Abstract
Objective
To evaluate MAGE tumor-associated antigen (TAA) expression in an extensive panel of normal and neoplastic tissues.
Summary Background Data
TAAs of the MAGE family represent targets of active specific immunotherapy. Limited-size studies indicate that they are expressed in normal testis and tumors of different histologies. High-throughput tissue microarray (TMA) technology and MAGE TAA-specific monoclonal antibodies now allow us to comprehensively evaluate their expression in large numbers of tissues and to address clinical correlations.
Methods
A TMA containing 3,520 samples from 197 different tissues and a non-small-cell lung cancer TMA including 301 specimens were stained using the MAGE TAA-specific monoclonal antibody 57B. For patients with squamous cell carcinoma of the lung, the dichotomous result (positive vs. negative) of MAGE TAA staining was used as a predictor variable along with other covariates in proportional hazard regression analysis of tumor-specific survival.
Results
MAGE TAAs are expressed with frequencies ranging between 22.7% (larynx) and 50% of cases (lung) in squamous cell carcinomas from different anatomic areas and in large cell carcinomas of the lung (37.9%). The authors provide here the first description of MAGE TAA expression in basalioma (48.1%). To investigate the clinical significance of MAGE expression in a frequently positive tumor type, a non-small-cell lung cancer, TMA was then studied. In this TMA 43.2% of tumors were 57B positive. In patients with squamous cell carcinoma, MAGE TAA positivity was significantly correlated with a shorter tumor-specific survival in the proportional hazard regression analysis model.
Conclusions
These data suggest novel potential therapeutic indications in different types of cancers. In lung squamous cell carcinoma, the significant association of MAGE TAA expression with poor prognosis suggests that patients with 57B-positive tumors may benefit from early, specific immunotherapy procedures.
Tumor-associated antigens (TAAs) of the Melanoma antigen E (MAGE) family were the first described in humans. 1 They belong to the so-called cancer/testis TAA subclass encoded by genes expressed in tumors of unrelated histologic origin and in a restricted number of healthy tissues. 2,3 Several epitopes derived from these TAAs and recognized by HLA class I restricted cytotoxic T cells have been identified. 1,4–6 Ongoing clinical trials suggest that specific immunization procedures could lead to clinically effective antitumor immune responses. 7,8
A small number of monoclonal antibodies (mAbs) specific for MAGE TAA gene products have been generated and used to confirm polymerase chain reaction data and to assess the extent of their expression in neoplastic cells from clinical samples. 2,9,10 Interestingly, they were also instrumental in demonstrating MAGE TAA in cancer cells such as Reed-Sternberg cells where unequivocal polymerase chain reaction data could not be generated. 11
Tissue microarray technology (TMA) takes advantage of tissue cylinders (diameter 0.6 mm) derived from hundreds of different primary tumor blocks and subsequently brought into one empty “recipient” paraffin block. Sections from such array blocks can then be used for simultaneous analysis of hundreds or thousands of tumors at the DNA, RNA, or protein level. Most importantly, specific TMA databases including relevant clinical information related to individual specimens have also been produced. Thus, TMAs offer the unique opportunity to combine large sets of pathologic and clinical data in an attempt to evaluate the potential significance of the expression of specific markers. 12
In this study we used a multitumor TMA containing 3,520 samples from 197 different tissues to comprehensively evaluate MAGE TAA expression as detectable by immunohistochemistry, taking advantage of a specific mAb. Furthermore, a non-small-cell lung cancer (NSCLC) TMA was used to address the prevalence of the expression of these TAAs and their correlation with histologic and clinical data in these tumors.
We report here that the exquisite tumor specificity of MAGE TAA expression is correlated with unfavorable prognosis in NSCLC.
