Abstract
Objective
To evaluate the clinical relevance of real-time quantitative polymerase chain reaction (qPCR) detection of CEA and CK20 transcripts, as potentially related to tumor cell dissemination, in blood and peritoneal lavage from patients undergoing surgery for colorectal cancer.
Summary Background Data
Dissemination of single colorectal cancer cells in the peritoneal cavity, as well as in tumor drainage and peripheral blood vessels, might play a role in the metastasis process, thus affecting the clinical course. However, this phenomenon needs further elucidation.
Methods
In a prospective study the authors evaluated the potential of qPCR in the detection of CEA and/or CK20 transcripts in the peritoneal lavage fluid and in the peripheral and mesenteric venous blood of 39 patients undergoing curative resection for colorectal cancer. Peritoneal lavage and peripheral blood was sampled before and after tumor resection; mesenteric venous blood was sampled from the major tumor-draining vein immediately before clamping. After RNA extraction and reverse transcription, qPCR was performed using specific cDNA primers and probes for CEA and CK20. The dichotomous results from the qPCR were used as a predictor along with other covariates in Cox proportional hazard regression models of long-term outcome (disease-free survival and overall survival).
Results
Of 39 patients, 11 were positive. The median follow-up at analysis was 31 months for all patients. The dichotomous qPCR covariate was significant, with P = .001 and .0035 for disease-free survival and overall survival, respectively, in the proportional hazard regression models with only qPCR. In seven patients, disseminated colorectal cancer cells were found in the peritoneal lavage fluid but not in blood specimens; five of these patients (71%) had recurrence.
Conclusions
These data suggest that detection of mRNA coding for CEA and/or CK20 using qPCR has potential clinical utility as a prognostic marker and should be evaluated in larger clinical studies. Identification of patients at high risk for metastatic disease after curative resection of colorectal cancer might be improved by analyzing peritoneal lavage specimens in addition to blood samples. This is based on the observation that in more than half of qPCR-positive patients, disseminated colorectal cancer cells were detected in peritoneal lavage specimens but not in blood samples, and that 71% of them had recurrence.
Colorectal cancer is a common and often life-limiting disease. Approximately 20% to 45% of colorectal cancer patients who undergo curative (R0) resection subsequently develop local tumor relapse or metastatic disease in lymph nodes, liver, lung, and peritoneum, implying a high probability of death. 1,2 Prognostic criteria include tumor staging and grading.
Detection of micrometastatic disease and dissemination of single cancer cells in lymph nodes, peripheral blood, peritoneal cavity, or bone marrow have been shown to have prognostic relevance in colorectal cancer patients. 3–6 The overall clinical value of these approaches remains a matter of debate and needs elucidation. However, in the future, these criteria might enable the selection of high-risk patients who would benefit from adjuvant treatment.
Numerous studies have confirmed the feasibility, reliability, and reproducibility of reverse transcriptase–polymerase chain reaction (RT-PCR), and its sensitivity was established at 1 to 10 neoplastic cells per 107 white blood cells. 7–11 In contrast, little has been published about real-time quantitative detection of disseminated colorectal cancer cells. No study has ever correlated disseminated tumor cells in blood and peritoneal lavage specimens as detected by real-time quantitative PCR (qPCR) with the clinical long-term course of patients undergoing surgery for colorectal cancer. Therefore, the aim of this study was to evaluate the prognostic relevance of qPCR-detected malignant cells in peripheral and mesenteric venous blood as well as in the peritoneal lavage fluid of patients undergoing resection for colorectal cancer. Moreover, the present pilot study was designed to provide basic information about the most appropriate specimen and time point of sampling for detection of disseminated colorectal cancer cells.
METHODS
Patients
Thirty-nine patients (16 women, 23 men; mean age 65.8 years [range 33–81]) with histologically proven colorectal cancer (16 rectal cancers, 23 colon cancers) were prospectively evaluated (Table 1). Exclusion criteria were previous surgical treatment for colorectal cancer and stage IV and secondary malignancy. All patients underwent curative (R0) resection. All resections were performed according international standards at more than 10 cm from the border of the primary tumor for colon and more than 2 cm for rectal carcinoma, with lymphadenectomy of pericolic, perirectal, and vascular truncal regions. The patients were consecutively enrolled in the study from July 1997 through December 1998.
Table 1. PATIENT CHARACTERISTICS

As negative controls, we investigated peripheral venous blood sampled before, during, and after resection and peritoneal lavage specimens obtained before and after resection from seven patients with benign abdominal disease (one laparoscopic and one open cholecystectomy for symptomatic cholecystolithiasis, four sigmoidectomies for sigmoid diverticulitis, and one ileocecal resection for Crohn’s disease).
Written informed consent was obtained from all patients enrolled in the investigation. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the guidelines of the regional ethical committees of Zurich, Switzerland, and Basel, Switzerland.
Tissue Sampling and Processing
Prior to and immediately after resection, 12 mL whole peripheral venous blood was collected, and before clamping of tumor-draining vessels, 12 mL mesenteric venous blood was collected. The blood samples were transferred to Ficoll gradient Vacutainer-CPT/Citrate tubes (BD, Franklin Lakes, NJ). The tubes were centrifuged for 20 minutes (1,500 g) at room temperature. After centrifugation, the isolated mononuclear cells were washed twice. The cell pellet was frozen at −70°C and later processed for total RNA extraction as described below.
