Abstract
Objective
The primary endpoint was to compare the impact of laparoscopic and open colorectal surgery on 30-day postoperative morbidity. Lymphocyte proliferation to mitogens and gut oxygen tension were surrogate endpoints.
Summary Background Data
Evidence-based proof of the effect of laparoscopic colorectal surgery on immunometabolic response and clinically relevant outcome variables is scanty. Further randomized trials are desirable before proposing laparoscopy as a superior technique.
Methods
Two hundred sixty-nine patients with colorectal disease were randomly assigned to laparoscopic (n = 136) or open (n = 133) colorectal resection. Four trained members of the surgical staff who were not involved in the study registered postoperative complications. Lymphocyte proliferation to Candida albicans and phytohemagglutinin was evaluated before and 3 and 15 days after surgery. Operative gut oxygen tension was monitored continuously by a polarographic microprobe.
Results
In the laparoscopic group the conversion rate was 5.1%. The overall morbidity rate was 20.6% in the laparoscopic group and 38.3% in the open group. Postoperative infections occurred in 15 of the 136 patients in the laparoscopic group and 31 of the 133 patients in the open group. The mean length of hospital stay was 10.4 ± 2.9 days in the laparoscopic group and 12.5 ± 4.1 days in the open group. On postoperative day 3, lymphocyte proliferation was impaired in both groups. Fifteen days after surgery, the proliferation index returned to baseline values only in the laparoscopic group. Intraoperative gut oxygen tension was higher in the laparoscopic than in the open group.
Conclusions
Laparoscopic colorectal surgery resulted in a significant reduction of 30-day postoperative morbidity. Lymphocyte proliferation and gut oxygen tension were better preserved in the laparoscopic group than in the open group.
After an adequate training period, laparoscopic colorectal resection may be performed with reasonable safety standards, 1,2 which are necessary but not sufficient for preferring laparoscopic to open surgery. In fact, besides the well-proven advantages of less postoperative pain, better cosmesis, and quicker patient mobilization, several other important issues remain debated. 3–8 In a recent review, Gupta and Watson 9 reported that the systemic immunometabolic response was better preserved after laparoscopic than open surgery, while laparoscopic surgery appeared to be associated with a greater suppression of intraperitoneal cell-mediated immunity, which may arouse caution in the management of cancer and sepsis by this technique.
Evidence-based advantages of laparoscopic surgery on clinically relevant outcome variables, such as postoperative morbidity, length of stay, cancer recurrence, and long-term survival, are even more scanty. Chapman et al 10 reviewed 52 clinical trials on laparoscopic colorectal resection: only 5 were properly randomized and in 2 of them intention-to-treat analysis was violated. All the remaining trials were case-control, cohort, historical control, or series studies. Thus, further randomized trials with adequate power and intent-to-treat data analysis are recommended before proposing laparoscopic colorectal surgery as a superior technique.
The primary endpoint of this randomized trial was to compare the impact of laparoscopic and open colorectal surgery on 30-day postoperative morbidity. The surrogate endpoint was to investigate the effect of laparoscopic and open surgery on lymphocyte proliferation of both common environmental antigen and polyclonal mitogen, and on gut oxygen tension.
METHODS
From February 2000 to November 2001, adult patients admitted to our department for colorectal disease were assessed for study eligibility. Inclusion criteria were age at least 18 years and suitability for elective surgery. Exclusion criteria were cancer infiltrating adjacent organs assessed by computed tomography or magnetic resonance imaging, cardiovascular dysfunction (New York Heart Association class > 3), respiratory dysfunction (arterial Po2 < 70 mmHg), hepatic dysfunction (Child-Pugh class C), ongoing infection, and plasma neutrophil level less than 2.0 × 109/L. The protocol was approved by the Ethical Committee of the San Raffaele Hospital. The potential participants had the study design explained and then were required to sign a written informed consent before randomization.
Eligible patients were randomly allocated to laparoscopic or open colorectal surgery. To obtain a balanced distribution of different surgical procedures in the two groups, five randomization lists according to the site of the lesion were generated by a computer program, assuming that all patients had the same probability of undergoing laparoscopic or open surgery. Randomization by individual random numbers was performed in blocks of 20 patients. Assignments were made by means of sealed sequenced masked envelopes that were opened, before the induction of anesthesia, by a nurse unaware of the trial design.
On hospital admission, demographics, nutritional status, and primary diagnosis were recorded in all patients. Undernutrition was defined as weight loss of more than 10% with respect to usual body weight in the 6-month period before admission. Obesity was defined as body mass index (weight in kilograms/height in meters2) more than 30. The presence of comorbidity factors was assessed according to the American Society of Anesthesiologists (ASA) score.
In all patients bowel preparation was carried out the day before operation by intestinal washout with an iso-osmotic solution (3 L). The evening before and the morning of the operation patients were given an enema. As antibiotic prophylaxis all patients received a single dose of cefotetan (2 g intravenously) during the induction of anesthesia. A second dose of the same antibiotic was administered intraoperatively if surgery lasted more than 4 hours. Deep vein thrombosis prophylaxis was carried out with low-molecular-weight heparin (50 IU/kg/d) in all patients. All patients underwent general anesthesia plus thoracic epidural anesthesia. Before induction, an epidural catheter was placed at T11–T12 level. General anesthesia was induced with fentanyl (1 μg/kg), thiopental (5 mg/kg), and atracurium (0.5 mg/kg). Anesthesia was maintained with air and oxygen (Fio2 0.5%), isoflurane (0.4–0.8%), and atracurium (0.5 mg/kg/h). Intraoperatively, ropivacaine 0.75% (1 mg/kg) was administered via the epidural catheter. To maintain normothermia, active intraoperative warming (forced air warming at 38°C on the thorax and upper limbs plus on-line heating) was instituted.