METHODS
Monoclonal Antibody
Monoclonal antibody 57B was generated using as immunogen recombinant MAGE-A3 protein. 13 Immunohistochemical studies, carried out in different laboratories, have emphasized that 57B identifies multiple MAGE gene products in transfected cells and prevailingly recognizes MAGE-A4 in paraffin-embedded sections. 9,10
Immunohistochemistry
Formalin-fixed paraffin-embedded tumor arrays (see below) were processed for immunohistochemistry according to standard methods. 14 Briefly, following deparaffinization, sections were heated in a microwave oven (30 minutes at 90°C) in citrate buffer for antigen retrieval. Then, they were washed in phosphate-buffered saline for 10 minutes and incubated overnight at 4°C in the presence of mAb 57B in the form of 1:100 diluted hybridoma supernatant or control reagents. Bound antibodies were visualized by using the avidin-biotin complex method according to the recommendations of the supplier (Vectastatin Elite ABC Kit; Vector Laboratories Inc., Burlingame, CA). Diaminobenzidine was used as chromogen. 57B staining was classified as follows: no staining; “weak,” indicating low-intensity staining regardless of positive cell percentages or medium intensity staining of no more than 20% of cells; “moderate,” indicating medium-intensity staining of more than 20% of cells or high-intensity staining of no more than 20% of cells; “strong,” indicating high-intensity staining of more than 20% of cells. Only moderately and strongly positive cases were considered positive. Examples of negative and positive cases from the multitissue TMA (see below) are shown in Figure 1.
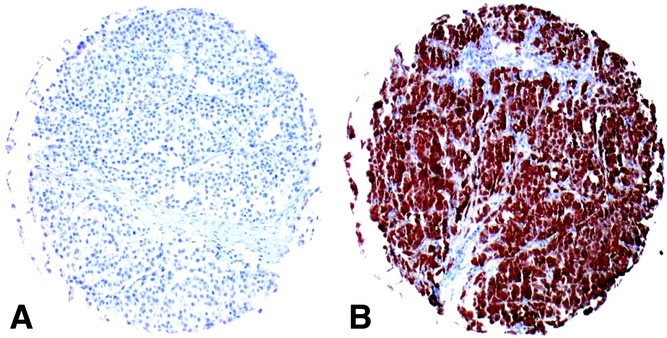
Figure 1. Examples of positive and negative tumors. Single punches from melanoma specimens included in the multitissue TMA. Shown are cases negative (a) and strongly positive (b) for 57B monoclonal antibody staining.
Tissue Microarrays and Clinical Follow-Up Data
The TMA construction has been described in detail elsewhere. 15,16 Formalin-fixed and paraffin-embedded tissues were obtained from the archives of the Institute of Pathology at the University of Basel. Two different types of TMAs were used in this study. The first TMA comprised 3,520 samples from normal (n = 253 from 38 different tissues) and tumoral (n = 3,267 from 159 different tumor types) tissues (Table 1). The second TMA consisted of 301 NSCLC samples from 297 patients. For this TMA, tumor stage and grade were defined according to International Union Against Cancer and World Health Organization classifications. 17 For a limited number of specimens (24 and 2, respectively) stage and grade could not be unequivocally established. Regarding clinical end points in squamous cell carcinoma, for patients having more than one tumor included in the array, only the first biopsies were used for further statistical analyses. Only patients with tumor-related causes of death were included in survival analysis. Average follow-up was 40 months (range 1–175).
