Abstract
Objective
To assess the outcome of laparoscopic Heller myotomy for achalasia using a specific quality of life (QoL) instrument for gastrointestinal disorders.
Summary Background Data
Current therapies for achalasia do not restore normal esophageal motility but aim at palliating dysphagia. However, many other symptoms may persist that cannot be assessed objectively by currently available symptom scores. Although generic QoL instruments have shown improvement in QoL after myotomy, disease-specific QoL instruments may be more responsive to change and therefore more reliable for comparing outcomes of therapeutic options for achalasia.
Methods
The Gastrointestinal Quality of Life Index (GIQLI) was studied before and after laparoscopic Heller myotomy associated with posterior partial fundoplication.
Results
Starting in January 1991, 73 consecutive patients were operated on laparoscopically for various clinical stages of achalasia. Since 1996, 40 patients completed a GIQLI questionnaire both preoperatively and after a minimum postoperative follow-up of 1 year. Median preoperative GIQLI score was 84 (range 34–129) out of a theoretical maximum score of 144. At a median follow-up of 31 months (range 12–54), the score had significantly improved to 119 (range 77–143), close to the range for the normal French population. Not only items assessing gastrointestinal symptoms but also the domains of physical, social, and emotional function were significantly improved. The most marked improvements were achieved in patients with the lowest preoperative scores.
Conclusions
The GIQLI allows us to objectify the impact of achalasia symptoms on health-related QoL. At medium-term follow-up, laparoscopic Heller myotomy, performed either as primary treatment or after endoscopic dilation, significantly improves most health-related QoL aspects. Short of randomized comparisons between the different therapeutic options available for achalasia, reported series could be made more comparable if validated QoL instruments specific for gastrointestinal disorders were used routinely for outcome evaluation.
Achalasia is a rare esophageal motor disorder with an estimated annual incidence of 1/100,000 in France. Characterized manometrically by aperistalsis or uncoordinated contractions of the esophageal body and impaired relaxation of a frequently hypertensive lower esophageal sphincter (LES), achalasia is secondary to irreversible degeneration of esophageal myenteric plexus neurons, the cause of which remains unknown. 1,2 As no currently available treatment can restore esophageal motility, all known therapeutic methods merely aim at palliation of dysphagia and other symptoms essentially related to esophageal stasis.
Short-term results seem to indicate that both myotomy and pneumatic dilation are equally efficient on dysphagia. According to the literature 1–4 and to the only available prospective randomized study comparing surgical myotomy with endoscopic dilation, 5 cardiomyotomy palliates dysphagia more efficiently than endoscopic dilation, especially in the long term. 6,7 The relatively high morbidity associated with myotomy performed via thoracotomy or laparotomy, 1,8,9 the reason why many patients and physicians still favor endoscopic dilation as the primary treatment option for achalasia, should be reduced with the advent of laparoscopic techniques.
In addition, there is a dire need to assess the outcome of surgical treatment using objective criteria such as weight loss and esophageal width changes and also in terms of symptoms such as regurgitation, heartburn, odynophagia, vomiting or coughing, sleep disorders, need for restrictive diets, slow eating, or maneuvers to improve food passage, all of which may affect the patient’s social functioning and emotional well-being before as well as after treatment. Such issues can be studied only by a validated tool for assessing quality of life (QoL).
While QoL studies after pneumatic dilation are lacking, recently three small surgical series 10–12 showed that laparoscopic Heller myotomy significantly improved QoL up to 2 years after operation using the well-validated generic QoL instrument Medical Outcome Study Short Form 36 item scale (SF-36). In contrast with the generic SF-36, the Gastrointestinal Quality of Life Index (GIQLI) has been designed to assess specifically the health-related QoL outcome in patients with gastrointestinal disorders and has been validated in English, German, Dutch, and French 13–16 for a variety of gastrointestinal conditions. 15–18 The aim of the present study was to evaluate health-related QoL after laparoscopic Heller myotomy and partial posterior fundoplication for achalasia using the GIQLI.
METHODS
Following the two very first patients operated on by the senior author (B.M.) in 1991 in another surgical center in Paris, 71 consecutive patients (38 women, 33 men) with a median age of 54 years (range 15–86) underwent a laparoscopic Heller myotomy for achalasia at the Department of Visceral Surgery A of the University Hospital of Montpellier between December 1993 and February 2001.
Preoperative Symptoms and Signs
The median preoperative duration of symptoms was 35 months (range 4–180). The median preoperative weight loss was 6 kg (range 0–30); 50 patients (70%) had lost 1 to 30 kg. The median preoperative body mass index (BMI) was 22.1 (range 17.7–35.9); four patients had a BMI of more than 30. All patients complained of dysphagia: three were aphagic, requiring total parenteral nutrition preoperatively. Some degree of regurgitation was present in 57 patients (80%), and 20 patients (28%) complained of heartburn; 9 (13%) took antacids on a regular basis. Thirty-two patients complained of pain (chest pain in 26 patients, upper abdominal pain in 6). Respiratory symptoms or complications were noted in 28 patients (39%). Two patients had a history of documented aspiration pneumonia and two patients were treated for asthma. Fourteen patients had chronic nocturnal or positional cough, while four patients complained of occasional suffocation. The remaining six patients had cervical bolus sensation or chronic sore throat.