All the operations were performed by the same surgical team (M. B., A. V., W. Z.), which was well trained in both laparoscopic and open colorectal surgery. 3,11,12 The learning curve for laparoscopic colorectal surgery was completed before starting the trial. 12 Pneumoperitoneum was induced by insufflation of CO2 and was maintained at 10 to 12 mmHg during the entire surgical procedure. Laparoscopic operations were performed according to the technique described by Milsom and Bohm. 13 In all patients the identification and division of the lymphovascular pedicle and the mobilization of colonic segments were carried out by the harmonic scalpel (Ethicon Endosurgery Inc., Cincinnati, OH). The specimen was always retrieved in an impermeable bag to prevent tumor spillage and/or wound contamination. A Knight-Griffen anastomosis was fashioned in all patients who underwent resection of the rectum or sigmoid or left colon. The anastomosis was performed intracorporeally in the laparoscopic group. An extracorporeal hand-sewn anastomosis was fashioned in all patients who underwent right hemicolectomy or transverse resection. Conversion to open surgery was defined as the need to perform an abdominal incision longer than 7 cm.
The following details of the surgical procedure were recorded in all patients: duration of operation, operative blood loss, amount of homologous blood transfused, and number of lymph nodes collected in cancer patients. The volume of operative blood loss was calculated by adding the blood aspirated to the weight of the gauzes used during surgery. Transfusion of blood products in the perioperative period was based on the hemoglobin level (<80 g/L) or on an individual basis according to the clinical condition.
All patients were treated on a strictly controlled protocol with regard to analgesic administration, feeding, and postoperative care. Postoperative analgesia was ensured by continuous epidural infusion of 0.2% ropivacaine (4–6 mL/h) and by intravenous morphine chloridrate (patient-controlled administration) at a maximum of 4 mg/h with a single dose of 1 mg and free interval of 10 minutes. Postoperative recovery of bowel function was evaluated by first flatus and bowel movement. No patient was allowed to resume oral feeding before the first flatus occurred.
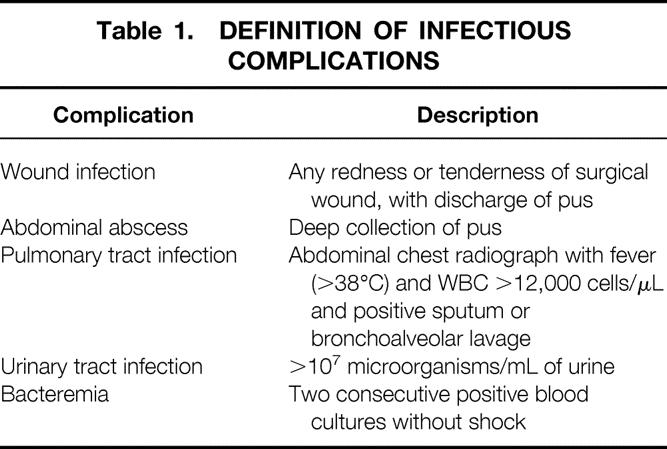
Four trained members of the surgical staff who were not involved in the study registered postoperative complications according to a priori definition. 14 They also decided the first day of solid food recovery and the day of hospital discharge. Details of infectious complications are given in Table 1. Microbiologic analysis and positive culture were used to diagnose infectious complications. Any anastomotic dehiscence with clinical and/or radiologic evidence has been considered. Patients were discharged after meeting the following conditions: bowel movement and full recovery of both ambulation and oral food intake. Follow-up for infectious and noninfectious complications was carried out for 30 days after hospital discharge by weekly office visits. Recovery of social and physical activity was evaluated every 30 days after surgery by a specific adaptation of the SF-36 health survey questionnaire 15 filled out by the patient. The main items are working ability, long-distance walking ability, climbing stairs, lifting heavy objects, and social activities with family, friends, neighbors, or groups.
Table 1. DEFINITION OF INFECTIOUS COMPLICATIONS
The proliferative ability of the peripheral blood-derived T cells in response to both antigen-specific and polyclonal mitogenic stimulation was evaluated in the first 10 cancer patients per group. Peripheral blood mononuclear cells (PBMCs) were drawn from patients the day before and 3 and 15 days after surgery and directly compared for proliferative responses in vitro. PBMCs were purified from whole blood by a Ficoll density gradient (Ficoll Hypaque, Pharmacia, Uppsala, Sweden). A fixed number of PBMCs (105) was then activated in triplicate wells of 96-well U-bottom plates with different proliferative stimuli:Candida albicans at 10 spores per T cell 16 and phytohemagglutinin-P (PHA, Roche, Nutley, NJ) at 2 μg/mL. After 3 days of culture at 37°C for PHA or 5 days for C. albicans, 1 μCi 3H-thymidine was added to each well and incubation was performed at 37°C for an additional 16 hours. Proliferation was determined by measuring 3H-thymidine incorporation using a liquid scintillation counter.
In the first 10 patients per group, intestinal tissue oxygen tension was assessed by a catheter oxygen polarographic microprobe (Type CC 1.2 Licox, GMC, Kiel-Mielendorf, Germany), which was placed into the thickness of the gut wall at the beginning of surgery, before any manipulation of the gut. 17 Intraoperative oxygen tension was monitored continuously. Values were recorded at four time points: beginning of surgery, after division of the vascular pedicle, before performing the anastomosis, and at the end of the operation.
The sample size of the trial was determined assuming a postoperative complication rate of 40% in the open surgery group. 3,18 A reduction to 20% would be considered to indicate the efficacy of treatment. Admitting a type I error level of 0.05 and a power of 0.90, at least 120 patients in each group were required. All patients were analyzed on an intention-to-treat basis. Descriptive data are reported as mean ± standard deviation, 95% confidence interval (CI), median and range, or number of patients and percentage. Comparison between groups for discrete variables was made by the chi-square test or the Fisher exact test when appropriate. The Student t test and analysis of variance for repeated measures were used to compare normally distributed variables. In other situations, nonparametric analysis was performed: the Mann-Whitney test was used to test differences between groups, whereas the Wilcoxon signed ranks test and the Friedman test were used for comparisons between and among paired data. Post-hoc multiple comparisons were adjusted by using the Bonferroni correction. P < .05 was considered to indicate statistical significance (two-tailed test). The SPSS package version 8.0 for Windows (SPSS Inc., Chicago, IL) was used for the statistical analysis.
