Abstract
Objective
To evaluate the strategies instituted by the authors’ center to decrease the time to transplantation and increase the rate of transplantation for African-Americans, consisting of a formal education program concerning the benefits of living organ donation that is oriented to minorities; a laparoscopic living donation program; use of hepatitis C-positive donors in documented positive recipients; and encouraging vaccination for hepatitis B, allowing the use of hepatitis B core Ab-positive donors.
Summary Background Data
The national shortage of suitable kidney donor organs has disproportional and adverse effects on African-Americans for several reasons. Type II diabetes mellitus and hypertension, major etiologic factors for end-stage renal disease, are more prevalent in African-Americans than in the general population. Once kidney failure has developed, African-Americans are disadvantaged for the following reasons: this patient cohort has longer median waiting times on the renal transplant list; African-Americans have higher rates of acute rejection, which affects long-term allograft survival; and once they are transplanted, the long-term graft survival rates are lower in this population than in other groups.
Methods
From March 1990 to November 2001 the authors’ center performed 2,167 renal transplants; 944 were in African-Americans (663 primary cadaver renal transplants and 253 primary Living donor renal transplants). The retransplants consisted of 83 cadaver transplants and 17 living donor transplants. Outcome measures of this retrospective analysis included median waiting time, graft and patient survival rates, and the rate of living donation in African-Americans and comparable non-African-Americans. Where applicable, data are compared to United Network for Organ Sharing national statistics. Statistical analysis employed appropriate SPSS applications.
Results
One- and 5-year patient survival rates for living donor kidneys were 97.1% and 91.3% for non-African-Americans and 96.8% and 90.4% for African-Americans. One- and 5-year graft survival rates were 95.1% and 89.1% for non-African-Americans and 93.1% and 82.9% for African-Americans. One- and 4-year patient survival rates for cadaver donor kidneys were 91.4% and 78.7% for non-African-Americans and 92.4% and 80.2% for African-Americans. One- and 5-year graft survival rates for cadaver kidneys were 84.6% and 73.7% for non-African-Americans and 84.6% and 68.9% for African-Americans. One- and 5-year graft and patient survival rates were identical for recipients of hepatitis C virus-positive and anti-HBc positive donors, with the exception of a trend to late graft loss in the African-American hepatitis C virus group due to higher rates of noncompliance, an effect that disappears with censoring of graft loss from that cause. The cadaveric renal transplant median waiting time for non-African-Americans was 391 days compared to 734 days nationally; the waiting time for African-Americans was 647 days compared to 1,335 days nationally. When looking at all patients, living and cadaver donor, the median waiting times are 220 days for non-African-Americans and 462 days for African-Americans.
Conclusions
Programs specifically oriented to improve volunteerism in African-Americans have led to a marked improvement in overall waiting time and in rates of living donation in this patient group. The median waiting times to cadaveric renal transplantation were also significantly shorter in the authors’ center, especially for African-American patients, by taking advantage of the higher rates of hepatitis C infection and encouraging hepatitis B vaccination. These policies can markedly improve end-stage renal disease care for African-Americans by halving the overall waiting time while still achieving comparable graft and patient survival rates.
Ever since the organ allocation system was first established, there has been controversy regarding the allocation policies. The Social Security Amendments of 1972 entitled almost all patients with end-stage renal disease (ESRD) in the United States to Medicare-funded dialysis or renal transplantation. 1 This funding implies equal access to renal transplantation. In 1998, the Department of Health and Human Services, using data from the Organ Procurement and Transplantation Network, reported the median waiting times for patients who underwent renal and liver transplantation between 1988 and 1994. This report revealed that African-American renal transplant recipients had median waiting times almost twice those of white recipients, and that this discrepancy had lengthened from 1988 to 1994. This same report revealed no difference for the same two ethnic groups when liver transplantation was considered. 2
As the immunosuppressive agents used to prevent acute rejection and the surgical techniques have improved, so have the graft and patient survival rates. Several studies have shown improved life expectancy for patients who have undergone renal transplantation as opposed to patients who have remained on dialysis. 3 This finding is true even if the recipient receives a marginal donor kidney, such as that from a diabetic, hypertensive, or elderly donor. 4 In addition, there is a cost benefit to patients who have undergone renal transplantation when compared to patients who remain on dialysis. After 2 years of functioning, a living donor renal transplant is less costly than maintaining the patient on hemodialysis. 5 The quality of life of recipients of successful renal transplants is superior to that of patients on dialysis. 6
Despite these facts strongly favoring transplantation over continued dialysis, African-Americans, compared to other groups, have dramatically lower rates of referral for renal transplantation compared to non-African-Americans. 7,8 African-Americans represent 36% of all patients awaiting renal transplantation in the United States but only 12% of the total population. This is causally related to the much higher incidence of hypertension and type 2 diabetes among African-Americans. The evidence also shows that compared to non-African-Americans, African-Americans are significantly less likely to be placed on transplant waiting lists, 9 to identify a living donor, 10 or to receive a cadaver transplant once placed on the waiting list. 11 Considering all factors combined, in a group of ESRD patients judged by an expert panel to fit the “ideal” for renal transplantation, the rate of transplantation of African-Americans was significantly less than that of non-African-Americans. 9 Of the patients on the waiting list, the time to transplantation has remained approximately twice as long for African-Americans over the last decade.