All but five patients completed preoperative esophageal manometry. Reasons for absence of manometry included one patient who underwent repeat laparoscopic myotomy, originally performed in another institution; two patients who had very tortuous grade 4 disease on esophagograms; and two patients who vomited repeatedly during the investigation. Aperistalsis of the esophageal body (0% propagated contractions) was present in 57 patients, including 3 with vigorous achalasia (hypertonic nonpropagated contractions). Nine further patients had less than 25% of propagated contractions. Whereas LES recordings were not possible because the probe did not pass the LES in nine patients, the median LES resting pressure for the remaining 57 patients was 35 cm H2O (range 1–80). Thirteen patients had complete absence of LES relaxation, 38 patients had LES relaxation of less than 50% of their baseline value, and 6 patients had complete LES relaxation (all 6 had esophageal body aperistalsis and LES resting pressure >40 cm H2O). All 71 patients underwent a preoperative upper gastrointestinal endoscopy, and a CT scan was performed in 12 patients to rule out secondary achalasia. A radiologic barium esophagogram was performed in 69 patients. Fourteen patients (20%) had normal or mildly dilated (<3 cm) esophagus (grade 1), 26 patients (38%) had moderate (3–6 cm) esophageal dilatation (grade 2), 22 patients (32%) had grade 3 dilatation (>6 cm), and 7 patients (10%) had a grade 4 sigmoid-shaped esophagus. A hiatal hernia was present in three patients (one patient with grade 2, two with grade 3 esophageal dilatation), including one patient with associated histologically proven Barrett’s esophagus.
Medical treatment was attempted in 23 patients (32%) before operation, including calcium channel blockers in 13 patients, isosorbide dinitrate in 4 patients, and prokinetic or anxiolytic drugs in the remaining 6, without any substantial symptomatic improvement. Eighteen patients (25%) had undergone a median of two (range 1–8) sessions of endoscopic dilation (14 pneumatic and 4 bougie dilations). None of the patients had been treated with Botulinum toxin injections. One 19-year-old female had already undergone a laparoscopic Heller myotomy and anterior Dor fundoplication elsewhere. Dysphagia recurred rapidly and an esophagogram suggested that the myotomy was insufficiently long.
Operative Technique
Patient and surgeon position and operative technique were similar to what has been described elsewhere. 19 All patients were operated on by or under the supervision of the senior author (B.M.). The anterior aspect of the esophagus was freed as high as possible into the mediastinum without detaching its posterior and lateral aspects. Myotomy was performed with plain laparoscopic scissors starting just above the gastroesophageal junction and progressing as high as possible into the mediastinum (minimum 6 cm), then downward across the gastroesophageal junction for at least 1 cm. Intraoperative endoscopic control was not performed except for a few patients in the early part of this series.
Considering that both myotomy of the LES and aperistalsis of the esophagus are factors for postoperative gastroesophageal reflux, a partial posterior fundoplication was routinely added except in patients in whom perforation of the esophageal mucosa occurred during myotomy, in which case an anterior fundoplication was used to cover the myotomy wound and the repaired mucosa.
The partial posterior fundoplication was performed without dividing the short gastric vessels. The gastric wrap was anchored on either side of the myotomy, providing moderate, lateral traction on the myotomy edges, potentially preventing late recurrence of dysphagia from fibrosis or scar tissue formation between the myotomy edges. At completion, mucosal integrity was tested by injecting air into the esophagus with the myotomy submerged in saline. Peritoneal cavity drainage was optional and the nasogastric tube was removed in the recovery room. A postoperative esophagogram was performed for the first 20 patients on postoperative day 1 but was subsequently omitted. A soft diet was started on the first postoperative day and maintained for 3 weeks.
Quality of Life Assessment
Health-related QoL was assessed prospectively with the GIQLI, 13 beginning in 1996 preoperatively and systematically after a minimum follow-up of 1 year postoperatively. The GIQLI explores the patient’s self-evaluation of the 2-week period before the questionnaire is filled out. It includes 36 items covering four domains: gastrointestinal symptoms (19 questions), physical function (7 questions), social function (4 questions), emotional function (5 questions), and one item about subjective treatment assessment. Every item is scored from 0 (least desirable option) to 4 (most desirable option). Summing the points, the GIQLI score theoretically ranges from 0 to 144. In the original report, a healthy control population scored 125.8 points (95% confidence interval: 121.5–127.5). 13,14 The French version of the questionnaire found a similar result of 126 points (95% confidence interval: 122–130) for a healthy control population. 16
Concerning the most typical symptoms of achalasia, item 29 of the questionnaire explicitly explores dysphagia (“How often during the past 2 weeks have you had trouble swallowing your food?”). Item 27 explores regurgitation (“How often during the past 2 weeks have you had trouble with fluid or food coming up into your mouth?”), item 35 explores heartburn (“How often during the past 2 weeks have you had trouble with heartburn?”), and item 1 explores abdominal pain (“How often during the past 2 weeks have you had pain in the abdomen?”). Other items explore issues directly related to achalasia such as the speed of eating (item 28) or frequent burping and belching (item 5).
Patients were free to add comments about the questionnaire, and we added a question about chest pain and the patient’s weight.
Statistical Analysis
The results for all items were expressed as means ± SD of the mean or median (range), as appropriate. The paired Student t test was used to compare each item before surgery and at follow-up. A two-tailed t test was used for comparison of total scores and scores in each subgroup of items, before operation and at follow-up. Values of P < .05 were considered significant. Preoperative GIQLI scores and their percentage of improvement at follow-up were assessed for possible correlation by measuring Pearson’s (r) coefficient of correlation, which was evaluated for significance within the 95% confidence interval.
RESULTS
The median length of myotomy was 10 cm (range 7–13). A partial posterior fundoplication was performed in 67 of 71 (94%) patients. There were no conversions to laparotomy. An anterior partial fundoplication was preferred to cover the suture line of a mucosal perforation in three patients. The patient who underwent a redo procedure had her anterior fundoplication dismantled and converted to a posterior partial fundoplication. In one 83-year-old patient, it was decided preoperatively not to perform any antireflux procedure to minimize the operative time. In three patients a hiatal repair was associated with myotomy, and one patient had an associated cholecystectomy. Median operative time was 120 minutes (range 85–240) and median postoperative hospital stay was 3 days (range 2–10).