RESULTS
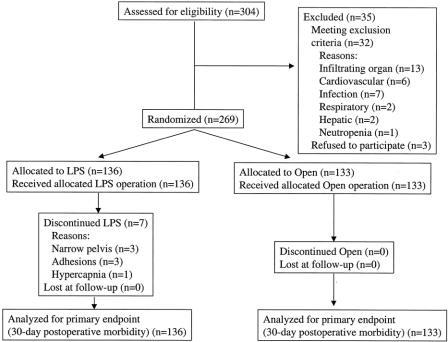
Figure 1 shows the diagram of the trial according to the CONSORT statement. 19 Of the 269 patients randomized, 136 were assigned to the laparoscopic group and 133 to the open group. All patients were analyzed on a intention-to-treat basis.
Figure 1. Study design according to the CONSORT statement. LPS, laparoscopy.
The two groups were well balanced for age, gender, ASA score, preoperative hemoglobin, and rate of undernutrition/obesity (minimum P = .24). There were 90 cancer patients (66.2%) in the laparoscopic group and 93 (69.9%) in the open group (P = .59). No difference between groups was found with respect to Dukes stage, distribution of benign diseases, and type of operation (minimum P = .85) (Table 2).
Table 2. DEMOGRAPHICS AND CLINICAL CHARACTERISTICS OF PATIENTS AND TYPE OF OPERATION PERFORMED
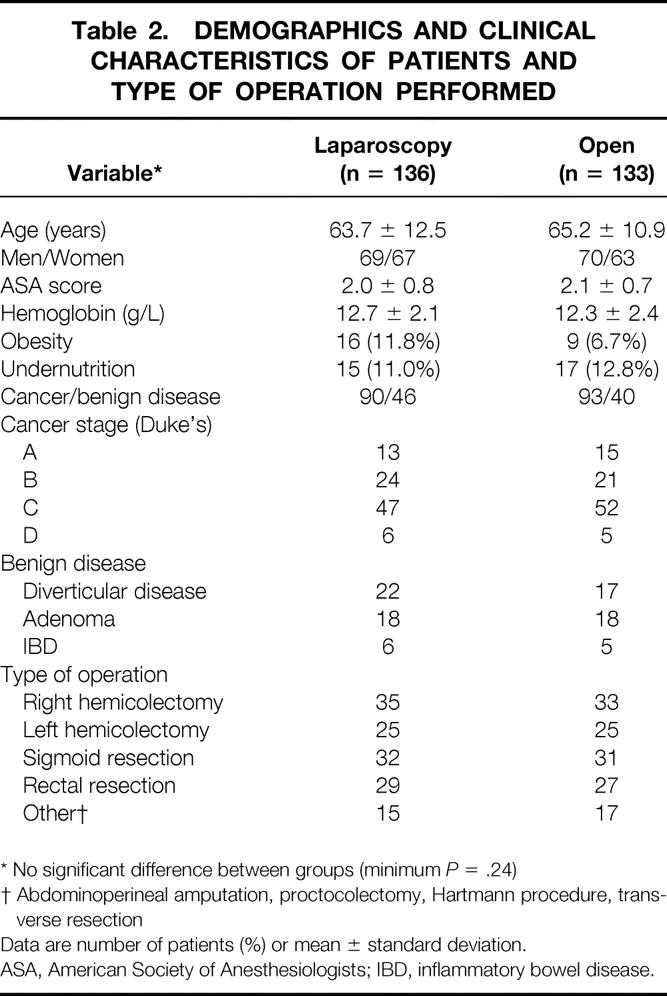
* No significant difference between groups (minimum P = .24)
† Abdominoperineal amputation, proctocolectomy, Hartmann procedure, transverse resection
Data are number of patients (%) or mean ± standard deviation.
ASA, American Society of Anesthesiologists; IBD, inflammatory bowel disease.
In seven (5.1%) patients in the laparoscopic group, conversion to open surgery was necessary (narrow pelvis, n = 3; adhesions, n = 3; development of hypercapnia, n = 1). These patients remained in the laparoscopic arm for data analysis. A primary diverting stoma was fashioned in 16 (11.8%) patients in the laparoscopic group and in 16 (12.0%) patients in the open group. The mean operative time was 222 ± 74 minutes in the laparoscopic group and 177 ± 56 minutes in the open group (P = .001). The mean operative blood loss was 170 ± 107 mL (median 100, range 50–1,600) in the laparoscopic group and 286 ± 242 mL (median 200, range 50–1,550) in the open group (P = .02). Twenty-seven (19.8%) patients in the laparoscopic group and 39 (29.3%) patients in the open group received homologous blood transfusion (P = .09). In transfused patients, the mean amount of homologous blood given was 439 ± 176 mL in the laparoscopic group and 487 ± 211 mL in the open group (P = .33). In cancer patients, the mean number of lymph nodes collected was 14.8 ± 7.6 in the laparoscopic group and 14.5 ± 7.2 in the open group (P = .90).
One patient (laparoscopic group) died in the late postoperative course of massive upper gastrointestinal bleeding. Table 3 shows that the overall morbidity rate was 20.6% (95% CI: 13.8–27.4%) in the laparoscopic group versus 38.3% (95% CI: 34.1–42.5%) in the open group (P = .003). Fewer patients had infectious complications in the laparoscopic group (11.0%; 95% CI: 5.7–16.3%) compared to the open group (23.3%; 95% CI: 16.1–30.5%). The difference in the infection rate between the two groups (12.3%) was significant (95% CI: 3.7–21.2%) (P = .01). Reoperation was necessary in 8 (5.9%) patients in the laparoscopic group (six anastomotic leaks, one adhesions, one bowel herniation through a 12-mm trocar site) and in 13 (9.8%) patients in the open group (eight anastomotic leaks, three adhesions, two bleeding) (P = .34).
