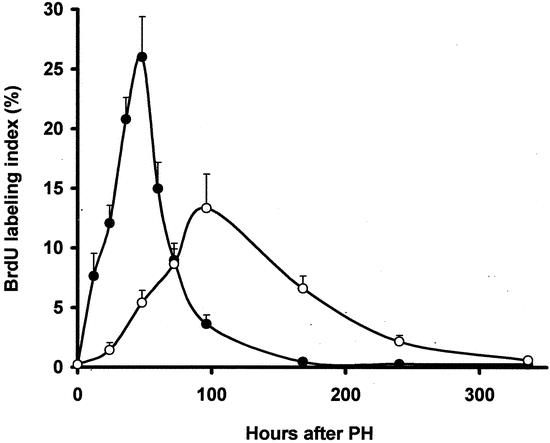
Figure 5. Effects of angiostatin on the proliferation of hepatocytes in resting and regenerating liver. BrdU uptake is represented by the ratio of labeled and nonlabeled hepatocytes times 100%. In normal resting liver the hepatocellular BrdU labeling index was approximately 0.2% (data not shown). Two days following 70% partial hepatectomy (PH group) a maximum of 26 ± 3.4% hepatocytes was labeled. In angiostatin-treated mice (PH/AS group) the BrdU labeling index peak was delayed to 96 hours after 70% partial hepatectomy. The BrdU peak was also significantly lower compared to the PH group (13.3 ± 2.9%;P < .001 vs. PH group).
To exclude any direct adverse effects of angiostatin on hepatocytes, we tested whether angiostatin affected the synthetic and detoxification capacity of cultured primary hepatocytes. The synthetic capacity of hepatocytes was not influenced by angiostatin. 4-Aminophenol was translated into glucuronide and sulfate in similar concentrations for all angiostatin concentrations tested (Fig. 6). In addition, angiostatin did not affect the detoxifying capacity of primary hepatocytes (data not shown). Finally, angiostatin did not induce programmed cell death (apoptosis). In control mice the apoptotic index was 0.27 ± 0.01 compared to 0.28 ± 0.42 in angiostatin-treated mice (P = NS). In addition, hematoxylin and eosin staining did not reveal hepatic necrosis in the liver remnant, implicating that impaired LR in the angiostatin-treated group is not caused by necrosis). As presented in Table 1, the serum levels of representative liver enzymes, particularly ALT, reflected the liver tissue damage (loss) following PH. Treatment with angiostatin did not affect these values in hepatectomized and sham-operated mice.
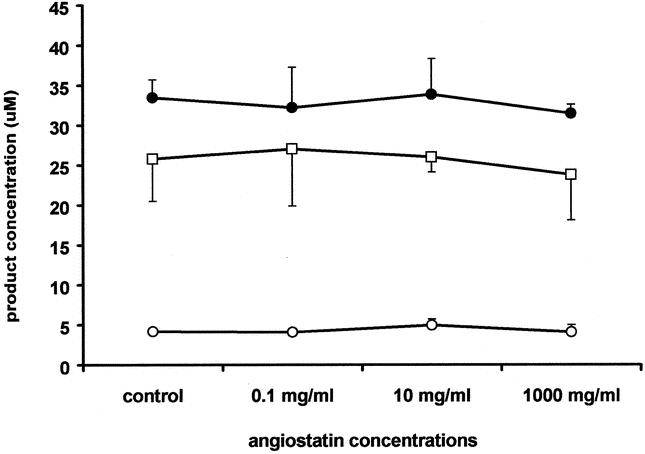
Figure 6. Effects of angiostatin on the synthetic capacity of cultured primary murine hepatocytes. The formation of the C-hydroxylated analine metabolite 4-aminophenol (μ) and its sulfate (ο) and glucuronide (λ) conjugates in primary cultures of mouse hepatocytes was used to investigate the possible effects of plasminogen-derived human angiostatin on these hepatocellular biotransformation processes. For all angiostatin concentrations tested, no significant differences were detectable between treated and control hepatocytes.
Table 1. EFFECTS OF ANGIOSTATIN ON SERUM LIVER BIOCHEMISTRY AT 2 DAYS AFTER PH
DISCUSSION
Our findings point to an important role of angiogenesis in physiologic LR following 70% PH. The observed development of hepatic angiogenesis, as represented by an increase in MVD during LR, corresponds with the general concept that formation of new blood vessels is required for adequate tissue repair. Mammalian cells need to be surrounded by a vascular network that is located no more than 100 to 200 μm away. 35 Within this distance, nutrition and oxygenation can be secured by diffusion. The increase in the number of cells seen during tissue repair results in expansion of tissue, leading to an increase in the distance between cells and the vascular network. To prevent the distance from surpassing the critical 100 to 200 μm, new blood vessels are recruited from preexisting vasculature. Similar to tumor angiogenesis, expansion of hepatic tissue is made possible not only because of paracrine stimulation by several (proangiogenic) growth factors, but also because of angiogenesis. According to Folkman, the lessons learned from tumor angiogenesis might be used to understand angiogenesis in many nonneoplastic and physiologic conditions. 3 From the data presented we conclude that LR is one of those physiologic conditions in which angiogenesis plays an important role.