For peritoneal lavage, 700 mL Ringer’s lactate was used. Four hundred milliliters of this lavage fluid was collected before and after resection and first pelleted for 10 minutes (350 g) at room temperature. Supernatants were decanted and the cell-containing pellets were carefully resuspended in 8 mL phosphate-buffered saline, transferred to Ficoll Gradient Vacutainer-CPT/Citrate tubes, and then processed like the blood samples.
Total RNA extraction was performed using the RNeasy Minikits (Quiagen, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, to 1 μL oligo-dT, 11 μL total RNA (representing 25% of the total extract) was added; the mixture was heated at 65°C for 5 minutes, then put on ice. The cDNA was synthesized in a 20-μL reaction mixture containing 4 μL 5× first strand buffer, 1 μL dNTP, 2 μL DTT buffer, and 1 μL Moloney leukemia virus reverse transcriptase. The reaction mixture was incubated at 37°C for 60 minutes and then at 94°C for 5 minutes to inactivate the reverse transcriptase. cDNA was stored at −20°C until qPCR was performed.
Gene expression was evaluated using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Rotkreuz, Switzerland). Primers and FAM-labeled probes designed with “Primer Express” software (Applied Biosystems) were synthesized by Applied Biosystems.
CEA (forward): CCACAGTCACGACGATCACAG
CEA (reverse): GGGTTGGAGTTGTTGCTGGT
CEA (probe): CTATGCAGAGCCACCCAAACCCTTCA
CK (forward): CTGAATAAAGACCTAGCTCTCCTCAAA
CK (reverse): TGTTGCCCAGATGCTTGTGT
CK (probe): GCCATCGACTTCCTCCTGATGCTCCT
The ready-to-use β-actin complete detection system (VIC-labeled probe, allowing multiplex detection) was provided by the same company and used as internal reference.
Each sample was processed as described above and tested for β-actin, CEA, and CK20 gene expression. The quantitative PCR was performed with 5 μL cDNA (representing 6.25% of the initial purified RNA) using the 2× concentrated Master Mix containing all necessary reagents for the qPCR (Applied Biosystems) for 45 cycles (95°C for 50 seconds; 60°C for 1 minute).
Patients were considered to display tumor cell dissemination if either CEA or CK20 was detected in at least one sample no later than after 40 PCR cycles. This threshold was defined in preliminary experiments as clearly below the level of false positivity (data not shown) and was confirmed by the analysis of samples from patients without cancer that were never detected below this level.
Real-time qPCR and clinical data were evaluated by independent investigators unaware of patient status.
Spiking Experiments
Cell spiking experiments were performed to test the potential sensitivity of the complete procedure (cell isolation, RNA purification, reverse transcription, cDNA amplification). Known cell numbers from two colorectal cancer cell lines (LS 180 and C205; ATCC CL-187 and CCL-222) were added to venous peripheral blood samples.
Statistical Analysis
The primary statistical analysis tool used was the Cox proportional hazard regression (PHR) 12 procedure of the Statistical Analysis System (SAS, Cary, NC). The Cox PHR procedure was used for univariate and multivariate analyses. The outcome variables were disease-free survival and overall survival from the day of surgery. The primary covariate (explanatory variable) of interest was the dichotomous qPCR result (positive or negative). Additional covariates available for analysis were stage (coded as III vs. I or II), positive node (yes or no), tumor location (colon or rectum), histologic grade (1–3), gender, and age (<68 vs. ≥68, split on median). The purpose of PHR modeling in this context was to explore whether the covariates carry prognostic information relative to outcomes (disease-free survival and overall survival). The primary interest in this study was whether qPCR carries additional prognostic information beyond that of the other covariates. PHR models of each covariate separately were estimated in a preliminary analysis. PHR models of all covariates were reduced successively by removing the least significant covariate with a significance level greater than 0.05 until all remaining covariates had significance levels of 0.05 or less. Models with interaction terms were computed where appropriate (sufficient data). Estimated distributions of time-to-event data are displayed as Kaplan-Meier plots.
Follow-Up
After surgical resection, all patients underwent standard therapeutic and follow-up measures according to recommended guidelines. 13–16 Postoperative surveillance consisted of medical history, physical examination, laboratory levels including CEA, abdominal ultrasound, and rectosigmoidoscopy/coloscopy.
RESULTS
In the preliminary spiking experiments, dose-dependent specific signals for CEA and CK20 were detected down to 10 cells/mL blood. Blood and peritoneal lavage specimens were analyzed from 39 patients with colorectal cancer and 7 patients with benign abdominal disease by qPCR using specific primers and probes for CK20 and CEA. None of the 35 specimens of the seven patients with benign abdominal diseases were found to be positive. Of the 39 patients with colorectal cancer, 11 had at least one sample positive for CEA or CK20. The qPCR-positive patients in relation to tumor stage are shown in Table 2. Six patients had evidence of disseminated colorectal cancer cells before and 10 patients after resection. A CEA qPCR amplification was detected in 8 patients and a CK20 qPCR amplification in 10 patients (Table 3). Nine of the 11 PCR-positive patients had recurrence (five distal metastases, four local metastases) after an average follow-up of 12 months, whereas only 2 of the 28 patients found to be qPCR negative had recurrence. The median follow-up for all patients was 31 months. Seven patients had disseminated colorectal cancer cells in the peritoneal lavage fluid but not in blood samples; five of these patients(71%) had recurrence.