Table 3. NUMBER OF PATIENTS WITH POSTOPERATIVE COMPLICATIONS
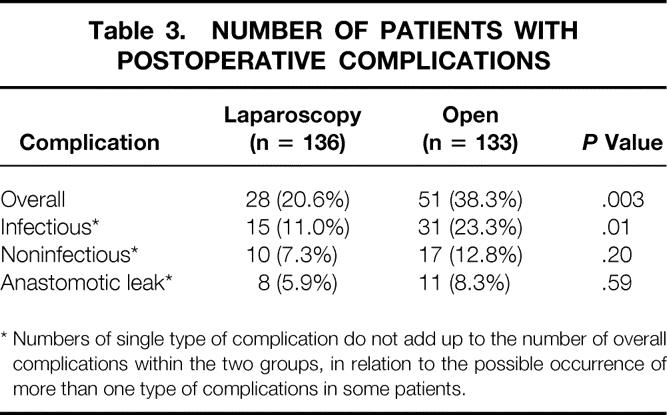
* Numbers of single type of complication do not add up to the number of overall complications within the two groups, in relation to the possible occurrence of more than one type of complications in some patients.
Table 4 reports the infectious and noninfectious postoperative complications in detail. The laparoscopic group had a lower wound infection rate (5.9%; 95% CI: 1.9–9.9%) than the open group (15.0%; 95% CI: 8.9–21.1%) (P = .02).
Table 4. POSTOPERATIVE INFECTIOUS AND NONINFECTIOUS COMPLICATIONS IN DETAIL
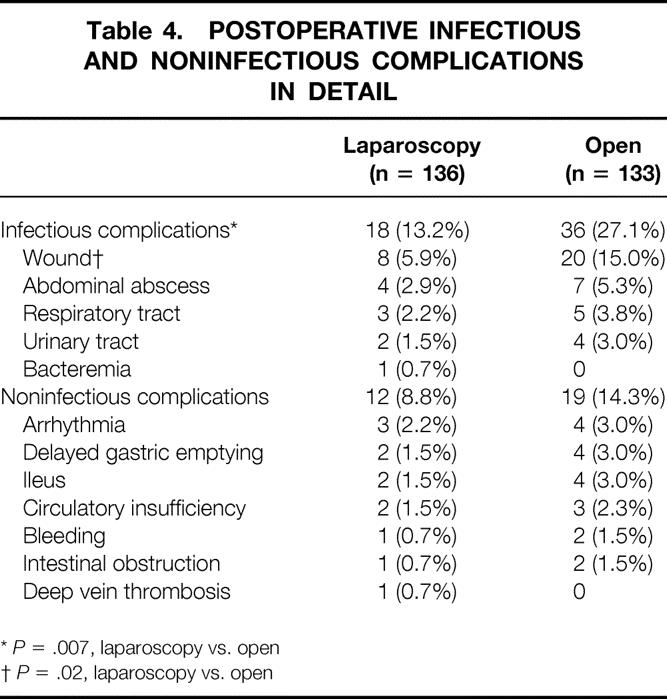
*P = .007, laparoscopy vs. open
†P = .02, laparoscopy vs. open
The mean length of hospital stay was 10.4 ± 2.9 days (median 8, range 5–83) in the laparoscopic group and 12.5 ± 4.1 days (median 10, range 6–80) in the open group (P < .0001).
Patients who underwent laparoscopic surgery had faster recovery of bowel function than patients who had open surgery. The first flatus occurred after 2.1 ± 0.2 days in the laparoscopic group versus 3.3 ± 0.6 days in the open group (P < .0001). The first bowel movement occurred after 4.7 ± 0.8 days in the laparoscopic group versus 5.7 ± 1.1 days in the open group (P < .0001). Recovery of oral food intake occurred after 3.7 ± 1.3 days in the laparoscopic group and 5.0 ± 2.0 days in the open group (P < .0001).
The mean hospital charge of laparoscopic resection was $931 higher than open surgery. This difference resulted from the additional cost of laparoscopic instruments and devices ($556 per patient) and from the estimated cost for the longer operative time in the laparoscopic group ($375 per patient). On the other hand, the mean estimated cost saved by the shorter hospital stay in the laparoscopic group was $840 per patient ($400 per day).
The mean time to recover physical and social activity was 32.1 ± 21.6 days in the laparoscopic group and 65.3 ± 33.2 days in the open group (P = .0001). Thirty-nine of 90 (43.3%) cancer patients in the laparoscopic group and 43 of 93 (46.2%) cancer patients in the open group completed 1-year follow-up. No port-site metastasis was found in the laparoscopic group, and one surgical wound metastasis was found in the open group.
Figure 2 shows that both laparoscopic and open surgery caused a significant impairment in the PBMCs proliferative response to C. albicans 3 days after operation. On postoperative day 15, PBMCs’ proliferative ability returned to preoperative values only in the laparoscopic group. Figure 3 shows that PBMCs’ proliferative response to PHA was slightly reduced on postoperative day 3 in both groups. On postoperative day 15, the laparoscopic group returned to preoperative values, whereas a further reduction was found in the open group.
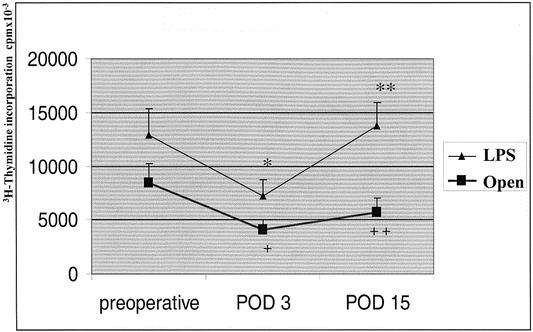
Figure 2. Proliferative response of T cells to C. albicans. Data are reported as mean and standard deviation. LPS, laparoscopic group; POD, postoperative day. *P = .05, POD 3 vs. preoperative (LPS); **P = .03, POD 15 vs. POD 3 (LPS); +P = .04, POD 3 vs. preoperative (open group); ++P = .005, LPS vs. open.
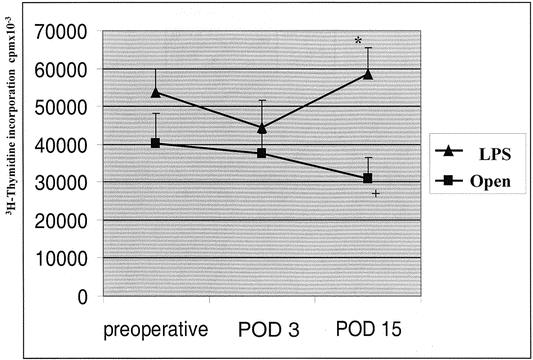
Figure 3. Proliferative response of T cells to phytohemagglutinin. Data are reported as mean and standard deviation. LPS, laparoscopic group; POD, postoperative day. +P = .06, POD 15 vs. preoperative (open group); *P = .006, LPS vs. open.