Table 1. MULTITISSUE TMA: MAGE A4 EXPRESSION IN NORMAL AND NEOPLASTIC TISSUES AS DETECTABLE BY 57B STAINING
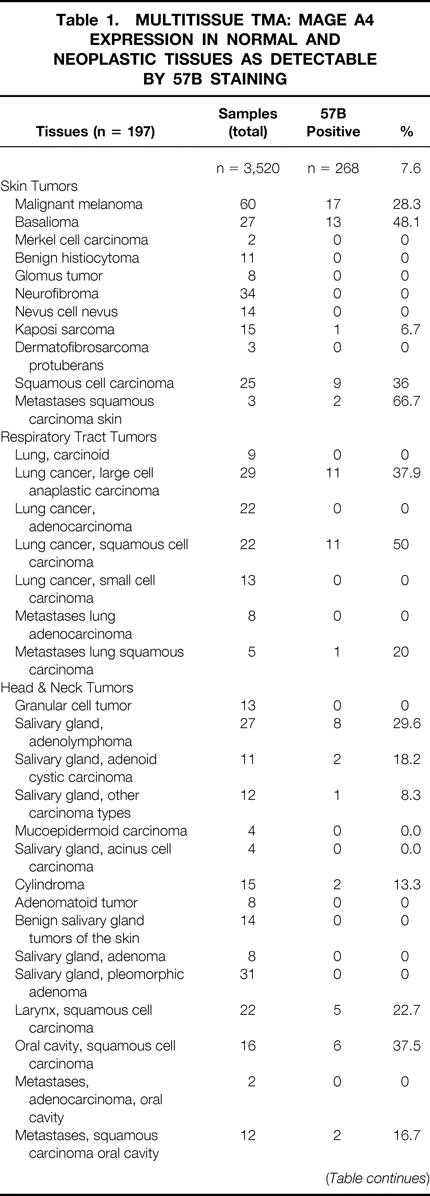
Table 1. Continued
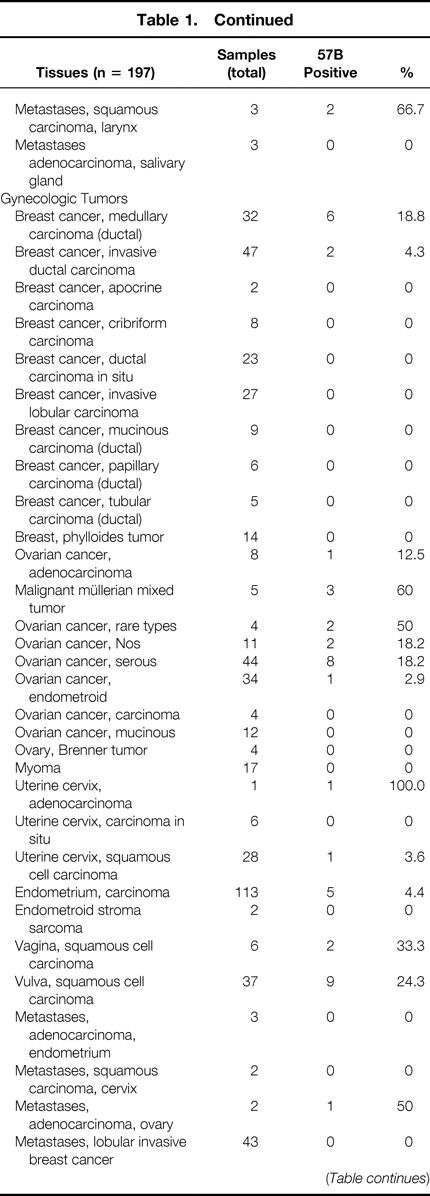
Table 1. Continued

Table 1. Continued
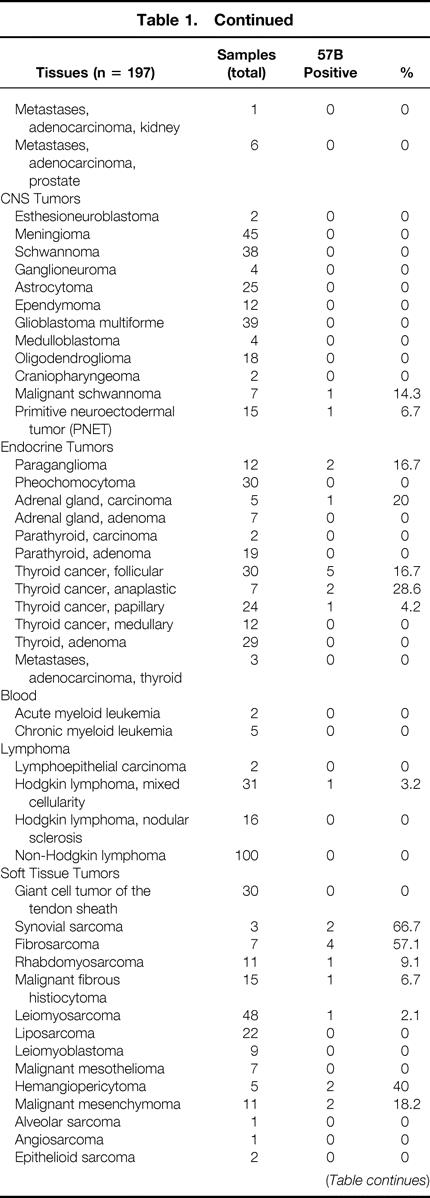
Table 1. Continued

Statistical Analysis
Contingency table analysis and chi-square tests were used to study the relationship between grade, stage, and MAGE TAA expression.