Table 2. REAL-TIME QUANTITATIVE PCR-POSITIVE PATIENTS IN RELATION TO TUMOR STAGE

Table 3. RELATION BETWEEN TUMOR STAGE, SAMPLING DATA, AND CLINICAL OUTCOME OF qPCR-POSITIVE PATIENTS
B1, peripheral venous blood before resection of colorectal cancer; B2, mesenteric venous blood from tumor draining vein before clamping; B3, peripheral venous blood after resection of colorectal cancer; L1, peritoneal lavage fluid before resection of colorectal cancer; L2, peritoneal lavage fluid after resection of colorectal cancer.
Tables 4 and 5 show the results from PHR models of each covariate separately on overall survival and disease-free survival, respectively. Models with stage dichotomously coded as I versus II and III and I and II versus III were computed, but only the coding of I and II versus III was significant; thus, in all further analyses stage is coded as I and II versus III. Only the covariates “qPCR,” “lymph node metastasis,” and “stage” were significant in this collection of models, and these covariates were significant for both disease-free survival (P = .001 for qPCR, P = .01 for lymph node metastases, P = .01 for TNM stage) and overall survival (P = .0035 for qPCR, P = .02 for lymph node metastases, P = .02 for TNM stage). The lack of significance of the other covariates could be due to the small sample size; therefore, we cannot conclude that these other covariates have no relationship to outcome.
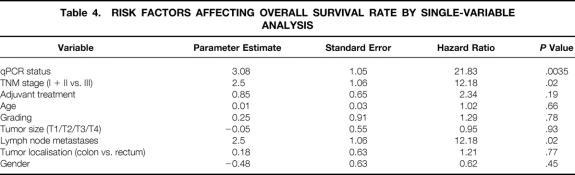
Table 4. RISK FACTORS AFFECTING OVERALL SURVIVAL RATE BY SINGLE-VARIABLE ANALYSIS
Table 5. RISK FACTORS AFFECTING DISEASE-FREE SURVIVAL BY SINGLE-VARIABLE ANALYSIS
PHR models with all covariates and then with successively fewer covariates were computed as described in the Methods section. The only remaining significant covariates for disease-free survival were “qPCR” and “stage (I and II vs. III)”; this model is shown in Table 6. For overall survival, only the qPCR variable remained following the stepdown procedure (see Table 5).
Table 6. ESTIMATES FROM PROPORTIONAL HAZARD REGRESSION MODEL OF DISEASE-FREE SURVIVAL
The Kaplan-Meier plots in Figures 1 and 2 show disease-free and overall survival rates by qPCR status (positive vs. negative). The Kaplan-Meier plots shown in Figures 3 and 4 show disease-free survival and overall survival by qPCR status and stage (I and II vs. III).

Figure 1. Kaplan-Meier life-table analysis of disease-free survival of qPCR-positive and qPCR-negative patients undergoing surgery for colorectal cancer. Rec, recurrences; pat, patients.

Figure 2. Kaplan-Meier life-table analysis of overall survival of qPCR-positive and qPCR-negative patients undergoing surgery for colorectal cancer. †, deaths; pat, patients.
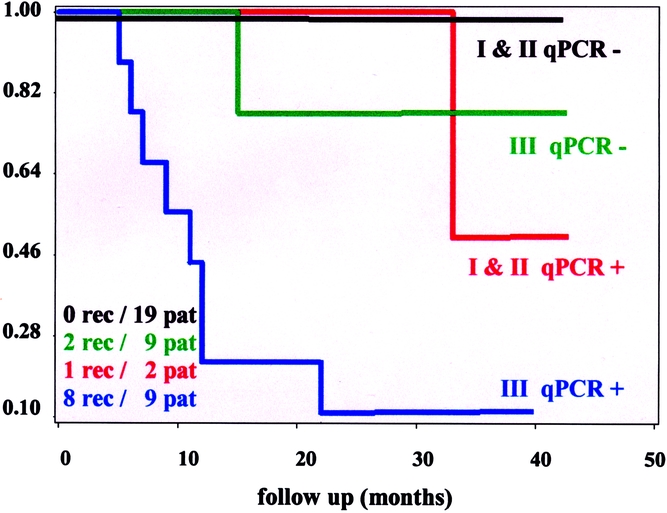
Figure 3. Kaplan-Meier life-table analysis of disease-free survival of the qPCR-positive and qPCR-negative high-stage (III) and low-stage (I + II) patients undergoing surgery for colorectal cancer. Rec, recurrences; pat, patients.