Intraoperative complications occurred in five patients (7%). Three esophageal mucosa perforations and one gastric fundus perforation were sutured laparoscopically with an uneventful postoperative course. One splenic capsule tear was treated conservatively. One patient with postoperative intraabdominal bleeding did not require reoperation, but this was the only patient of the series to receive a blood transfusion. Other postoperative complications included left lower lobe pneumonia (inhalation at anesthesia induction), superficial wound hematoma, urine retention, and paranoid delirium in one case each. One patient required endoscopic removal of a gastric phytobezoar 20 months after myotomy. There were no postoperative deaths: one patient aged 86 years at the time of operation died 15 months postoperatively of chronic heart failure.
Follow-up was completed in 70 of 71 patients (99%), with one patient lost because of emigration. At a median overall follow-up of 31 months (range 12–80), dysphagia was efficiently palliated in 58 patients (83%): 31 patients had no residual episodes of dysphagia, 18 patients had only rare episodes of dysphagia, and 9 patients had occasional dysphagia during the 2 weeks preceding evaluation. Twelve patients (17%) were improved but still had some degree of dysphagia during most or all of their meals. Median weight gain was 3 kg (range −24 to +23 kg). Weight increased in 50 patients (71%), was unchanged in 9 patients (13%), and decreased in 11 patients (16%). Ten patients had lost 1 to 5 kg at follow-up, while one patient without any residual dysphagia or reflux had voluntarily lost 24 kg through dieting. Significant regurgitation was reported in nine patients (13%) (“frequently” in four patients and “most of the time” in five patients). Significant heartburn was reported in eight patients (11%) (“most of the time” in seven and “always” in one patient who was pregnant when she initially filled out the questionnaire [despite normal pH-metry recordings, she still complains of the same symptoms 5 years after operation]), but only five patients took antacids compared with nine patients preoperatively. Chest pain more than once per week was reported in five patients (7%), all of whom had had daily chest pain preoperatively. “Occasional” chest pain was noted in 10 patients (14%). Some degree of upper abdominal pain was noted in 13 patients (19%). Pulmonary symptoms had completely disappeared at follow-up in all 28 patients who had preoperative respiratory symptoms.
Quality of Life Assessment
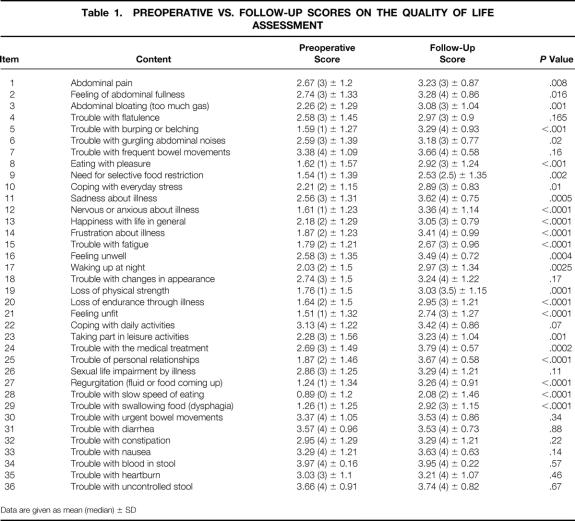
Since 1996, gastrointestinal QoL was prospectively assessed in 40 patients who completed both pre- and postoperative questionnaires with a minimum of 1 year of follow-up. Median follow-up for this subgroup was 30 months (range 12–54). Two questionnaires were excluded because of three or more unanswered questions, leaving 38 patients with usable pre- and postoperative QoL assessments for evaluation. Their median GIQLI at follow-up was 119 points (range 77–143) and was significantly higher than the preoperative score of 84 (range 34–129) (P < .00001) (Fig. 1). Scores for each item before myotomy and at follow-up are compared in Table 1. Significantly higher subtotals were found at follow-up for each domain: symptoms (P = .00001), physical function (P = .00001), social function (P = .00003), emotional function (P = .00001) as well as for subjective treatment assessment (item 24;P = .006) (Table 2). Comparisons of each of the 38 patients’ preoperative GIQLI to its percentage of improvement at follow-up showed a strong negative correlation (r = −0.84, P < .01) (Fig. 2), indicating that QoL scores improved most in the patients with the lowest preoperative scores.
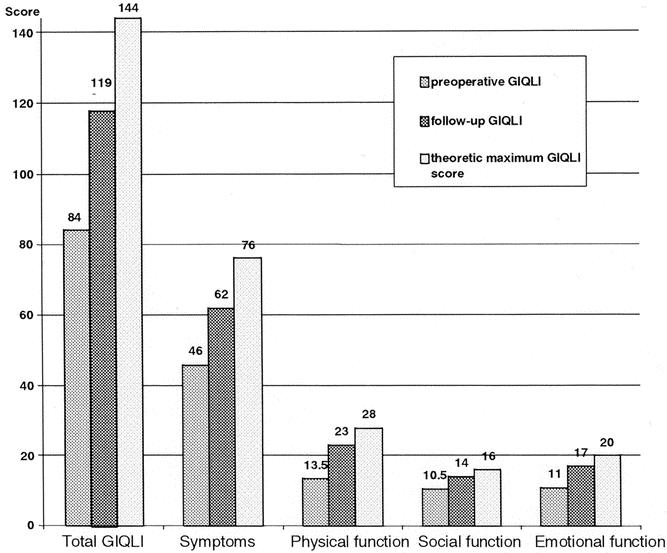
Figure 1. Preoperative Gastrointestinal Quality of Life Index (GIQLI) vs. follow-up GIQLI vs. theoretical maximum score.