Several groups have reported the upregulation of many proangiogenic factors in the remnant liver following PH. 17,36 Furthermore, Sato et al have demonstrated an increase in mitotic activities of sinus endothelial cells (SECs) after PH, which suggests that angiogenesis might occur during liver regeneration. 16 However, in our opinion the actual formation of new blood vessels, as judged by an increase in the microvascular density, has never been documented before.
Two other studies have investigated the effects of antiangiogenic agents on liver regeneration. However, neither included critical end points regarding LR and angiogenesis (i.e., wet liver weight and microvascular density). In accordance with our present findings, Taniguchi et al reported that neutralization of VEGF by anti-VEGF antibodies significantly inhibited the proliferative activity of hepatocytes at 48 and 96 hours after 70% PH. 15 In contrast, Tanaka et al reported that moderate antiangiogenic treatment with TNP-470 did not induce inhibition of liver regeneration as judged by BrdU incorporation in rabbits subjected to 45% PH. 37 However, in this study only two time points were studied and the course of liver weight was not documented. Therefore, we cannot exclude the possibility that a delay in LR was missed, particularly since the peak of BrdU lasts for only 3 days. 11 In addition, species-related differences (rabbits vs. mice) might also be responsible for the apparent differences, since the rapidity of LR is proportional to the amount of tissue resection. 38 A 45% PH, as used by Tanaka et al, is difficult to compare with the standard 70% used in our study.
Wack et al investigated the difference of porosity of SECs during the revascularization process in a regenerating liver. 39 They elegantly revealed that changes in the porosity of SECs play a critical role in the sieving function of the sinusoids and contribute to the progression of the regenerative process following PH. At 72 hours after PH they described the compression of sinusoids adjacent to avascular zones of islands of hepatocytes. We observed a similar sinusoidal compression in a regenerating liver both in the presence and absence of angiostatin. In addition, using scanning electron microscopy, they demonstrated the growth and migration of SECs into clusters of regenerating hepatocytes, suggesting neovascularization during LR. These observations provide further confirmation of LR-associated angiogenesis.
Previously, we reported the inhibitory effects of angiostatin on accelerated growth of hepatic metastases, subcutaneous tumor growth, corneal angiogenesis, and retinal angiogenesis. 24,26 However, the exact mechanisms responsible for the antiangiogenic action of angiostatin have not been fully resolved. Stack et al 40 suggested that tissue plasminogen activator (t-PA), when occupied by angiostatin, is prevented in a ternary complex formation between t-PA, plasminogen, and matrix protein. This results in the inhibition of plasminogen activation and a reduced endothelial cell migration and invasion. Since matrix breakdown by proteases such as plasmin is known to play an important role in LR, it is conceivable that the observed inhibitive effects of angiostatin on LR may be explained by interference in the fibrinolytic cascade. Alternatively, Moser et al attributed the antiangiogenic effects of angiostatin to binding to the α/β subunits of ATP synthase on the cell surface of endothelial cells, resulting in the downregulation of endothelial cell proliferation and migration. 41 Finally, Lucas et al proposed that the antiangiogenic activity of angiostatin may be ascribed to its apoptotic effect on endothelial cells. 42 In our opinion, this latter hypothesized mechanism does not appear to be important in regeneration-associated angiogenesis, since apoptosis was not observed in hepatocytes nor in SECs at any time point. In addition, we could not discern any direct adverse effects of angiostatin treatment on hepatocytes in terms of hepatocellular metabolism and detoxifying capacity.
In this study we used manual counting of intrahepatic blood vessels as a measure of angiogenesis in the liver. This is based on the pioneering work of Weidner et al 43 on MVD, currently the most widely used method to asses tumor-associated angiogenesis. Although manual counting of MVD suffers from inter- and intraobserver variation, computer image analysis of MVD is no more accurate than manual counting. 44 We found remarkably little variation in intrahepatic vascular counts between two independent observers. Sato et al 16 reported data on relative sinusoidal surface area as a vascular parameter in rat livers. We believe that such values should be treated with caution since their correlation with the number of blood vessels may be problematic. This is particularly true in conditions in which the surrounding hepatic tissue is subject to volume changes (i.e., hyperplasia during LR). An increase in volume and/or number of the hepatocytes may result in compression of blood vessels (see Fig. 2). Compression of blood vessels in combination with hyperplasia of hepatocytes, together with our observation of increased MVD, can be explained only by the formation of new blood vessels (i.e., angiogenesis). Determination of angiogenesis by measuring the surface area of the compressed vascular structures would be an underestimation compared to a counting method, which is independent of volume changes.