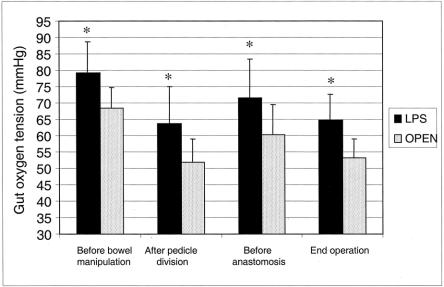
Figure 4 shows that gut oxygen tension was significantly higher in the laparoscopic group than in the open group at all time points measured. During surgery, gut oxygenation declined in both groups, and the lowest values were recorded after pedicle division and at the end of operation.
Figure 4. Operative gut oxygen tension. Data are reported as mean and standard deviation. LPS, laparoscopic group. Overall P for repeated measures = .001. *Minimum P = .03, LPS vs. versus open.
DISCUSSION
The feasibility and safety of laparoscopic colorectal resection have been repeatedly reported. The rate of conversion to open surgery is low when strict eligibility criteria are applied and the surgical team is well trained. 1–3 The highest conversion rates were reported in series resulting from early experiences. 20,21 In the present trial, the conversion rate was 5.1%, similar to that of other large series. 22,23
Some concerns remain about the oncologic adequacy of laparoscopy, although the data published so far are promising. No difference was found in the exfoliation of cancer cells in the peritoneal lavage before and after colon resection by comparing laparoscopic and open surgery. 24 The number of lymph nodes collected was similar for both techniques, 23,25–28 probably because the same oncologic principles for lymphovascular pedicle division and the extent of colonic resection were applied. With the exception of the earliest series, 29,30 the port-site recurrence rate is not different from wound recurrence rate reported following open colectomy. 31 Furthermore, the few studies focusing on cancer recurrence and patient survival did not report a substantial difference between laparoscopic and open surgery. 32–35
Laparoscopic colorectal surgery seems to be associated with less tissue injury than open surgery. Thus, some hypothetical benefits can be expected, such as better preservation of systemic immune function, a less pronounced postoperative inflammatory response, reduced postoperative pain, and faster recovery of intestinal motility and function. This might translate into an improved outcome. In contrast, the potential disadvantages of laparoscopic surgery are the longer operative time and the higher charges for surgical devices and instruments compared to open surgery. Moreover, two studies reported that laparoscopic surgery caused a higher mental strain for surgeons. 36,37
It is difficult to draw any firm conclusions as to the advantage of laparoscopic surgery on clinically relevant variables because the results of published trials are often conflicting. 25,28,33,38 This may be due to several reasons: few and underpowered randomized clinical trials have been performed, intention-to-treat analysis of results has been occasionally violated, no a priori definition of complications has been given (particularly important in nonblinded studies), and criteria for patient selection often differ. To overcome these potential biases, the power of the study was calculated a priori based on the complication rate of our previous trials, 3,18 five separate randomization lists according to the site of the lesion were generated to obtain a balanced distribution of different surgical procedures, strict inclusion and exclusion criteria were used, postoperative complications were rigorously defined a priori, 14 and the analysis of results was carried out on an intention-to-treat basis.
The analysis of the operative variables confirmed that the laparoscopic operation was longer than open surgery, according to previous studies. 3,4,25,26,38 Our results showed that blood loss was significantly lower in the laparoscopic group than in the open group. This finding is consistent with the results by Psaila et al, 27 while other authors found no difference in the operative blood loss by comparing the two techniques. 25,26,39,40 However, the lower blood loss observed in the laparoscopic group was not associated with a significant reduction in the homologous transfusion rate.
In the present trial, the laparoscopic group had a significantly lower postoperative complication rate compared to the open group. This is consistent with data recently reported by Milsom et al, 41 who randomized 60 patients undergoing ileocolic resection for refractory Crohn’s disease, and by Liang et al 42 in a series of patients undergoing sigmoid resection for complex polyps. In particular, the laparoscopic technique significantly reduced the incidence of wound infections, possibly because of minimal wound contamination, the shorter incision, and less manipulation of the intestine. The wound infection rate found in the present series is similar to the pooled rate calculated by Chapman et al in a systematic review of trials on laparoscopic colorectal resection. 10 The relatively high morbidity rate registered in the open group suggests some caution in the generalizability of the present data. However, the postoperative infection rate in the open group was consistent with intent-to-treat analysis from our previous trials. 18,43,44 A strict 30-day follow-up is a key point to obtain reliable data on the incidence of postoperative infections. 45,46 In fact, in the present series about 30% of postoperative infections (most of them were surgical wound infections) occurred after discharge. The rate of anastomotic leak and reoperation was not significantly different between groups. The incidence of clinically and/or radiologically evident anastomotic leak in the open group was comparable with our previous reports. 11,44 In both groups, anastomotic leak was the more frequent cause of reoperation. This reflects our policy in patients with clinically evident anastomotic dehiscence, which consists of both lavage of the peritoneal cavity and construction of a proximal ostomy.
Previous studies comparing laparoscopic and open colorectal surgery found a significant shorter hospital stay following laparoscopy. 25,26,33,40 In the present series, the lower postoperative complication rate combined with the earlier recovery of both bowel function and oral feeding may represent important determinants for the reduced length of hospital stay in the laparoscopic group. Other factors that could explain the shorter hospital stay following laparoscopy are the lower postoperative pain/consumption of analgesic drugs 3,4,27,38 and the earlier recovery of self-care score 26 and full ambulation ability. 47 In Italy, the hospital stay is usually longer than in the United States. This is due to the lack of outpatient guesthouses; thus, the patients completed postoperative recovery in the hospital before being discharged. In our study, patients who underwent laparoscopic surgery recovered full physical and social activity about 30 days earlier than the patients in the open group.