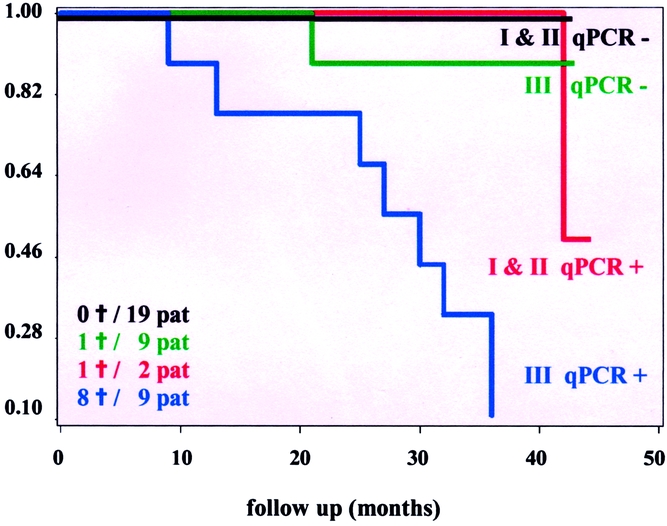
Figure 4. Kaplan-Meier life-table analysis of overall survival of the qPCR-positive and qPCR-negative high-stage (III) and low-stage (I + II) patients undergoing surgery for colorectal cancer. †, deaths; pat, patients.
DISCUSSION
The present investigation correlates for the first time qPCR detection of disseminated colorectal cancer cells with the clinical course of the disease. The results of our analysis suggest that qPCR detection of CEA and/or CK20 transcripts in blood and peritoneal lavage specimens has prognostic relevance in patients undergoing curative resection of colorectal cancer. Indeed, for both disease-free and overall survival, qPCR-detected neoplastic cells in blood and peritoneal lavage fluid represent an independent prognostic factor.
Detection of micrometastases and disseminated cancer cells in patients with tumors undergoing surgery for cure is a challenging field in oncology because dissemination of neoplastic cells is the main determinant of distant relapse and cancer-related death. Conventional methods such as cytology and immunohistochemistry have been reported. 17–20 There are numerous publications about conventional RT-PCR detection of tumor cells in peripheral blood, 6,18,21,22 mesenteric venous blood, 23,24 lymph nodes, 4,25–27 and bone marrow, 5,28,29 whereas reports about qPCR detection of disseminated cancer cells are rare. Although some investigations have reported a negative impact of RT-PCR-detected colorectal cancer cells in blood on recurrence rate and survival, 6,30 its significance remains a matter of debate. 8,30 Bessa et al reported that in 95 patients with stage I to IV colorectal cancer, disease-free and overall survival rates during a median follow-up of 42 months were identical for patients with and without disseminated cancer cells in peripheral blood. 31 The results of the present investigation suggest that the outcome for patients with stage III and positive qPCR is worse than that for negative stage III patients or stage I and II patients, who seem to have similar outcomes (see Figs. 3 and 4). This pattern of outcome suggests that the addition of an interaction term would improve the PHR models with qPCR and stage. However, because of the small sample size and the pattern of associations between qPCR and stage models, the interaction term could not be computed, so verification of this data-directed suggestion could not be verified statistically. Nonetheless, this graphical analysis suggests that qPCR is an additive prognostic factor in patients with stage III disease, but not necessarily a prognostic factor in patients with stage I or II disease.
Many target genes have previously been used to detect micrometastases of colorectal cancer, including CEA, 7,9,23,32 mucin 1 (MUC1), 33 cytokeratin 8, 21 cytokeratin 18, 34 cytokeratin 19, 21,35 and cytokeratin 20. 21,23 There is no specific marker for colorectal cancer, 9,36 and detection of disseminated neoplastic cells is based on epithelial markers such as CK20 and CEA. CK20 mRNA is considered a reliable target for detection of disseminated colorectal cancer cells, 5,11,21 and the frequency of false-positive results is reportedly rarer (0–8%) as compared to CEA (0–33%). 9,23,37,38 Although considered a good target for detection of disseminated colorectal cancer cells, 23,31 in one study CEA mRNA was found to be positive as well in patients with inflammatory bowel disease, but not in healthy volunteers. 9 To overcome the issue of false positivity, Yamaguchi et al 23 defined conventional RT-PCR positivity as the situation where both CK20 and CEA were positive. Conversely, in a recent study, Patel et al used CEA and CK20 to detect disseminated colorectal cancer cells by conventional PCR and defined positivity if the patients were PCR positive for either CEA or CK20 in any of three blood samples taken at 1-minute intervals. 39 Similarly, in the present investigation, based on the high specificity of our detection system as verified on samples from patients without tumors, qPCR was considered positive if either CEA or CK20 or both was found to be positive in at least one patient sample. Currently, neither the ideal time point of sampling, the ideal body compartment, nor the ideal marker (or combination of markers) for the detection of disseminated colorectal cancer cells is known. Given this lack of knowledge, we analyzed two different markers, specimens from different body compartments, at three different time points. qPCR positivity can be interpreted as systemic disease, independently of the body compartment, marker, and time point of sampling. Moreover, it is well known that not all colorectal cancers, nor all individual colorectal cancer cells, express the markers under investigation. If we assessed only one marker, disseminated colorectal cancer cells expressing either CEA or CK20 might have remained undetected.