The proportional hazard regression (PHR) 18 procedure of the Statistical Analysis System (SAS, Cary, NC) was used for univariate and multivariate analyses. The outcome variable was tumor-specific survival. The primary covariate (explanatory variable) of interest was the dichotomous result (positive/negative) from 57B staining. Additional covariates available for analysis included lymph node status (positive vs. negative), histologic grade (well-differentiated vs. undifferentiated), tumor stage (pT1, pT2, pT3, pT4), and metastases (present vs. absent). PHR models of each covariate were evaluated separately as a preliminary analysis (single-variable analysis). PHR models of all covariates were successively reduced by removing the least significant covariate with a significance level greater than 0.05 until all remaining covariates had significance levels of 0.05 or less. Models with interaction terms were then computed. Estimated distributions of time-to-event data are displayed as Kaplan-Meier plots. 19
RESULTS
Multitissue Array
Of the 3,520 specimens contained in the multitissue array (see Table 1), 253 were derived from healthy tissues. Eleven of these samples showed evidence of 57B staining. As expected, all seven testis specimens were strongly positive. One of nine placental tissues also scored strongly positive. Unexpected positivities (n = 3) were also occasionally detectable in fetal adrenal glands (1/5), vagina (1/6), and parathyroid tissue (1/6).
Regarding neoplastic tissues, 257 of 3,267 (7.9%) were 57B positive. For 49 different tumor types, representative numbers of cases (n > 20) were available within the array. Ten of these entities showed evidence of MAGE TAA expression in more than 20% of cases. This group included melanoma (28.3%), seminoma (28.6%), and squamous cell carcinomas of different histologic origin (skin 36%; vulva 24.3%; larynx 22.7%). Similar percentages of 57B-positive tumors were detectable among large cell anaplastic lung carcinomas (37.9%), salivary gland adenolymphomas (29.6%), and invasive (pT2–4) transitional cell carcinomas of the urinary bladder (25.8%). The highest percentages of positive cases were found in basaliomas (48.1%) and, in keeping with previous data, 20 in squamous cell carcinomas of the lung (50%).
NSCLC Tumor Array
Prompted by the high frequency of MAGE TAA expression in lung cancers, we analyzed a specific NSCLC TMA including information on clinical end points. This TMA comprised 301 cases (SCC, n = 178; adenocarcinoma, n = 65; large cell, n = 38; bronchoalveolar carcinoma, n = 10; neuroendocrine tumors, n = 4; other tumors, n = 6). This series included 76 well- or moderately differentiated low-grade (G1) cases and 223 poorly differentiated, high-grade (G2) cases.
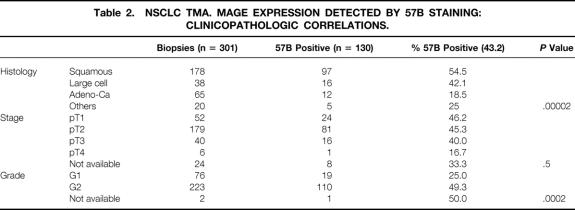
In 130 of 301 (43.2%) NSCLC biopsies, MAGE gene products were detectable by 57B mAB. According to histologic classification, 97 of 178 (54.5%) squamous cell carcinomas and 16 of 38 (42.1%) large cell carcinomas, but only 12 of 65 (18.5%) adenocarcinomas, were 57B positive (P < .00002). Among positive squamous cell carcinomas (n = 97), 87 were strongly positive and 10 were moderately positive. Among large cell carcinomas (n = 16), 14 were strongly positive and 2 were moderately positive. All positive adenocarcinomas were strongly positive. The relationships between overall MAGE positivity and tumor histology, staging, and grading were explored in detail (Table 2). 57B staining did not appear to significantly correlate with tumor stage. However, a clear correlation between MAGE expression and tumor grade was observed.