According to Liang et al, 42 this study showed that laparoscopy was more expensive than open surgery, although the shorter hospital stay nearly compensated for the additional costs of surgical instruments and devices and the longer operative time. To better evaluate the cost/benefit balance of laparoscopy, a precise quantification should be made of both the healthcare resources consumed by postoperative complications and the impact of the social cost of the faster recovery of full physical and social activity.
The real advantage of laparoscopic colorectal operation on the immune and inflammatory responses is still debated. 3–5,9 The conflicting results of previous trials are probably due to the different immune parameters investigated and the different techniques used to measure postoperative immune response. In the present study, both polyclonal stimulation and antigen-specific stimulation were used to evaluate the immune status of patients. PHA stimulates the proliferation of the whole T-cell population, while C. albicans selectively stimulates the Th1 inflammatory type of CD4+ T cells, 16 which secrete IFN-gamma and TNF-alfa and migrate selectively from the circulation into inflamed tissue following the release of soluble mediators of inflammation. PHA stimulation shows that the suppressive effect of open surgery on the whole T-cell population was more evident 15 days after operation, whereas no suppressive effect was found in the laparoscopic group. The selective analysis of Th1 proliferation showed a significant impairment in both groups early after surgery. This is likely due to the migration of Th1 cells from the circulation into the injured tissue. To explain the quicker recovery of Th1 cells’ proliferative ability in the laparoscopic group, it may be hypothesized that laparoscopy induced a less pronounced local inflammation than open surgery. This might result in a faster return of Th1 cells from tissues into the blood, with consequent recovery of their proliferation ability on postoperative day 15. This hypothesis is consistent with the lower levels of proinflammatory mediators observed after laparoscopy compared to open surgery. 3,4,6–8
High-pressure pneumoperitoneum has been shown to impair splanchnic perfusion and oxygenation in animal models. 48 Potential determinants of such effect are hypercapnia and its systemic effects on hemodynamics, reduced venous return, elevated diaphragm, and increased thoracic pressure. In humans, only one trial has investigated the impact of laparoscopy on bowel microperfusion by the laser Doppler technique. The authors found a 44% reduction in the colonic microperfusion during laparoscopic surgery, but it was promptly reversed by the interruption of pneumoperitoneum. 49 No data have been published so far about the effect of laparoscopy on gut oxygen tension in humans. In the present study, a higher bowel oxygen tension was found at the beginning of surgery before any gut manipulation, and during the entire surgical procedure in the laparoscopic group. Many factors could explain this finding, such as the less traumatic abdominal incision, less traction on the mesentery, and the relative low-pressure pneumoperitoneum used. The clinical impact of these data remains unknown. Nevertheless, it may be speculated that the higher oxygen tension during laparoscopic surgery plays an important role in improving the systemic host response, early recovery of gut function, and local wound repair.
In conclusion, the laparoscopic technique resulted in a reduction of both the overall morbidity rate and the length of hospital stay, and in a faster recovery of physical and social activity. The surgery-related impairment of lymphocyte proliferation and gut oxygen tension was less pronounced in the laparoscopic than in the open group.
Discussion
Prof. A. L. Fingerhut: Thank you for the opportunity to read your paper. Congratulations for an excellent, labor-intensive study which seems to be one of the largest mono-institutional studies on laparoscopic surgery for colonic disorders. I have several comments and questions.
It has been shown that many of the criteria you have used and found to be “statistically significantly different” are in fact culture-related, payer-related, surgeon-related. The well-known Majeed study on laparoscopic cholecystectomy has shown that when the patient and evaluator were blinded to which technique had been performed, many of these criteria differences disappeared. Could you comment on this?
My second question concerns the so-called learning curve: were all surgeons participating in this study experienced in both types of surgery (laparoscopic and open), and do you have any opinion of what the learning curve should be for laparoscopic colonic surgery, not only for the surgeons in your unit, who are obviously motivated and academic surgeons, but for the ordinary surgeon performing laparoscopic surgery who endeavors in all aspects of laparoscopic surgery? This is an expert center study, and I want to know your thoughts on how you think laparoscopic colonic surgery can be applied by the overall general population of surgeons.
Last, you have shown statistically significant differences for several results. The question I have is: What is the clinical significance you associated with these differences, because in your paper, although the P values are statistically significant, the confidence intervals overlap slightly.
Thank you for the opportunity to review and comment on this very interesting paper.
Prof. M. Braga: Prof. Fingerhut, I appreciated your comments. The study is not blinded; however, postoperative complications were registered by four trained surgeons who were not involved in the study, according to a priori definition.
Positive culture proved all infectious complications and, in my opinion of primary importance, the follow-up was carried out throughout a 30-day period. This is a key point to obtain reliable data on the incidence of postoperative complications. In the present series about 30% of postoperative infections occurred after hospital discharge.
We performed more than 40 laparoscopic colorectal resections before starting the present trial. There are few papers focusing on the learning curve; however, there is a consensus that 40 laparoscopic operations represents a cutoff value to obtain good technical skills.
Most of the patients who met exclusion criteria underwent open surgery. The 13 patients excluded because of locally advanced cancer were treated by neoadjuvant radiochemotherapy and 7 of them were successfully operated by laparoscopic technique.
Finally, when we evaluated the difference in the infection rate between groups, the 95% confidence interval did not overlap.
Prof. C. E. Broelsch: Colorectal surgery is a standard procedure in many institutions, although we see that several reoperations referred from the outside reflect a wide range in standards. When I look at your data, I find there two really distinctively different groups, particularly looking at the blood loss, the infectious complications, and the anastomotic leaks in the open groups. I have some doubts to believe they belong to the same teams, or the one team as you have expressed. I am asking myself whether the open group was operated by the anesthesiologist. You were saying there was a long recovery in the operated group. Was this due to the blood loss in that group, and why did these people use 60 days for the same level of recovery? I think these are biased observations in the group with open laparotomy.
The question I have is, is there a difference in the results in the group operated on the rectum, whether the colon was infectious or transversectomy or so on? Looking at the infectious complications, was there preparation for this operation in both groups or the same antibiotic preoperative regimen?