Real-time quantitative PCR as used in the present investigation is advantageous over conventional RT-PCR with respect to specificity. A clear advantage of qPCR is represented by the possibility to establish a clear cut-off reference value based on highly objective evaluation criteria. Quantitative real-time PCR allowed us to establish threshold levels of gene expression based on data obtained from cell lines and from surgically treated patients with pathologies other than cancer (data not shown). This is opposed to potentially biased scoring of faint gel bands in conventional PCR. A further advantage of qPCR over conventional PCR is that it relies not only on primers (as for conventional PCR) but also on internal probes that specifically hybridize to the amplified sequences. Moreover, due to continuous measurement (real-time) of the amplified signal, false-positive results, which produce an abnormally shaped, nonlinear amplification curve, could be easily identified and removed. We suggest that the excellent correlation between qPCR positivity and poor clinical outcome is due to the accurate differentiation between positive and negative results provided by qPCR. However, two patients for whom specific gene amplification was found to be positive had no relapse after an average follow-up of 44 months (see Table 3). A possible explanation for this observation is that the vast majority of circulating tumor cells have been regarded as being rapidly destroyed in the bloodstream. 40,41 Moreover, cells expressing the gene markers under investigation could still be endowed with different metastasizing potential, possibly related to other mechanisms, including the expression of adhesion molecules or chemokine receptors. Furthermore, metastatic outgrowth capacity is known to be different in clones derived from the same cell line, and the genetic background of this differential behavior represents a field of active research. Clearly, the identification of surrogate markers of metastatic capacity will result in more focused evaluation of disseminated tumor cells. Circulating CK20 or CEA transcripts were not detected in a total of 35 samples from seven patients with benign abdominal disease or in blood samples from healthy donors. Amplification of these specific targets may thus be considered to indicate the presence of cancer cells in blood. This investigation suggests that qPCR is not only a highly sensitive but also a very specific diagnostic tool for disseminated colorectal cancer cells.
To our knowledge, this is the first study of real-time quantitative detection of disseminated colorectal cancer cells in blood and peritoneal lavage specimens. The vast majority of investigations analyzed single disseminated colorectal cancer cells in one anatomic compartment only. The present investigation suggests that identification of patients at high risk for recurrence after curative resection of colorectal cancer can be improved by analyzing peritoneal lavage specimens in addition to blood samples. This is based on the observation that in more than half of positive patients, disseminated colorectal cancer cells were detected in peritoneal lavage specimens but not in blood samples, and 71% of them had recurrence.
A frequently discussed issue is the presence of circulating neoplastic cells in association with the time point of sampling during surgery. Different series have reported intraoperative tumor cell dissemination 7,11,42–45 associated with different surgical procedures. In contrast, in this study disseminated tumor cells were found before the resection in 6 of 11 positive patients. This contradicts the hypothesis that surgical manipulation enhances tumor cell dissemination during curative resection of colorectal cancer.
The data from the present investigation suggest that detection of mRNA coding for CEA and/or CK20 using qPCR in patients undergoing curative resection for colorectal cancer has potential clinical utility as a prognostic marker. The presence of qPCR-detected disseminated colorectal cancer cells might be a useful indicator for the screening of patients at high risk for recurrence who would benefit from adjuvant chemotherapy. Furthermore, identification of patients at high risk for metastatic disease can be improved by analyzing peritoneal lavage specimens in addition to peripheral and mesenteric venous blood samples.
There is a need to evaluate specimens of peripheral and mesenteric venous blood, peritoneal lavage fluid, and bone marrow taken at different time points during colorectal cancer resection in larger patient populations. This might enable us to define the most representative specimen and time point of sampling for detection of disseminated colorectal cancer cells to identify patients at high risk of recurrence who would have the greatest potential benefit from adjuvant therapy.
Discussion
Prof. R. Margreiter: Since I didn’t have a chance to see your manuscript, I first have a technical question. Why did you use quantitative PCR if you just look for positivity or negativity and disregard the number of copies? I think the most crucial slide was the one at which time-point tumor cells were detected either in the lavage fluid or in the blood. If I got it correctly, you draw the samples before resection and after resection. It was my impression that most patients became positive after and were negative before resection. That would imply that surgery made them positive. If this would not be the case, you could even tell beforehand from the blood sample that this patient has a poor prognosis, and one would do only minimal surgery. But if this is only after surgery that they become positive, we have to think about how to avoid or control tumor cell spread.
Dr. W. R. Marti: About your first question, the technical question. It is correct that we are not actually using the quantification mode to define the numbers of mRNA copies. However, a clear advantage of quantitative real-time as compared with conventional PCR is represented by the possibility to establish clear cut-off reference values. The quantity of amplified DNA is continuously measured during qPCR, and all amplification curves can be displayed. Only a curve with a logarithmic increase of DNA amount is consistent with a specific gene amplification. In contrast, conventional PCR provides an end point analysis and does not allow the assessment of the amplification process. In the present investigation, nonlinear qPCR amplification curves were considered negative. These false-positive results would not have been identified and removed from the analysis using conventional PCR. A further advantage of qPCR over conventional PCR is represented by a higher specificity, relying not only on primers as for conventional PCR but also on internal probes hybridizing to the amplified sequences.