Our data show an important role of angiogenesis in physiologic conditions of tissue repair such as LR. Blocking this role might have important implications for prolonged therapy. Both in animals and humans surgical hepatic resection (e.g., for liver tumors or metastases) results in LR until the full original liver weight has been regained. A major problem is the recurrence of liver tumors due to accelerated tumor growth of micrometastases in the remnant liver, as previously described by our group. 24 Angiostatin was able to inhibit this accelerated tumor growth without completely blocking LR. Apart from the delay in LR, no clinical or biochemical side effects were noted in this study. 24 Regarding the potential clinical use of angiostatin in the perioperative setting in metastatic liver surgery, we could speculate that a brief postoperative discontinuation of angiostatin treatment could facilitate early LR. Such an intermission in antiangiogenic treatment could still be considered oncologically effective. 45
In conclusion, LR following 70% PH is associated with angiogenesis as judged by an increase in MVD. Inhibition of this phenomenon leads to a delay in LR, suggesting that LR is at least partial dependent on angiogenesis.
Acknowledgments
The authors thank Dr. Vecchi (Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy) for providing monoclonal antibodies against CD31 and Dr. van de Kant (Laboratory of Cell Biology, University Medical Center Utrecht, The Netherlands) for using laboratory facilities to perform and learning the BrdU pulse-labeling technique.
Discussion
Prof. P. Neuhaus: How specific is the biologic effect of angiostatin in mice; did you test the effects in other species as well? Is the inhibition of liver regeneration as demonstrated induced by a specific effect of angiostatin on hepatic angiogenesis or on hepatocyte proliferation?
Dr. T. Drixler: The inhibitory effects of angiostatin in mice have been reported by the group of Folkman and many others. The strong antiangiogenic effects of angiostatin in other species, such as rats and rabbits, have been reported as well, suggesting that its effect is species-independent. We have tested angiostatin in mice only. In several murine models, we have demonstrated that angiostatin inhibited corneal angiogenesis, retinal angiogenesis, and the outgrowth of colorectal hepatic metastasis in both resting and regenerating liver.
Possible negative effects of angiostatin on hepatocytes were excluded by subjecting in vitro isolated murine hepatocytes to angiostatin inoculation. Angiostatin did not affect both detoxifying and synthetic capacity of hepatocytes. In addition, angiostatin did not induce apoptosis in vivo either. These data are suggestive that angiostatin has no direct effect on hepatocytes. However, the number of blood vessels, as judged by microvascular density, was decreased significantly in angiostatin-treated mice. Therefore, we conclude that the inhibition of liver regeneration is induced by a specific effect on the microvasculature.
Prof. P. Neuhaus: Did you see a rebound phenomenon when you stopped your study? Did you see more angiogenesis?
Dr. T. Drixler: We have not evaluated a possible rebound phenomenon. Three weeks following partial hepatectomy the experiment was finished by terminating the mice.
Prof. P.-A. Clavien: This is a very nice paper. I think liver regeneration is currently becoming one of the most important topics in the field of liver transplantation and surgery.
Evidence that angiogenesis is involved in regeneration has been provided earlier. For example, it has been shown in models of major hepatectomy in rats that VEGF expression (Shimizu et al, reference 17 of the paper) and mitotic activity of the sinusoidal endothelial cells (Sato et al, reference 16 of the paper) increase rapidly after surgery. Reduced hepatocellular proliferation by anti-VEGF antibodies would also corroborate the importance of angiogenesis in liver regeneration. The novelty of your study lies in the question of whether hepatocyte proliferation depends on angiogenesis. In other words, you hypothesize that endothelial cell proliferation precedes and somewhat controls hepatocyte proliferation. To do so, you have used only one agent (angiostatin) to inhibit angiogenesis. My question is, how specific is angiostatin as an antiangiogenic agent? It is possible that angiostatin may block hepatocyte proliferation through mechanisms unrelated to angiogenesis? In a previous study from our group (Gastroenterology 1997: 113:1692–1700) suggesting that angiogenesis-like mechanisms trigger sinusoidal endothelial cell injury during cold preservation, we used three different inhibitors since none of the inhibitors alone would convincingly incriminate angiogenesis in the pathway of injury.