Table 2. NSCLC TMA. MAGE EXPRESSION DETECTED BY 57B STAINING: CLINICOPATHOLOGIC CORRELATIONS.
Prognostic Relevance of MAGE Expression in Lung Squamous Cell Carcinoma
The high incidence of positive 57B staining in lung squamous cell carcinoma and the presence of a substantial number of cases in the NSCLC TMA led us to investigate relationships with defined end points.
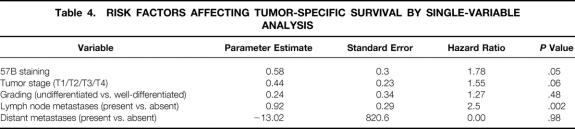
Histopathologicparameters and follow-up data were available for 153 of 178 patients (Table 3). Table 4 shows the results of PHR models of each covariate separately tested on tumor-specific survival. Only the covariates “lymph node metastases” and “57B staining” were significant in this model.
Table 3. SQUAMOUS CELL CARCINOMA PATIENTS: CLINICOPATHOLOGIC CHARACTERISTICS (N = 153)
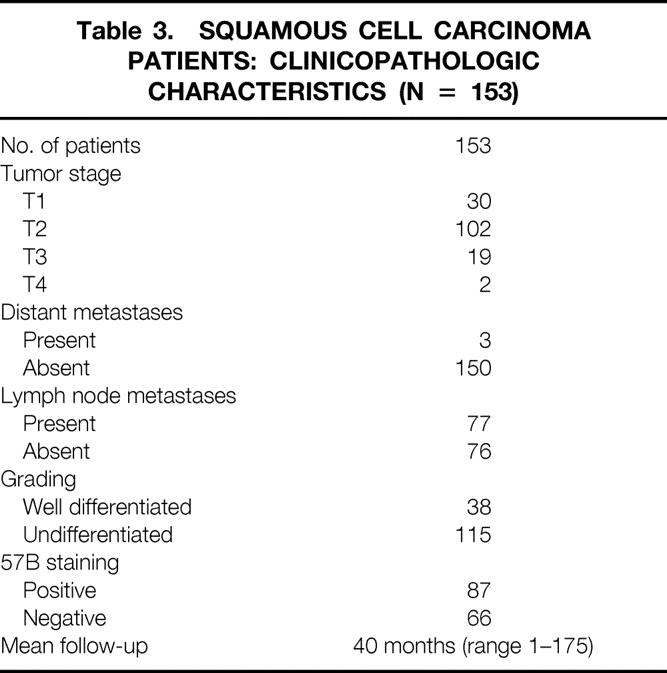
Table 4. RISK FACTORS AFFECTING TUMOR-SPECIFIC SURVIVAL BY SINGLE-VARIABLE ANALYSIS
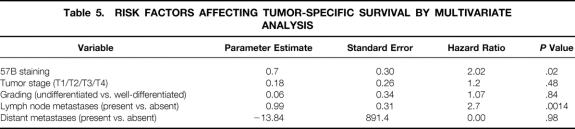
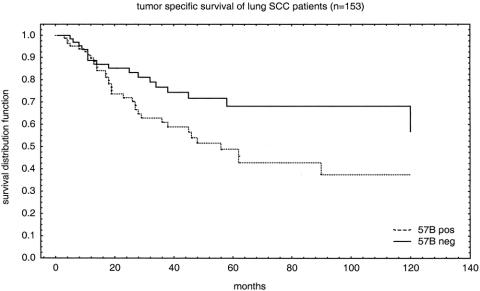
PHR models with all covariates (Table 5) and then with successively fewer covariates were computed as described in the Methods section. Only “lymph node status” and “MAGE TAA staining” remained significant following the stepdown procedure (Table 6). Interaction terms did not reach the threshold of statistical significance (data not shown). The Kaplan-Meier plot (Fig. 2) shows tumor-specific survival by MAGE TAA status (positive vs. negative).