Table 1. PREOPERATIVE VS. FOLLOW-UP SCORES ON THE QUALITY OF LIFE ASSESSMENT
Data are given as mean (median) ± SD
Table 2. TOTAL GASTROINTESTINAL QUALITY OF LIFE INDEX (GIQLI) AND DOMAIN SCORES
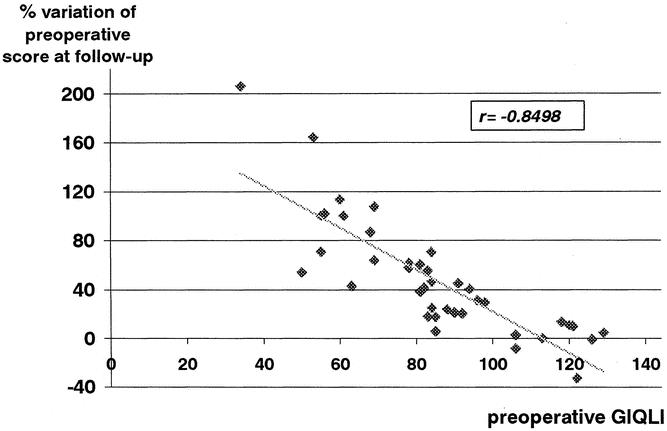
Figure 2. Correlation between the preoperative Gastrointestinal Quality of Life Index (GIQLI) and the percentage of improvement after a median follow-up of 31 months.
In seven patients with a “normal” preoperative GIQLI scores (i.e., within two standard deviations of the score for a “normal” German or French population 14,16: 116–134 points), follow-up scores increased by 11 points (9%) from a median of 122 (range 118–129) to 133 (range 82–135) (P = .68). The 33 patients with preoperative scores below this normal range improved their follow-up score by 33.5 (42%), from 79.5 (range 34–113) to 113 (range 77–143) (P < .0001).
Three patients (7%) had a negative variation of their follow-up GIQLI at, respectively, 53, 35, and 24 months (Fig. 3). All three patients had preoperative GIQLI scores within the “normal” range (respectively 122, 126, and 129). The first patient had a slightly decreased intermediate GIQLI at 14 months of follow-up (107) without residual dysphagia but increased heartburn and regurgitation compared with preoperative findings. At 53 months dysphagia recurred and an esophagogram suggested peptic stenosis. His GIQLI was 87 (−40), he was overweight (BMI 34), and he refused reintervention. The second patient had a follow-up GIQLI of 125 at 24 months (−1 point). He had occasional residual episodes of dysphagia, but his preoperative chronic cough had disappeared. The third patient had a stable intermediary GIQLI of 129 points at 23 months. She had only rare episodes of dysphagia and regurgitation but no heartburn. At 35 months of follow-up her GIQLI had fallen to 97 (−32 points). Symptom relief was maintained, but she was in mourning for the death of her husband, and GIQLI deterioration was confined mainly to the domains of emotions and physical function.
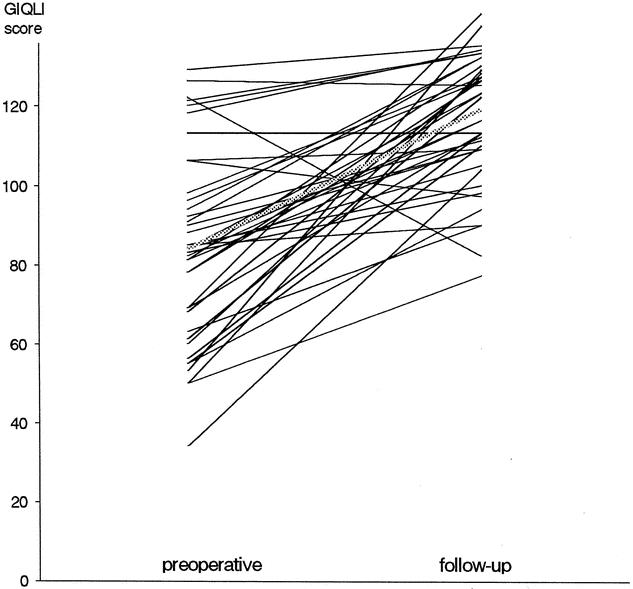
Figure 3. Preoperative vs. follow-up Gastrointestinal Quality of Life Index (GIQLI) scores.
Preoperative dilatation had no influence on GIQLI scores, as preoperative scores were similar for the 6 patients with preoperative dilatation compared to those 34 operated primarily (83 vs. 83.5;P = .83). Follow-up scores improved significantly in both groups to 118 and 116, respectively (P < .0001 and P = .013, respectively).
Repeat GIQLI assessments were available at different follow-up intervals for 16 of these 40 patients: their postoperative score decreased from 118 points at a median of 12 months (range 4–26) to 110 at a median of 40 months (range 13–54) (P = .03). Although this decrease was statistically significant (P = .039), the final score of 110 was still significantly higher than the preoperative score (P = .029).
In univariate analysis of the 40 prospectively assessed patients, preoperative QoL scores were not significantly different between patients with or without preoperative chest pain (79 vs. 84;P = .16), abdominal pain (83 vs. 84;P = .87), preoperative heartburn (78.5 vs. 85;P = .13), or pulmonary symptoms (83 vs. 83.5;P = .5). Neither were preoperative GIQLI scores different between those patients with or without preoperative weight loss (78.5 vs. 88.5;P = .16), preoperative endoscopic dilatation (n = 6; 82.5 vs. 83.5;P = .87), or stage IV esophageal dilation versus stages I—III (69 vs. 84;P = .21).