Prof. M. Braga: Both laparoscopic and open operations were performed by the same surgical team. The mean operative blood loss in the open group was relatively low. In fact, most of patients who were given homologous blood transfusion had preoperative anemia. Both infectious complication and anastomotic leak rates in the open group were comparable with our previous studies. As I previously mentioned, a 30-day follow-up is a key point to obtain reliable data on the incidence of infectious complications. Most of the leaks occurred in rectal cancer patients, and I would also emphasize that we include any dehiscence with clinical or radiologic evidence.
Not surprisingly, full performance recovery occurred earlier in the laparoscopic group. This resulted from a subjective judgment by the patients which was focused on their working ability, long-distance walking ability, climbing stairs, social activities with family and friends. No difference between groups in postoperative hemoglobin values was found.
All patients had the same bowel preparation and also the same antibiotic prophylaxis. We did not perform any separate analysis according to the type of surgery.
Prof. A. G. Johnson: Congratulations on doing a randomized trial like this. In the UK we have a large multicenter MRC trial which closes this year, with over 700 patients randomized. The primary endpoint in that trial is the oncological data, because what matters in cancer is that the cancer is cleared. Have you got any data on the resection margins? You mentioned a number of lymph nodes, but local cancer clearance is very important. Second, you mentioned the long stay before going back to work; what were the patients told about when they could leave hospital and return to work? Our evidence is that what you tell the patients influences how quickly they recover, and the family doctor who signs them back to work has a huge influence.
Prof. M. Braga (closing): In all patients resection margins were cancer-free. Most of our patients completed 1-year follow-up, and no port site recurrence was found in the laparoscopic group.
The majority of our patients are retired, so in this case we asked them when they recovered their preillness performance. We did not influence the patients concerning how quickly they get back to work. In our country, patients usually decided to return to work when they experienced a complete recovery of full performance.
Footnotes
Correspondence: Marco Braga, MD, Department of Surgery, San Raffaele University, Via Olgettina 60, 20132 Milan, Italy.
E-mail: braga.marco@hsr.it
Accepted for publication April 2002.
References
- 1.Wexner SD, Reissman P, Pfeifer J, et al. Laparoscopic colorectal surgery. Analysis of 140 cases. Surg Endosc 1996; 10: 133–136. [DOI] [PubMed] [Google Scholar]
- 2.Bruch HP, Schiedeck TH, Schwandner O. Laparoscopic colorectal surgery. A five-year experience. Dig Surg 1999; 16: 45–54. [DOI] [PubMed] [Google Scholar]
- 3.Braga M, Vignali A, Zuliani W, et al. Metabolic and functional results after laparoscopic colorectal surgery. A randomized controlled trial. Dis Colon Rectum 2002; 45: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt PM, IP SM, Kwok SP, et al. Laparoscopic-assisted vs. open surgery for colorectal cancer: comparative study of immune effects. Dis Colon Rectum 1998; 41: 901–909. [DOI] [PubMed] [Google Scholar]
- 5.Nishiguchi K, Okuda J, Toyoda M, et al. Comparative evaluation of surgical stress of laparoscopic and open surgeries for colorectal carcinoma. Dis Colon Rectum 2001; 44: 223–230. [DOI] [PubMed] [Google Scholar]
- 6.Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg 2000; 231: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwenk W, Jacobi C, Mausmann U, et al. Inflammatory response after laparoscopic and conventional colorectal resections. Results of a prospective randomized trial. Langenbecks Arch Surg 2000; 385: 2–9. [DOI] [PubMed] [Google Scholar]
- 8.Kishi D, Nezu R, Ho T, et al. Laparoscopic-assisted surgery for Crohn’s disease: reduced surgical stress following ileocolectomy. Surg Today 2000; 30: 219–222. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Watson DI. Effect of laparoscopy on immune function. Br J Surg 2001; 88: 1296–1306. [DOI] [PubMed] [Google Scholar]
- 10.Chapman AE, Levitt MD, Hewett P, et al. Laparoscopic-assisted resection of colorectal malignancies. A systematic review. Ann Surg 2001; 234: 590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignali A, Gianotti L, Braga M, et al. Microperfusion of the rectal stump as predictive index of anastomotic leak. Dis Colon Rectum 2000; 43: 76–82. [DOI] [PubMed] [Google Scholar]
- 12.Braga M, Vignali A, Zuliani W, et al. Training period in laparoscopic colorectal surgery. A case-matched comparative study with open surgery. Surg Endosc 2002; 16: 31–35. [DOI] [PubMed] [Google Scholar]
- 13.Milsom JW, Bohm BL. Laparoscopic colorectal surgery. New York: Springer-Verlag, 1996.