Table 5. RISK FACTORS AFFECTING TUMOR-SPECIFIC SURVIVAL BY MULTIVARIATE ANALYSIS
Table 6. FINAL MODEL OF MULTIVARIATE ANALYSIS INCLUDING ONLY COVARIATES BELOW P = .05
Figure 2. MAGE TAA immunostaining and prognosis. The association between 57B staining and tumor-specific survival is shown for all squamous cell carcinomas included in the non-small-cell lung cancer TMA. Follow-up data were available for 153 patients.
DISCUSSION
TAAs of the MAGE gene family are of particular interest in tumor immunology since they encompass a relatively large number of antigenic epitopes recognized by specific T cells 1,4,6 and are expressed in different cancers but only in a limited range of nontransformed cells. 2,3 Remarkably, some of them are already used in the context of dedicated clinical trials. 7,8,21 57B mAb, produced by our group, has been widely used to address MAGE TAA expression in different tumor types. 2,9–11,13
TMA technology permits fast and accurate screening of large numbers of samples for the expression of discrete markers at the gene or protein level. 12,15,16,22 Most importantly, TMA data can be evaluated in combination with databases containing clinical information related to individual specimens.
In this study we took advantage of TMA technology and 57B mAb to screen a large series of tissues (>3,000) for MAGE TAA expression to identify pathologies of peculiar interest. For one of them, lung squamous cell carcinoma, the availability of a specific array also permitted us to address clinical end points as related to 57B positivity.
Samples from 38 different normal tissues (n = 253) were included in the array. In these specimens, 57B positivity was observed in testis 1,2 and placenta, 23 as expected. In agreement with a previous report, 2 positive staining was also detectable in one adrenal gland specimen, possibly due to fixation artifacts, and in one normal parathyroid and one normal vagina tissue sample. We have no obvious explanation for these unexpected findings.
More than 20 specimens were available in the TMA under investigation for 49 different neoplastic entities. For 10 of them, the incidence of 57B positivity exceeded 20%. Data on seminomas, melanomas, and transitional bladder carcinomas are largely in agreement with previous reports. 24–26 Highly frequent positive stainings (37.9%) were also observed in large cell anaplastic carcinomas of the lung. Remarkably, a high frequency of MAGE TAA expression was observed in basaliomas (13/27 [48.1%]). To our knowledge, expression of cancer/testis antigens has never been reported before in these low-malignancy tumors. Additional polymerase chain reaction studies are needed to extend these findings. Data on 57B staining in adenolymphomas of the salivary glands (8/27 [29.6%]) should be evaluated cautiously, considering that these tumors are rich in cells expressing high amounts of Fc receptors.
Of particular interest is the frequent expression of MAGE TAA in squamous cell carcinomas of different histologic origin, with positivities ranging between 22.7% (larynx) and 50% (lung). These data suggest the possibility of common mechanisms in squamous cell oncogenesis and of common immunotherapeutic approaches.
The multitumor TMA data led us to address correlations between MAGE TAA expression and the clinical course of discrete neoplastic diseases. 57B positivities were highly frequent in NSCLC and usually involved more than 20% of the tumor cells present in individual specimens. Considering their epidemiologic relevance and the availability of a lung cancer-specific TMA including a clinical database, we expanded our investigation for this tumor type.
This second analysis confirmed that among NSCLCs, expression of MAGE TAA gene products is most frequently detectable in squamous cell carcinoma and large cell carcinomas, and it is significantly associated with a higher histologic grade. An interesting and potentially relevant finding of this study is the detection of a significant (P = .02) impact of MAGE TAA expression on tumor-specific survival for squamous cell carcinoma patients in the PHR analyses. Future prospective studies are warranted to confirm the findings of this investigation.
The physiologic role of MAGE gene products is unknown. However, activation of MAGE genes has been reported in early carcinogenesis of the lung. 27 Remarkably, it has been demonstrated that DNA demethylating agents induce the expression of MAGE genes. 28,29 Future studies should further address in clinical materials the relationship between MAGE TAA expression and DNA demethylation, potentially leading to the transcription of normally silent oncogenes and tumor progression.