Preoperative endoscopic dilation (P = .54), stage IV esophagus (P = .51), preoperative chest pain (P = .20) or abdominal pain (P = .61), preoperative heartburn (P = .82), or pulmonary symptoms (P = .36) did not show any significant negative impact on follow-up GIQLI scores.
Follow-up GIQLI scores were significantly higher in the 28 patients with no or only rare episodes of persisting dysphagia compared to the 12 patients with at least occasional persisting episodes of dysphagia (124 vs. 109, P = .046). Follow-up scores were also significantly higher in the 32 patients without any chest pain at follow-up compared to the 8 patients with some degree of persisting chest pain (102.5 vs. 123;P = .035) and in those aged 50 or more at the time of operation (112 vs. 125.5;P = .047).
There were, however, no differences in follow-up GIQLI between patients with and without postoperative heartburn (111.5 vs. 116;P = .44), with and without persisting abdominal pain (110 vs. 122.5;P = .23), with and without weight gain (123 vs. 111, P = .19), or with and without perioperative perforation (117.5 vs. 116;P = .51).
Median follow-up GIQLI in patients with stage IV sigmoid esophagus was significantly higher compared to their preoperative scores (69 vs. 109;P = .01).
In addition to these 40 prospectively assessed patients, only follow-up QoL questionnaires were obtained in 19 further patients operated on before the routine introduction of the GIQLI. This group scored a median of 122 points (range 64–136) at 59 months (range 13–80) of follow-up.
DISCUSSION
No currently available treatment for achalasia, whether medical or surgical, can restore normal esophageal motility. In the short term, both progressive pneumatic dilatation and surgical myotomy can improve dysphagia in most patients. 1,2 With follow-up beyond 5 to 10 years, only 26% to 49% of patients after pneumatic dilatation 1,4,6–8 and 33% to 79% after surgical myotomy 4,6,7,20,21 still have complete relief of dysphagia. However, 33% to 38% of patients with recurrent dysphagia do not seek further medical help, 7 and therefore the best therapeutic method should be primarily applied in every patient. According to the literature 1,2 and the only available prospective randomized comparison of surgical myotomy with pneumatic dilation, 5,6 surgical myotomy is more efficient on dysphagia than pneumatic dilation at 5 years of follow-up. Nevertheless, the controversy hovering over the best treatment for achalasia is not about to be resolved, as both the endoscopic and surgical techniques have evolved since the only randomized study was performed. Starting from the early 1990s, Heller myotomy has been performed using a transthoracic (thoracoscopic) (VATS) or transabdominal (laparoscopic) approach. 22 The laparoscopic transabdominal approach soon became the technique of choice as it has the same short-term efficiency on dysphagia as open cardiomyotomy while reducing the pulmonary and parietal morbidity of laparotomy. 17,23–28 However, the long-term results of minimal-access Heller myotomy are still unknown. In most retrospective surgical and endoscopic series, outcome assessment has been based on a variety of symptom scores evaluating the main symptoms of achalasia (e.g., dysphagia, regurgitation, or heartburn). The different criteria used for these symptom scores render comparisons between retrospective series methodologically unreliable. 2 The severity and mix of symptoms may change as much from one patient to another as does the manometric pattern of esophageal motricity in achalasia. 29 The physical, psychological, and social consequences of these symptoms, and hence the QoL impairment they produce, should not be underestimated but cannot be reliably evaluated using classical symptom scoring systems.
To best assess therapy for achalasia, self-questionnaires of QoL may represent a more reliable outcome assessment measure than individual physician’s evaluation. To the best of our knowledge, only one study has ever assessed QoL after pneumatic dilation. 8 Fifty-two patients (41 pneumatic dilations and 11 open myotomies) were studied using a 12-item questionnaire that had not been formally validated. At follow-up, 69% of patients had some form of residual dysphagia, 59% had some degree of heartburn, and 56% required dietary restrictions. This study concluded that residual symptoms with a potential impact on the patient’s lifestyle such as restriction of sports activities were most noticeable in patients treated by open cardiomyotomy. Recently, three small surgical series have used a well-validated generic QoL tool (SF-36) to assess QoL after laparoscopic Heller myotomy. 10–12 Ben-Meir et al evaluated 19 patients 21 months after laparoscopic myotomy associated with posterior partial fundoplication. 10 The heartburn score was unchanged after operation, but the overall SF-36 score was significantly improved at follow-up. The domains of general health, emotional function, and mental health were unchanged, but physical function, bodily pain, vitality, and social function were significantly improved. Luketich et al studied 53 patients (2 myotomies without fundoplication, 40 partial posterior fundoplications, 8 anterior and 3 Belsey partial fundoplications), but there were no preoperative data with which postoperative data could be compared. 11 Eight patients underwent reoperation during follow-up and at 19 months; all eight domains of SF-36 data scored at least equal or better than a “normal” U.S. population used for comparison. Katilius et al studied 15 patients after laparoscopic and eight after open myotomy. 12 Three of the former were converted to laparotomy and one patient required reoperation. Nevertheless, the general health domain of SF-36 improved significantly, and patients with a successful laparoscopy had the best follow-up scores in the domains of physical function and bodily pain.