In 6 of 11 positive patients, mRNA-encoding tumor-associated genes could be detected before resection. This does not support the hypothesis that surgical manipulation markedly enhances tumor spread. In the present investigation 10 of 39 patients were qPCR positive in their lavage specimens and 4 patients were qPCR positive in their blood samples. Therefore, the majority of positivities would have been missed by checking blood samples only.
There are unfortunately no good data in the literature about how to avoid or influence the cell dissemination during or even before resection of colorectal cancer. In the context of disseminated colorectal cancer cells, this question needs to be addressed in a prospective setting investigating a much larger series of patients.
Prof. R. Margreiter: Since you find more tumor cells in lavage fluid as compared to the blood, you could even do a lavage beforehand through a peritoneal dialysis catheter, which should not be a major technical problem.
Dr. W. R. Marti: Yes, it would be feasible. However, in 10 of 11 positive patients, we found mRNA of tumor-associated genes CEA or CK20 in the peritoneal cavity, but only 6 of these patients were positive before resection. Therefore, it would not be advisable to rely on preresectional lavage only.
Sir P. Morris: I will only ask one question, and that is would you consider doing portal vein sampling during the resection? Studies like that have been done spasmodically looking for tumor cells or bacteria. Certainly in both cases one can demonstrate tumor cells or bacteria during the resection, even with the inferior mesenteric vein clamped, and it would be quite interesting with this far more sophisticated technique to look at samples within the portal vein during the resection. I suspect the results may be quite high. It is interesting that you are getting positivity in the peripheral blood, where obviously the cells have to get through the liver in the first instance.
Dr. W. R. Marti: Central portal vein blood sampling is feasible. In our study we tended to avoid any supplementary preparation for fear of additional morbidity. Therefore, we sampled blood from the tumor-draining mesenteric vein. However, we were surprised to find there only in 1 of 10 positive patients mRNA of tumor-associated genes. We too expected to find more frequent evidence of disseminated tumor cells there. Nevertheless, this finding may reflect a lack of knowledge in the sense that currently, neither the ideal time point of sampling, nor the ideal body compartment (blood or peritoneal lavage), nor the ideal amount of tissue to be analyzed for disseminated colorectal cancer cells is known.
Sir P. Morris: I understood that is before resection, what is the actual timing procedure that samples taken from the mesenteric blood?
Dr. W. R. Marti: Yes, we used the “no touch” technique. The central ligation of the tumor-draining vessel was done before mobilization of the gut itself.
Sir P. Morris: That is why I am saying, portal vein or mesenteric vein sampling during the resection?
Dr. W. R. Marti: Maybe sampling of portal venous blood after resection may lead to a higher frequency of detection of mRNA of tumor-associated genes. This has not been investigated in this study.
Prof. P. Neuhaus: I am aware of some studies that involved T1 and T2 tumors, where also cytokeratin-positive cells in the bone marrow have been detected at the time of operation. Could you put these findings into context with your personal findings? Have all your tumors been T3 tumors, and have most of them been as I saw with positive lymph nodes? Or do you also have results in early T1 and T2 tumors?
Dr. W.R. Marti: We included patients with stage I to III in our study. However, only 2 of 18 patients in stage I or II were positive. Therefore, the sample size is too small to draw conclusions for early-stage patients. To my knowledge we are the first group presenting data applying the novel technique of quantitative real-time PCR detection of mRNA from CEA and CK20 markers in colorectal cancer patients together with long-term follow-up data (mean follow-up 31 months). It is correct that other investigators applying cytology, immunohistochemistry, or classical PCR found evidence of disseminated colorectal cancer cells in the bone marrow, which might have a clinical relevance too. However, there are too many variables as compared to our study such as different techniques applied, search for cancer cells in bone marrow, to allow a proper comparison to our own results.
Prof. P. Neuhaus: In clinics the cytokeratin immunostaining is a widely used method. Is your real-time RT-PCR technique now the method of choice, and does it really give more reliable results?
Dr. W. R. Marti: There are different techniques to detect disseminated tumor cells. Originally, historically it is cytology only, and then combined with immunocytology a very specific method with limited sensitivity. Then the technique of classic RT-PCR became available, which has a higher sensitivity at the price of low specificity. There is a remarkable risk of false-positive results. According to the literature up to more than 20% are false-positive, even in recent literature. The latest technique now is the real-time quantitative RT-PCR where only few results are published correlating the results of tumor antigen detection with the follow-up of patients. However our data suggest that real-time quantitative RT-PCR is a reliable method for antigen detection as indirect evidence for disseminated tumor tumor cells. It has the advantage of an excellent specificity and a high sensitivity similar to the classic RT-PCR.
Dr. R. Poon: I have two questions for you. One question is on the quantitation of disseminated cancer cells by RT-PCR. In this study you use a definition of positive or negative result based on detection of CEA and CK20 transcripts following 40 PCR cycles, but other studies have shown that the actual quantitative level of the transcripts by real-time quantitative RT-PCR has a significance in determining whether the patient is likely to have recurrence. Do you think you should use the actual real-time quantitative RT-PCR levels to further refine the power of the detection of disseminated cancer cells and prediction of recurrence?