Alternatively, our data may suggest that patients bearing MAGE-positive tumors could take advantage of specific vaccination protocols in early phases of treatment, possibly in disease-free conditions. Notably, MAGE A1, A2, A3, A4, A6, and A12 genes are frequently expressed in clusters, 3,23 and no fewer than 20 different epitopes derived from these TAAs and recognized by cytotoxic T cells within discrete HLA restrictions have been identified. 6
Finally, this study underlines the high effectiveness of TMA technology in the collection of molecular data from large sets of well-characterized tumors. 57B staining could be rapidly evaluated in thousands of tumors, and strong associations could be readily determined. The TMA format appears to be an effective way to test sets of molecular markers in large numbers of tissue specimens, thus facilitating translational cancer research.
Discussion
Prof. M. W. Büchler: In relation with previous papers, do you think that immunohistochemistry alone, without any other method such as Western blotting or Northern blotting or PCR, is enough in the setting you investigated your patients? Would you not think that you should add another method such as Western blotting to support your data?
Dr. M. Bolli: In the past we have studied by RT-PCR and Western blots the expression of MAGE genes and proteins in limited patient series. Indeed, regarding NSCLC we observed analogous frequencies of positive cases. Furthermore, in the literature there are data from other groups confirming the high frequency of MAGE expression in these tumors. On the other hand, it is difficult to apply RT-PCR technology to large numbers of paraffin-embedded specimens.
Prof. J. M. Mendes de Almeida: In your conclusion you tell us that early specific immunotherapy directed against these epitopes may be good. But regarding your abstract and your slides, you do not have any data or reference that permit these words. Why do you come to this conclusion?
Dr. M. Bolli: MAGE proteins are known to encompass several epitopes recognized by cytotoxic T cells and can therefore serve as targets for specific immunotherapy. Furthermore, in this context, they are particularly attractive since they are expressed almost exclusively in neoplastic cells.
Prof. G. C. O’Sullivan: There is currently considerable interest in MAGE expression in tumors. Do you see uniform staining within these tumors? This is important when considering prognostic significance, as we need to be sure that the negatively stained regions behave similarly to the positively stained components of the same tumor. Do you have any information about MAGE expression in metastases? It would have been better if you had given us multiple regression analysis examining the influence of disease grade, stage, age, and gender variables as well as MAGE expression. This would allow you to consider the contribution of each variable to prognosis.
Dr. M. Bolli: What do you mean with contribution to the variants?
Prof. G. C. O’Sullivan: The question was, if you have a tumor with marked MAGE expression in one area and no evidence of it in other areas, what does this mean to you? If you see MAGE expression at all, does the significance differ for the degree of expression or uniformity of expression?
Dr. M. Bolli: Yes, indeed, focal expression of MAGE tumor-associated antigens has been reported. However, in the current NSCLC series, a large majority of positive cases were “strongly” positive (high-intensity staining of >20% of tumor cells). Clearly, given the small size of sections, tissue microarray technology is not adequate for thorough investigations of tumor heterogeneity. Regarding your second question, we have included stage, grade, histology, and presence or absence of metastases in our multivariate regression analysis. Future tissue arrays will obviously have to be accompanied by larger clinical databases.
Footnotes
Partially supported by the Swiss National Fund for Scientific Research (grant no. 31-57473.99 to G.C.S.).
Correspondence: Martin Bolli, MD, Department of Surgery, Division of Research, University Hospital Basel, ZLF, Lab. 401, Hebelstrasse 20, 4031 Basel, Switzerland.
E-mail: mbolli@uhbs.ch
Accepted for publication April 2002.