Dr. T. Drixler: We have used angiostatin because it is a major antiangiogenic agent. Indeed, inclusion of other antiangiogenic agents would support the concept that liver regeneration is an angiogenesis-dependent phenomenon. Actually, recent work by our group has revealed that, similar to angiostatin-treated mice, both liver regeneration and microvascular density were impaired in mice deficient for plasminogen. Since plasminogen plays an important role in the degradation of the extracellular matrix during angiogenesis, knocking out the plasminogen gene can be interpreted as an antiangiogenic strategy. These data support our concept.
Indeed, toxicity can be evaluated in a more simple fashion such as AST. Actually, we have evaluated liver enzymes such as AST, ALT, bilirubin, and LDH. Angiostatin treatment did not affect these enzymes in the hepatectomized and sham mice. The reason why we have not presented these data are that we believe that more specific assays exist (as presented here) to evaluate possible toxic effects. Finally, in H/E-stained liver slides we did not see apoptosis. Necrosis was absent as well, suggesting that liver regeneration was not impaired by angiostatin.
At this moment there is no consensus about the mechanism of angiostatin. So far, three possibilities have been put forward. One of these implies that angiostatin can form a complex with tissue plasminogen activator (t-PA). This complex binds to plasminogen, which in turn cannot be converted to the activated form (plasmin). This prevents the extracellular matrix from being broken down. That means that proliferating endothelial cells cannot migrate and invade, resulting in an impaired angiogenesis. From this point of view we evaluated whether knocking out the plasminogen gene would inhibit liver regeneration analogous to angiostatin-treated mice. Since this second antiangiogenic strategy produced similar results, we believe it strongly suggests that liver regeneration is an angiogenesis-dependent phenomenon.
Prof. C. E. Broelsch: I have a similar question regarding the mechanism of inhibition. Some of us have looked at liver regeneration for 20 years and we know that hepatocytes regenerate by hyperplasia. Now you are telling us the stories about the interrelationship of angiogenesis with regeneration, but we do not know what is the egg and what is the hen here. In your experiments you have not shown really absence of regeneration totally, but only a delay of 50%, but still there is regeneration there without angioproliferation.
I think the real clue is how to find what is the effect of angiogenesis on regeneration. Can you get better regeneration, for example, by initiating angiogenesis rather than inhibiting angiogenesis during delayed regeneration? Can we get data by reversing your experiment and ask the appropriate questions?
Dr. T. Drixler (closing): I think based on the literature, many growth factors have been shown to be involved in liver regeneration. We have not investigated the effects of stimulating liver regeneration in this study by proangiogenic factors, because we have been focusing on the effects of antiangiogenic treatment for cancer on physiologic angiogenesis.
Footnotes
Supported by the Wijnand M. Pon Foundation, the Netherlands Digestive Diseases Foundation (grant WS 96–29 to I.H.M.B.R.), and the Dutch Cancer Society (grant 99–2114 to E.E.V.).
Correspondence: Inne H. M. Borel Rinkes, MD, PhD, Department of Surgery (G04.228), University Medical Center Utrecht, PO Box 85500, 3508 GA, Utrecht, The Netherlands.
E-mail: i.h.m.borelrinkes@azu.nl
Accepted for publication April 2002.