The second question is whether the CEA and CK20 transcripts are detected before or after resection of the tumors. In certain cancers, like liver cancer, patients may have circulating cancer cells detected after resection rather than before resection, and this is important in determining the risk of recurrence. This can occur because manipulation of the cancers can lead to dissemination of cancer cells during the operation. I wonder if you have measured the transcripts in the draining mesenteric vein samples before manipulation of the tumor, and then compared it with the levels after mobilization of the tumor, before clamping of the vein, and maybe even afterwards. This may give us a better understanding of the mechanism of dissemination of cancer cells and its prognostic implication in these patients. Thank you.
Dr. W. R. Marti: It is somewhat simplified to say quantitative real-time PCR is very specific and sensitive. In fact, it depends on several variables. The design of the probe and the design of the primer are very crucial. Not everybody has the same design of probe, even if you look for the same gene transcripts CK20 or CEA. Therefore, whatever you use, you have to define the cut-off point or your level, considering negative or positive, in your own lab, depending on how you decide on primers and probes. So, we designed it on our level according to our material.
Now the different time point for looking for circulating cells: that is still open and a matter of debate, only focused on the perioperative time point. Indeed, if it is possible to start the search previous to the intervention, during the intervention, and even after (and there are hints in the literature that maybe after the intervention), the most cells are circulated. Therefore, we should expand the time points in the future to further prolonged after the intervention, even in the first or second postoperative day.
Footnotes
Supported by the Regional Cancer Leagues of Glarus, Schaffhausen and Zurich, Switzerland.
Correspondence: Walter Richard Marti, MD, Department of Surgery, University of Basel, Spitalstrasse 21, CH-4031 Basel, Switzerland.
E-mail: wrmarti@uhbs.ch
Accepted for publication April 2002.
References
- 1.Boring CC, Squires TS, Tong T. Cancer statistics, 1993. CA Cancer J Clin 1993; 43: 7–26. [DOI] [PubMed] [Google Scholar]
- 2.Safi F, Beyer HG. The value of follow-up after curative surgery of colorectal carcinoma. Cancer Detect Prev 1993; 17: 417–424. [PubMed] [Google Scholar]
- 3.Schott A, Vogel I, Krueger U, et al. Isolated tumor cells are frequently detectable in the peritoneal cavity of gastric and colorectal cancer patients and serve as a new prognostic marker. Ann Surg 1998; 227: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liefers GJ, Cleton-Jansen AM, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med 1998; 339: 223–228. [DOI] [PubMed] [Google Scholar]
- 5.Soeth E, Roder C, Juhl H, et al. The detection of disseminated tumor cells in bone marrow from colorectal-cancer patients by a cytokeratin-20-specific nested reverse-transcriptase-polymerase-chain reaction is related to the stage of disease. Int J Cancer 1996; 69: 278–282. [DOI] [PubMed] [Google Scholar]
- 6.Soeth E, Vogel I, Roder C, et al. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res 1997; 57: 3106–3110. [PubMed] [Google Scholar]
- 7.Mori M, Mimori K, Ueo H, et al. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer 1996; 68: 739–743. [DOI] [PubMed] [Google Scholar]
- 8.Wyld DK, Selby P, Perren TJ, et al. Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer 1998; 79: 288–293. [DOI] [PubMed] [Google Scholar]
- 9.Castells A, Boix L, Bessa X, et al. Detection of colonic cells in peripheral blood of colorectal cancer patients by means of reverse transcriptase and polymerase chain reaction. Br J Cancer 1998; 78: 1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamori S, Kameyama M, Furukawa H, et al. Genetic detection of colorectal cancer cells in circulation and lymph nodes. Dis Colon Rectum 1997; 40 (10 Suppl): S29–36. [DOI] [PubMed] [Google Scholar]
- 11.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res 1998; 4: 343–348. [PubMed] [Google Scholar]
- 12.Cox D. Regression models and life tables. J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- 13.Desch CE, Benson AB, 3rd, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol 1999; 17: 1312. [DOI] [PubMed] [Google Scholar]
- 14.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997; 112: 594–642. [DOI] [PubMed] [Google Scholar]
- 15.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med 1994; 330: 1136–1142. [DOI] [PubMed] [Google Scholar]
- 16.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet 2000; 355: 395–399. [DOI] [PubMed] [Google Scholar]
- 17.Leather AJ, Gallegos NC, Kocjan G, et al. Detection and enumeration of circulating tumour cells in colorectal cancer. Br J Surg 1993; 80: 777–780. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld A, Kruger KH, Gomm J, et al. The detection of micrometastases in the peripheral blood and bone marrow of patients with breast cancer using immunohistochemistry and reverse transcriptase polymerase chain reaction for keratin 19. Eur J Cancer 1997; 33: 854–861. [DOI] [PubMed] [Google Scholar]