References
- 1.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–1647. [DOI] [PubMed] [Google Scholar]
- 2.Jungbluth AA, Busam KJ, Kolb D, et al. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer 2000; 85: 460–465. [PubMed] [Google Scholar]
- 3.Chomez P, De Backer O, Bertrand M, et al. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 2001; 61: 5544–5551. [PubMed] [Google Scholar]
- 4.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med 1996; 183: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffour MT, Chaux P, Lurquin C, et al. A MAGE-A4 peptide presented by HLA-A2 is recognized by cytolytic T lymphocytes. Eur J Immunol 1999; 29: 3329–3337. [DOI] [PubMed] [Google Scholar]
- 6.Renkvist N, Castelli C, Robbins PF, et al. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother 2001; 50: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer 1999; 80: 219–230. [DOI] [PubMed] [Google Scholar]
- 8.Schuler-Thurner B, Dieckmann D, Keikavoussi P, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol 2000; 165: 3492–3496. [DOI] [PubMed] [Google Scholar]
- 9.Landry C, Brasseur F, Spagnoli GC, et al. Monoclonal antibody 57B stains tumor tissues that express gene MAGE-A4. Int J Cancer 2000; 86: 835–841. [DOI] [PubMed] [Google Scholar]
- 10.van Baren N, Brasseur F, Godelaine D, et al. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood 1999; 94: 1156–1164. [PubMed] [Google Scholar]
- 11.Chambost H, Van Baren N, Brasseur F, et al. Expression of gene MAGE-A4 in Reed-Sternberg cells. Blood 2000; 95: 3530–3533. [PubMed] [Google Scholar]
- 12.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4: 844–847. [DOI] [PubMed] [Google Scholar]
- 13.Kocher T, Schultz-Thater E, Gudat F, et al. Identification and intracellular location of MAGE-3 gene product. Cancer Res 1995; 55: 2236–2239. [PubMed] [Google Scholar]
- 14.Gudat F, Zuber M, Durmuller U, et al. The tumour-associated antigen MAGE-1 is detectable in formalin-fixed paraffin sections of malignant melanoma. Virchows Arch 1996; 429: 77–81. [DOI] [PubMed] [Google Scholar]
- 15.Richter J, Wagner U, Kononen J, et al. High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol 2000; 157: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallioniemi O, Wagner U, Kononen J, et al. Tissue microarray technology for high throughput molecular profiling of cancer. Hum Mol Genet 2001; 10: 657–662. [DOI] [PubMed] [Google Scholar]
- 17.Sobin LH, Wittekind C, eds. IUCC TNM Classification of Malignant Tumors, 6th ed. New York: John Wiley & Sons, 2002.
- 18.Cox D. Regression models and life tables. J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 20.Fischer C, Gudat F, Stulz P, et al. High expression of MAGE-3 protein in squamous-cell lung carcinoma. Int J Cancer 1997; 71: 1119–1121. [DOI] [PubMed] [Google Scholar]
- 21.Sadanaga N, Nagashima H, Mashino K, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 2001; 7: 2277–2284. [PubMed] [Google Scholar]
- 22.Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999; 5: 1966–1975. [PubMed] [Google Scholar]
- 23.De Plaen E, Arden K, Traversari C, et al. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 1994; 40: 360–369. [DOI] [PubMed] [Google Scholar]
- 24.Aubry F, Satie AP, Rioux-Leclercq N, et al. MAGE-A4, a germ cell specific marker is expressed differentially in testicular tumors. Cancer 2001; 92: 2778–2785. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer GFL, Schaefer C, Noppen C, et al. MAGE-3 immunoreactivity in formalin-fixed paraffin-embedded primary and metastatic melanoma. Am J Pathol 1997; 151: 1549–1553. [PMC free article] [PubMed] [Google Scholar]
- 26.Patard JJ, Brasseur F, Gil-Diez S, et al. Expression of MAGE genes in transitional-cell carcinomas of the urinary bladder. Int J Cancer 1995; 64: 60–64. [DOI] [PubMed] [Google Scholar]
- 27.Jang SJ, Soria JC, Wang L, et al. Activation of melanoma antigen tumor antigens occurs early in lung carcinogenesis. Cancer Res 2001; 61: 7959–7963. [PubMed] [Google Scholar]
- 28.Weber J, Salgaller M, Samid D, et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2”-deoxycytidine. Cancer Res 1994; 54: 1766–1771. [PubMed] [Google Scholar]
- 29.Janssen BL, van de Locht LT, Fourkour A, et al. Transcription of the MAGE-1 gene and the methylation status of its Ets binding promoter elements: a quantitative analysis in melanoma cell lines using a real-time polymerase chain reaction technique. Melanoma Res 1999; 9: 213–222. [DOI] [PubMed] [Google Scholar]