References
- 1.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71. [PubMed] [Google Scholar]
- 2.Kerbel RS. Clinical trials of antiangiogenic drugs: opportunities, problems, and assessment of initial results. J Clin Oncol 2001; 19: 45S–51S. [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 4.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995; 1: 149–153. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann NY Acad Sci 1992; 667: 101–111. [DOI] [PubMed] [Google Scholar]
- 6.Polverini PH, Schmizu K, Solt DB. Genetic control of expression of the angiogenic phenotype. Northwest Dent Res 1989; 1: 4–7. [PubMed] [Google Scholar]
- 7.Dvorak HF. Tumors: wounds that do not heal. Similarity between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1658. [DOI] [PubMed] [Google Scholar]
- 8.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer 1984; 49: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J 1992; 6: 886–892. [PubMed] [Google Scholar]
- 10.Brown LF, Yeo KT, Berse B, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992; 176: 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997; 276: 60–66. [DOI] [PubMed] [Google Scholar]
- 12.Stolz DB, Mars WM, Petersen BE, et al. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res 1999; 59: 3954–3960. [PubMed] [Google Scholar]
- 13.Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995; 9: 1527–1536. [DOI] [PubMed] [Google Scholar]
- 14.Mars WM, Liu ML, Kitson RP, et al. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology 1995; 21: 1695–1701. [PubMed] [Google Scholar]
- 15.Taniguchi E, Sakisaka S, Matsuo K, et al. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 2001; 49: 121–130. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, El Assal ON, Ono T, et al. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol 2001; 34: 690–698. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol 2001; 34: 683–689. [DOI] [PubMed] [Google Scholar]
- 18.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 19.Mochida S, Ishikawa K, Toshima K, et al. The mechanisms of hepatic sinusoidal endothelial cell regeneration: a possible communication system associated with vascular endothelial growth factor in liver cells. J Gastroenterol Hepatol 1998; 13 (Suppl): S1–5. [PubMed] [Google Scholar]
- 20.Tomiya T, Tani M, Yamada S, et al. Serum hepatocyte growth factor levels in hepatectomized and nonhepatectomized surgical patients. Gastroenterology 1992; 103: 1621–1624. [DOI] [PubMed] [Google Scholar]
- 21.Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 1991; 13: 743–750. [PubMed] [Google Scholar]
- 22.Aoki M, Morishita R, Taniyama Y, et al. Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. J Atheroscler Thromb 2000; 7: 71–76. [DOI] [PubMed] [Google Scholar]
- 23.Aoki M, Morishita R, Taniyama Y, et al. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, etc. Gene Ther 2000; 7: 417–427. [DOI] [PubMed] [Google Scholar]
- 24.Drixler TA, Rinkes IH, Ritchie ED, et al. Continuous administration of angiostatin inhibits accelerated growth of colorectal liver metastases after partial hepatectomy. Cancer Res 2000; 60: 1761–1765. [PubMed] [Google Scholar]
- 25.O’Reilly MS, Holmgren L, Chen C, et al. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996; 2: 689–692. [DOI] [PubMed] [Google Scholar]
- 26.Drixler TA, Rinkes IH, Ritchie ED, et al. Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci 2001; 42: 3325–3330. [PubMed] [Google Scholar]
- 27.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–328. [DOI] [PubMed] [Google Scholar]
- 28.Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol 1931; 187–202. [Google Scholar]
- 29.Van de Kant HJG, Van Pelt AMM, Vergouwen RPFA, et al. A rapid immunogold-silver staining for detection of bromodeoxyuridine in large number of plastic sections, using microwave irradiation. Histochem J 1990; 22: 321–326. [DOI] [PubMed] [Google Scholar]
- 30.Vecchi A, Garlanda C, Lampugnani MG, et al. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol 1994; 63: 247–254. [PubMed] [Google Scholar]
- 31.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol 1976; 13: 29–83. [DOI] [PubMed] [Google Scholar]
- 32.Evelo CT, Versteegh JF, Blaauboer BJ. Kinetics of the formation and secretion of the aniline metabolite 4-aminophenol and its conjugates by isolated rat hepatocytes. Xenobiotica 1984; 14: 409–416. [DOI] [PubMed] [Google Scholar]
- 33.Boot JH, Van Holsteijn CW, Seinen W, et al. Effects of sulphydryl reagents on the formation of the aniline metabolite 4-aminophenol and its sulphate and glucuronide conjugates in primary cultures of rat hepatocytes. Xenobiotica 1989; 19: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257. [DOI] [PubMed] [Google Scholar]
- 36.Pediaditakis P, Lopez-Talavera JC, Petersen B, et al. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology 2001; 34: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka H, Taniguchi H, Mugitani T, et al. Angiogenesis inhibitor TNP-470 prevents implanted liver metastases after partial hepatectomy in an experimental model without impairing wound healing. Br J Surg 1996; 83: 1444–1447. [DOI] [PubMed] [Google Scholar]
- 38.Fausto N. Liver regeneration: from laboratory to clinic. Liver Transplant 2001; 7: 835–844. [DOI] [PubMed] [Google Scholar]
- 39.Wack KE, Ross MA, Zegarra V, et al. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology 2001; 33: 363–378. [DOI] [PubMed] [Google Scholar]
- 40.Stack MS, Gately S, Bafetti LM, et al. Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced plasminogen activation. Biochem J 1999; 340: 77–84. [PMC free article] [PubMed] [Google Scholar]
- 41.Moser TL, Stack MS, Asplin I, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA 1999; 96: 2811–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas R, Holmgren L, Garcia I, et al. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood 1998; 92: 4730–4741. [PubMed] [Google Scholar]
- 43.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 44.Fox SB, Leek RD, Weekes MP, et al. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol 1995; 177: 275–283. [DOI] [PubMed] [Google Scholar]
- 45.Boehm T, Folkman J, Browder T, et al. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–407. [DOI] [PubMed] [Google Scholar]