- 19.Leinung S, Wurl P, Weiss CL, et al. Cytokeratin-positive cells in bone marrow in comparison with other prognostic factors in colon carcinoma. Langenbecks Arch Surg 2000; 385: 337–343. [DOI] [PubMed] [Google Scholar]
- 20.Broll R, Lembcke K, Stock C, et al. [Tumor cell dissemination in bone marrow and peritoneal cavity. An immunocytochemical study of patients with stomach or colorectal carcinoma]. Langenbecks Arch Chir 1996; 381: 51–58. [DOI] [PubMed] [Google Scholar]
- 21.Burchill SA, Bradbury MF, Pittman K, et al. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer 1995; 71: 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zach O, Kasparu H, Krieger O, et al. Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via a nested reverse transcriptase polymerase chain reaction assay for mammaglobin mRNA. J Clin Oncol 1999; 17: 2015–2019. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Takagi Y, Aoki S, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 2000; 232: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita S, Sugano K, Fukayama N, et al. Detection of K-ras point mutations in mesenteric venous blood from colorectal cancer patients by enriched polymerase chain reaction and single-strand conformation polymorphism analysis. Jpn J Clin Oncol 1996; 26: 417–421. [DOI] [PubMed] [Google Scholar]
- 25.Masuda N, Tamaki Y, Sakita I, et al. Clinical significance of micrometastases in axillary lymph nodes assessed by reverse transcription-polymerase chain reaction in breast cancer patients. Clin Cancer Res 2000; 6: 4176–4185. [PubMed] [Google Scholar]
- 26.Schoenfeld A, Luqmani Y, Smith D, et al. Detection of breast cancer micrometastases in axillary lymph nodes by using polymerase chain reaction. Cancer Res 1994; 54: 2986–2990. [PubMed] [Google Scholar]
- 27.Mori M, Mimori K, Ueo H, et al. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription-polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 1998; 16: 128–132. [DOI] [PubMed] [Google Scholar]
- 28.Vannucchi AM, Bosi A, Glinz S, et al. Evaluation of breast tumour cell contamination in the bone marrow and leukapheresis collections by RT-PCR for cytokeratin-19 mRNA. Br J Haematol 1998; 103: 610–617. [DOI] [PubMed] [Google Scholar]
- 29.Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 1994; 12: 725–729. [DOI] [PubMed] [Google Scholar]
- 30.Funaki NO, Tanaka J, Ohshio G, et al. Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 1998; 77: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bessa X, Elizalde JI, Boix L, et al. Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology 2001; 120: 1084–1092. [DOI] [PubMed] [Google Scholar]
- 32.Futamura M, Takagi Y, Koumura H, et al. Spread of colorectal cancer micrometastases in regional lymph nodes by reverse transcriptase-polymerase chain reactions for carcinoembryonic antigen and cytokeratin 20. J Surg Oncol 1998; 68: 34–40. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi S, Aihara T, Nakamori S, et al. The detection of breast carcinoma micrometastases in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Cancer 1994; 74: 1595–1600. [DOI] [PubMed] [Google Scholar]
- 34.Neumaier M, Gerhard M, Wagener C. Diagnosis of micrometastases by the amplification of tissue-specific genes. Gene 1995; 159: 43–47. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi S, Aihara T, Motomura K, et al. Histologic characteristics of breast cancers with occult lymph node metastases detected by keratin 19 mRNA reverse transcriptase-polymerase chain reaction. Cancer 1996; 78: 1235–1240. [DOI] [PubMed] [Google Scholar]
- 36.Funaki NO, Tanaka J, Itami A, et al. Detection of colorectal carcinoma cells in circulating peripheral blood by reverse transcription-polymerase chain reaction targeting cytokeratin-20 mRNA. Life Sci 1997; 60: 643–652. [DOI] [PubMed] [Google Scholar]
- 37.Jonas S, Windeatt S, O-Boateng A, et al. Identification of carcinoembryonic antigen-producing cells circulating in the blood of patients with colorectal carcinoma by reverse transcriptase polymerase chain reaction. Gut 1996; 39: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko Y, Klinz M, Totzke G, et al. Limitations of the reverse transcription-polymerase chain reaction method for the detection of carcinoembryonic antigen-positive tumor cells in peripheral blood. Clin Cancer Res 1998; 4: 2141–2146. [PubMed] [Google Scholar]
- 39.Patel H, Le Marer N, Wharton RQ, et al. Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg 2002; 235: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss L. Metastatic inefficiency. Adv Cancer Res 1990; 54: 159–211. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ. The biology of human cancer metastasis. Acta Oncol 1991; 30: 668–675. [DOI] [PubMed] [Google Scholar]
- 42.Nishizaki T, Matsumata T, Kanematsu T, et al. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res 1990; 49: 92–97. [DOI] [PubMed] [Google Scholar]
- 43.Eschwege P, Dumas F, Blanchet P, et al. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995; 346: 1528–1530. [DOI] [PubMed] [Google Scholar]
- 44.Brown DC, Purushotham AD, Birnie GD, et al. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery 1995; 117: 95–101. [DOI] [PubMed] [Google Scholar]
- 45.Weitz J, Koch M, Kienle P, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg 2000; 232: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]