Compared to these three previous QoL studies, the present study population is larger and more homogeneous, as 95% of patients had the same operative technique and all operations were performed by the same surgeon or under his strict supervision. Moreover, follow-up was twice as long (median 31 months) and twice as many patients were evaluated both preoperatively and at follow-up (n = 40) than in previous studies. 10–12 In agreement with Rasanen et al, 30 a minimum interval of 12 months was used between surgery and QoL evaluation to exclude potential interference of immediate postsurgical sequelae on QoL. Furthermore, the QoL instrument used here is specific for gastrointestinal disorders. Higher responsiveness of specific rather than generic QoL instruments 31 should render the results of this study usable for comparison with studies of other treatment modalities for achalasia, provided that the same QoL instrument is used.
Overall conclusions of the present study are limited by the medium-term follow-up (31 months): 5- to 10-year results are needed. Our present results indicate, nevertheless, that health-related QoL is significantly improved by laparoscopic Heller myotomy with posterior partial fundoplication. The median follow-up GIQLI score of 119 is close to the 95% confidence intervals of the GIQLI for a healthy control population found by Eypasch et al 13 and Slim et al 16, 121.5 to 127.5 and 122 to 130, respectively. All domains of the GIQLI were improved: symptoms, physical function, and social and emotional function. Considering only the patients with data from both preoperative and follow-up questionnaires, significant improvement was maintained for up to 3 years of follow-up. QoL scores were most improved in the patients with the lowest preoperative scores. Conversely, when symptoms were mild and did not significantly alter preoperative QoL, there was only a small (9%) and statistically nonsignificant improvement of QoL afterward. In patients with high preoperative QoL scores, there is even a risk of deteriorated QoL when the operation creates side effects such as reflux. Should the GIQLI be used before therapeutic decisions, a QoL score within the “normal” range might argue in favor of primary pneumatic dilation.
Analysis of all 36 items (see Table 1) showed that not only items directly related to achalasia but also swallowing difficulties, the speed of eating, food selection, regurgitation, belching, abdominal pain, or heartburn are improved by laparoscopic Heller myotomy. This analysis also revealed how greatly achalasia affects general problems such as loss of physical strength, fatigue, frustration, and even personal relationships, and how these aspects are improved by the operation. Regarding achalasia, one major shortcoming of the GIQLI questionnaire is the absence of an item analyzing chest pain and esophageal spasms, which are frequent symptoms in achalasia patients. We therefore completed our follow-up evaluation with a specific question about chest pain. Eleven of the 40 prospectively assessed patients complained of chest pain preoperatively, with three having typical manometric features of vigorous achalasia. Eight patients (two with vigorous achalasia) still had occasional to frequent episodes of chest pain at follow-up, and these patients had significantly lower follow-up GIQLI scores than the 32 patients with complete relief of chest pain.
We observed three cases in which the follow-up GIQLI scores worsened after operation: in one case only, the negative score was clearly due to a poor functional outcome (reflux stenosis). The second patient had a nonsignificant 1-point drop but was still within the “normal” range (125 points at 24 months). The third patient had no residual symptoms of achalasia, and the GIQLI deterioration clearly was not due to her health. This indicates that even system- or disease-specific QoL instruments may be affected by non-health-dependent overall QoL aspects such as professional, marital, or financial problems. 32
Our results in patients with several follow-up questionnaires show that there is a slight drop in QoL scores between 1 and 3 years after surgery. While this might be the initial indication of functional decay observed in some long-term follow-up studies, it might also reflect the fact that shortly after a Heller myotomy, most patients become euphoric about their ability to eat relatively normally again and thus may overestimate their own overall health status. 32 If, however, one considers that the operation is palliative and does not restore normal esophageal function, it may well be that QoL scores at 6 months to 1 year are abnormally high, while the scores after 3 to 5 years reflect the “true” outcome once the initial euphoria has settled and patients become used to their improved rather than normalized condition. This is underlined by the fact that scores in patients with 5 years of follow-up seem to remain stable (122 points).
Our study included seven patients with grade 4 esophageal dilatation: none were excluded. Follow-up GIQLI scores were significantly improved in all, and none so far has required an esophagectomy. This confirms previous reports that massive dilatation and sigmoid esophagus are not contraindications to laparoscopic myotomy, and that routine resection is not always required in these patients to achieve a symptomatic improvement. 11,33
QoL instruments such as the GIQLI that are designed to study health-related QoL issues in specific diseases or systems seem to be helpful in assessing outcome after surgical therapy for rare functional disorders such as achalasia. The systematic use of such instruments might improve the reliability of comparisons between retrospective studies and should also serve as study endpoints in future prospective trials comparing laparoscopic myotomy to nonsurgical therapies such as pneumatic dilation.
Discussion
Prof. A. P. Peracchia: Dr. Millat (Montpellier) must be congratulated on his excellent presentation. The topic is not new, but the methodology of the work, based on a pre- and postoperative assessment of quality of life, is indeed original.
This presentation further highlights the view that laparoscopic surgery is nowadays the gold standard of therapy for achalasia. Many issues regarding surgical technique are still open to discussion. One of these is the type of fundoplication to be performed after myotomy, together with the length of myotomy on the gastric side, which is a factor that may have an impact on the postoperative reflux.
Dr. Millat reported a 12% incidence of severe heartburn and a 13% incidence of regurgitation after using Toupet fundoplication. In my experience, with about 200 laparoscopic procedures, the incidence of reflux detected by 24-hour pH monitoring is less than 10%. In fact, I believe that by leaving intact the posterior gastroesophageal junction, as you are able to do with the Dor procedure and not with Toupet, you may further protect from reflux. Can you comment on this aspect?
Prof. B. L. M. Millat: In our study, we were concerned about the patient’s self-evaluation regarding a symptom called “heartburn,” but this should and cannot be interpreted as always being directly related to reflux. pH-metry investigations were not performed routinely in our patients postoperatively, but several patients reporting “heartburn” actually had normal pH-metry recordings. As shown in our results, the proportion of patients with heartburn symptoms improved from 26% preoperatively to 12% postoperatively. It seems obvious that all patients with achalasia reporting “heartburn” preoperatively do not always have gastroesophageal reflux, and that changes reported in our study regarding this symptom might be related to myotomy itself.