- 14.Bozzetti F, Braga M, Gianotti L, et al. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 2001; 358: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J 1992; 305: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romani L, Mencacci A, Cenci E, et al. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med 1996; 183: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braga M, Gianotti L, Gentilini O, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001; 29: 242–248. [DOI] [PubMed] [Google Scholar]
- 18.Gianotti L, Braga M, Nespoli L, et al. A randomized controlled trial on preoperative immunonutrition in patients with gastrointestinal cancer. Gastroenterology 2002; 122: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Schulz KF, Altman DG, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-randomized trials. JAMA 2001; 285: 1987–1991. [DOI] [PubMed] [Google Scholar]
- 20.Molenaar CB, Bijnen AB, de Ruiter P. Indications for laparoscopic colorectal surgery: results from the medical centre Alkmaar, The Netherlands. Surg Endosc 1998; 12: 42–45. [DOI] [PubMed] [Google Scholar]
- 21.Delgado F, Bolufer JM, Grau E, et al. Laparoscopic colorectal cancer resection, initial follow-up results. Surg Laparosc Endosc 1999; 9: 91–98. [PubMed] [Google Scholar]
- 22.Leung KL, Yiu RYC, Lai PBS, et al. Laparoscopic-assisted resection of colorectal carcinoma: five-year audit. Dis Colon Rectum 1999; 42: 327–332. [DOI] [PubMed] [Google Scholar]
- 23.Franklin ME, Rosenthal D, Abrego MD, et al. Prospective comparison of open vs. laparoscopic colon surgery for carcinoma. Five-year results. Dis Colon Rectum 1996; 39: S35–46. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Milsom JW, Gramlich TL, et al. Does laparoscopic vs. conventional surgery increase exfoliated cancer cells in the peritoneal cavity during resection of colorectal cancer? Dis Colon Rectum 1998; 41: 971–977. [DOI] [PubMed] [Google Scholar]
- 25.Lacy AM, Carcia-Valdecasas JC, Piquè; MJ, et al. Short-term outcome analysis of a randomised study comparing laparoscopic vs open colectomy for colon cancer. Surg Endosc 1995; 9: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 26.Stage JG, Schulze S, Moller P, et al. Prospective randomized study of laparoscopic versus colonic resection for adenocarcinoma. Br J Surg 1997; 84: 391–396. [PubMed] [Google Scholar]
- 27.Psaila J, Bulley SH, Ewings P, et al. Outcome following laparoscopic resection for colorectal cancer. Br J Surg 1998; 85: 662–664. [DOI] [PubMed] [Google Scholar]
- 28.Stocchi L, Nelson H, Young Fadok TM, et al. Safety and advantages of laparoscopic vs. open colectomy in the elderly: matched-control study. Dis Colon Rectum 2000; 43: 326–332. [DOI] [PubMed] [Google Scholar]
- 29.Nduka CC, Monson JR, Menzies GN, et al. Abdominal wall metastases following laparoscopy. Br J Surg 1994; 81: 648–652. [DOI] [PubMed] [Google Scholar]
- 30.Berends FJ, Kazemier G, Bonjer HJ, et al. Subcutaneous metastases after laparoscopic colectomy [letter]. Lancet 1994; 344: 58. [DOI] [PubMed] [Google Scholar]
- 31.Pearlstone DB, Mansfield PF, Curley SA, et al. Laparoscopy in 533 patients with abdominal mailgnancies. Surgery 1999; 125: 67–72. [PubMed] [Google Scholar]
- 32.Lacy AM, Delgado S, Garciavaldecasas JC, et al. Port site metastases and recurrence after laparoscopic colectomy: a randomized trial. Surg Endosc 1998; 12: 1039–1042. [DOI] [PubMed] [Google Scholar]
- 33.Leung KL, Meng WCS, Lee JFY, et al. Laparoscopic-assisted resection of right-sided colonic carcinoma: a case-control study. J Surg Oncol 1999; 71: 97–100. [DOI] [PubMed] [Google Scholar]
- 34.Poulin EC, Marnazza J, Schlachta CM, et al. Laparoscopic resection does not adversely affect early survival curves in patients undergoing surgery for colorectal adenocarcinoma. Ann Surg 1999; 229: 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartley JE, Mehigan BJ, MacDonald AW, et al. Patterns of recurrence and survival after laparoscopic and conventional resections for colorectal carcinoma. Ann Surg 2000; 232: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohm B, Rotting N, Schwenk W, et al. A prospective randomized trial on heart rate variability of the surgical team during laparoscopic and conventional sigmoid resection. Arch Surg 2001; 136: 305–310. [DOI] [PubMed] [Google Scholar]
- 37.Berguer R, Smith WD, Chung YH. Performing laparoscopic surgery is significantly more stressful for the surgeon than open surgery. Surg Endosc 2001; 15: 1204–1207. [DOI] [PubMed] [Google Scholar]
- 38.Tate JJT, Kwok S, Dawson JW, et al. Prospective comparison of laparoscopic and conventional anterior resection. Br J Surg 1993; 80: 1396–1398. [DOI] [PubMed] [Google Scholar]
- 39.Goh YC, Eu KW, Seowchoen F. Early postoperative results of a prospective series of laparoscopic vs. open anterior resections for rectosigmoid cancers. Dis Colon Rectum 1997; 40: 776–780. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima R, Kawamura YJ, Saito H, et al. Interleukin-6 and stress hormone responses after uncomplicated gasless laparoscopic-assisted and open sigmoid colectomy. Dis Colon Rectum 1996; 39:Suppl. S. [DOI] [PubMed]
- 41.Milsom JW, Hammerhofer KA, Bohm B, et al. Prospective, randomized trial comparing laparoscopic vs. conventional surgery for refractory ileocolic Crohn’s disease. Dis Colon Rectum 2001; 44: 1–9. [DOI] [PubMed] [Google Scholar]
- 42.Liang JT, Shieh MJ, Chen CN, et al. Prospective evaluation of laparoscopy-assisted colectomy versus laparotomy with resection for management of complex polyps of the sigmoid colon. World J Surg 2002; 26: 377–383. [DOI] [PubMed] [Google Scholar]
- 43.Braga M, Gianotti L, Radaelli G, et al. Perioperative immunonutrition in patients undergoing cancer surgery. Results of a randomized double-blind phase 3 trial. Arch Surg 1999; 134: 428–433. [DOI] [PubMed] [Google Scholar]
- 44.Braga M, Gianotti L, Vignali A, Di Carlo V. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery (in press). [DOI] [PubMed]
- 45.Melling AC, Alì; B, Scott ME, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomized controlled trial. Lancet 2001; 358: 876–880. [DOI] [PubMed] [Google Scholar]
- 46.Keeling NJ, Morgan MWE. Inpatient and postdischarge wound infections in general surgery. Ann R Coll Surg Engl 1995; 77: 245–247. [PMC free article] [PubMed] [Google Scholar]
- 47.Bokey EL, Moore JW, Chapuis PH, et al. Morbidity and mortality following laparoscopic-assisted right hemicolectomy for cancer. Dis Colon Rectum 1996; 39: S24–28. [DOI] [PubMed] [Google Scholar]
- 48.Schafer M, Krahenbuhl L. Effect of laparoscopy on intra-abdominal blood flow. Surgery 2001; 129: 385–389. [DOI] [PubMed] [Google Scholar]
- 49.Schilling MK, Redaelli C, Krahenbuhl L, et al. Splanchnic microcirculation changes during CO2 laparoscopy. J Am Coll Surg 1997; 184: 378–382. [PubMed] [Google Scholar]