You are right when you suggest that leaving the posterior attachments of the gastroesophageal junction intact by performing a Dor rather than a Toupet procedure might be a better approach to prevention of reflux after operation for achalasia. In my opinion, however, leaving intact the gastroesophageal junction does not tell the whole story, because these attachments are also untouched in patients undergoing dilatation or laparoscopic Heller myotomy only, without any antireflux procedure. When we look at the results in terms of reflux in these patients, we can see that up to 30% of patients report postprocedural reflux symptoms. Although not in achalasia but in the specific situation of reflux disease, at least one randomized comparison between the Toupet and Dor procedures has shown that the Toupet operation was a better antireflux procedure than the Dor wrap. Getting back to achalasia, however, the Toupet procedure is not exclusively an antireflux procedure in patients with esophageal aperistalsis and an inefficient lower esophageal sphincter: as the posterior fundoplication is sutured to both edges of the myotomy, it should help keep the edges open, potentially avoiding postoperative scarring and shrinking. I agree with your suggestion that there is still room for a randomized comparison between the Dor and the Toupet procedure. Such a trial would gain a great deal if this kind of quality of life tool were used to assess clinical outcome, obviously associated with routine preoperative and postoperative pH-metry.
Prof. T. M. E. Lerut: I very much enjoyed this elegant study, which I think is a very good study and relevant to this problem.
In my experience, despite the fact that the number of patients are free from dysphagia, some of them either continue or start to have problems with thoracic pain due to spasm-related problems, which is of course clearly handicapping them in their QoL. Now your questionnaire is in my mind the same as you are using for reflux, and it is a general questionnaire of QoL, which definitely does not include specific attention to the problem of thoracic pain, which can be very handicapping. So my question is, is this questionnaire the right instrument to use for this type of problem?
Prof. B. L. M. Millat: I think that the problem of evaluating the quality of life is in part the choice of an appropriate tool. When starting this evaluation in 1996 we had to choose a questionnaire which had been validated in the normal French population, and this was the best and most pragmatic choice at that time. With regard to the pertinence of this tool for achalasia and thoracic pain, I totally agree that the specific issue of thoracic pain is not addressed by the GIQLI score; this is why we added a specific question about chest pain. I agree also that it seems unrealistic to imagine that all patients will be completely asymptomatic postoperatively, because the Heller myotomy is only a palliative procedure, and patients will still have problems with the aperistalsis or nonpropagated contractions in the esophageal body. Postoperative thoracic pain is probably related to this motility disorder.
Prof. A. G. Johnson: I am interested in the group who had previous dilatation from one to eight times. What were the criteria that made you decide to operate, and did this group behave differently from the rest?
Second, it is important to realize that the normal so-called normal population has quite a lot of symptoms, and as you mentioned sometimes we are expected to make them perfect. Everybody has some social problems and it is therefore important to compare with a “normal” group, not a perfect or ideal group.
Prof. B. L. M. Millat: Professor Johnson, I agree that we cannot consider the maximum score as a reference. This theoretic maximal score, equal to 144, has no clinical significance: the “normal” score in the French population is 122.
Regarding our indications for surgery, you have seen that we had a high number of patients undergoing primary surgery: none of our patients had Botox injection preoperatively, and several patients had between one and eight dilations before operation. All of these indications were decided by the gastroenterologists, never by myself or my team. Surgery was decided mainly because of recurrence of dysphagia after dilatation or, in the young patients, as a primary procedure. In France there were at least two evaluations, one of them randomized, showing better results in the group with dilatation when compared to Botox injection and the second study showing worst results for dilatation in young patients.
Prof. A. G. Johnson: Did that group do worse in your study?
Prof. B. L. M. Millat (closing): No, preoperative dilation had no influence on GIQLI scores, as preoperative scores were similar for the six patients with preoperative dilation compared to 34 operated primarily (83 vs. 83.5, respectively). Both groups significantly improved their follow-up scores to 118 and 116, respectively.
Footnotes
Correspondence: Bertrand Millat, MD, PhD, Head of Department of Surgery A, University Hospital Center Montpellier, Hôpital Saint-Eloi, F-34295 Montpellier Cedex 5.
E-mail: b-millat@chu-montpellier.fr
Accepted for publication April 2002.
References
- 1.Vantrappen G, Hellemans J. Treatment of achalasia and related motor disorders. Gastroenterology 1980; 79: 144–154. [PubMed] [Google Scholar]
- 2.Spiess AE, Kahrilas PJ. Treating achalasia. From whalebone to laparoscope. JAMA 1998; 280: 638–643. [DOI] [PubMed] [Google Scholar]
- 3.Andreollo NA, Earlam RJ. Heller’s myotomy for achalasia: is an added anti-reflux procedure necessary? Br J Surg 1987; 74: 765–769. [DOI] [PubMed] [Google Scholar]
- 4.Okike N, Spencer Payne W, Neufeld DM, et al. Oesophagomyotomy versus forceful dilation for achalasia of the oesophagus: results in 899 patients. Ann Thorac Surg 1979; 28: 119–25. [DOI] [PubMed] [Google Scholar]
- 5.Csendes A, Velasco N, Braghetto I, et al. A prospective randomized study comparing forceful dilation and esophagomyotomy in patients with achalasia of the esophagus. Gastroenterology 1981; 80: 789–795. [PubMed] [Google Scholar]
- 6.Csendes A, Braghetto I, Henriques A, et al. Late results of a prospective randomised study comparing forceful dilation and oesophagomyotomy in patients with achalasia. Gut 1989; 30: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torbey CF, Achkar E, Rice TW, et al. Long-term outcome of achalasia treatment: the need for closer follow-up. J Clin Gastroenterol 1999; 28: 125–130. [DOI] [PubMed] [Google Scholar]
- 8.Meshkinpour H, Haghighat P, Meshkinpour A. Quality of life among patients treated for achalasia. Dig Dis Sci 1996; 41: 352–356. [DOI] [PubMed] [Google Scholar]
- 9.Abid S, Champion G, Richter JE, et al. Treatment of achalasia: the best of both worlds. Am J Gastroenterol 1994; 89: 979–985. [PubMed] [Google Scholar]
- 10.Ben-Meir A, Urbach DR, Khajanchee YS, et al. Quality of life before and after laparoscopic Heller myotomy for achalasia. Am J Surg 2001; 181: 471–474. [DOI] [PubMed] [Google Scholar]
- 11.Luketich JD, Fernando HC, Christie NA, et al. Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg 2001; 72: 1909–1913. [DOI] [PubMed] [Google Scholar]
- 12.Katilius M, Velanovich V. Heller myotomy for achalasia: quality of life comparison of laparoscopic and open approaches. JSLS 2001; 5: 227–231. [PMC free article] [PubMed] [Google Scholar]
- 13.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995; 82: 216–222. [DOI] [PubMed] [Google Scholar]
- 14.Eypasch E, Wood-Dauphinée S, Williams JI, et al. Der Gastrointestinale Lebensqualitätsindex (GLQI). Ein klimetrischer Index zur Befindlichkeitsmessung in der gastroenterologischen Chirurgie. Chirurgie 1993; 64: 264–274. [PubMed] [Google Scholar]
- 15.Nieveen Van Dijkum EJ, Terwee CB, Oosterveld P, et al. Validation of the gastrointestinal quality of life index for patients with potentially operable periampullary carcinoma. Br J Surg 2000; 87: 110–115. [DOI] [PubMed] [Google Scholar]
- 16.Slim K, Bousquet J, Kwiatkowski F, et al. Première validation de la version française de l’index de qualité de vie pour les maladies digestives (GIQLI). Gastroenterol Clin Biol 1999; 23: 25–31. [PubMed] [Google Scholar]
- 17.Sailer M, Bussen D, Debus ES, et al. Quality of life in patients with benign anorectal disorders. Br J Surg 1998; 85: 1716–1719. [DOI] [PubMed] [Google Scholar]
- 18.Bülent Mentes B, Akin M, Irkörücü; O, et al. Gastrointestinal quality of life in patients with symptomatic or asymptomatic cholelithiasis before and after laparoscopic cholecystectomy. Surg Endosc 2001; 15: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 19.Hunter JG, Trus TL, Branum GD, et al. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 1997; 225: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonavina L, Nosadini A, Bardini R, et al. Primary treatment of esophageal achalasia. Long-term results of myotomy and Dor fundoplication. Arch Surg 1992; 127: 222–227. [DOI] [PubMed] [Google Scholar]
- 21.Ellis FH. Oesophagomyotomy for achalasia: a 22-year experience. Br J Surg 1993; 80: 882–885. [DOI] [PubMed] [Google Scholar]
- 22.Shimi S, Nathanson LK, Cuschieri A. Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edimb 1991; 36: 152–154. [PubMed] [Google Scholar]
- 23.Ancona E, Anselmino M, Zaninotto G, et al. Esophageal achalasia: Laparoscopic versus conventional open Heller-Dor operation. Am J Surg 1995; 170: 265–270. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey DT, Kalan MH, Gerson RS, et al. Comparison of outcomes following open and laparoscopic esophagomyotomy for achalasia. Surg Endosc 1999; 13: 747–750. [DOI] [PubMed] [Google Scholar]
- 25.Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia. An 8-year experience with 168 patients. Ann Surg 1999; 230: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart KC, Finley RJ, Clifton JC, et al. Thoracoscopic versus laparoscopic modified Heller myotomy for achalasia: efficacy and safety in 87 patients. J Am Coll Surg 1999; 189: 164–169. [DOI] [PubMed] [Google Scholar]
- 27.Beckingham IJ, Callanan M, Louw JA, et al. Laparoscopic cardiomyotomy for achalasia after failed balloon dilation. Surg Endosc 1999; 13: 493–496. [DOI] [PubMed] [Google Scholar]
- 28.Diener U, Patti MG, Molena D, et al. Laparoscopic Heller myotomy relieves dysphagia in patients with achalasia and low LES pressure following pneumatic dilation. Surg Endosc 2001; 15: 687–690. [DOI] [PubMed] [Google Scholar]
- 29.Hirano I, Tatum RP, Shi G, et al. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology 2001; 120: 789–798. [DOI] [PubMed] [Google Scholar]
- 30.Rasanen JV, Niskanen MM, Miettinen P, et al. Health-related quality of life before and after gastrointestinal surgery. Eur J Surg 2001; 167: 419–425. [DOI] [PubMed] [Google Scholar]
- 31.Velanovich V. Using quality-of-life instruments to assess surgical outcomes. Surgery 1999; 126: 1–4. [DOI] [PubMed] [Google Scholar]
- 32.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA 1998; 272: 619–626. [PubMed] [Google Scholar]
- 33.Patti MG, Feo CV, Diener U, et al. Laparoscopic Heller myotomy relieves dysphagia in achalasia when the esophagus is dilated. Surg Endosc 1999; 13: 843–847. [DOI] [PubMed] [Google Scholar]