In 1991, Callender published the results of a concerted effort to improve minority donation in the District of Columbia. 12,13 This work found that same-ethnicity educators, ethnically appropriate audiovisual education material, and a systematic effort at public education were highly successful strategies to improve minority family consent rates for cadaver organ donation. This program resulted in an increase in the level of awareness of the success of transplantation in African-Americans from 10% to 32% after the educational intervention. The signing of organ donor cards increased from 7% to 24%. Based on this work, we reasoned that the same factors would be successful in educating African-Americans about the choice between dialysis and transplantation and issues surrounding living organ donation. These strategies were put in place formally in 1994 as an education program at the University of Maryland.
The use of organs from donors with evidence of previous exposure to hepatitis B (HBV) infection and with past or current hepatitis C (HCV) infection in appropriate recipients has gradually gained acceptance. Wachs et al demonstrated that kidneys from donors with positive antibody serology to HBV core antigen (anti-HBc) can safely be transplanted into naive hosts. 14 In contrast, livers from anti-HBc-positive donors had a significantly higher risk of HBV transmission. We have since encouraged the routine administration of HBV vaccine to all dialysis patients. Several centers have also adopted a policy of restricting the use of organs from donors with serologic evidence of HCV for recipients with past HCV. One report suggested that the use of pulsatile perfusion may even allow safe transplantation of HCV seropositive donor organs in seronegative recipients. 15 Since African-Americans have infection rates for HCV that are twice that of non-African-Americans, we reasoned that transplantation of anti-HBc- and HCV-positive organs into African-Americans and non-African-Americans equally might actually preferentially benefit African-Americans on the waiting list. This report of a decade of experience with renal transplants focuses on the effects of these strategies in improving access to safe renal transplantation for African-Americans.
METHODS
Patients
The study population consists of 2,167 recipients of 1,541 cadaveric and 816 living donor kidney transplants in 1,223 non-African-American and 944 African-American patients between April 3, 1991, when the program was restructured, until November 30, 2001. The study compares the outcome of African-American and non-African-American recipients with respect to median waiting time to transplantation as they were affected by strategies designed to improve access to transplantation for African-American patients, since this population represents 48% of all referrals for renal transplant evaluation at the University of Maryland.
Education and Outreach
Settings for education included presentations at dialysis units, support groups, and grand rounds at local and regional community hospitals. Evaluation of patients, particularly those with impediments to travel to a transplant center, was performed in referring physicians’ offices. In addition to verbal and written material, two short video presentations were played that give information about the risks, benefits, and, in one video, the responsibilities of kidney transplant recipients. The other video depicted the life experiences of actual living donors. Both videos were designed to portray representative experiences of the population of patients we serve, one that is predominantly African-American and elderly. Regarding live kidney donation, emphasis is placed on candid discussion regarding the time lost from work and family activity after donation, possible lost wages, and potential complications and mortality. The superior outcome of live kidney donation and its effect, especially on minority waiting time, is described. Before September 1994, there was no formalized education about living donation. After that time, only a specifically trained coordinator using prepared material delivered the formal education program.
Laparoscopic Donor Nephrectomy
Since March 1996, our program has used the technique of laparoscopic donor nephrectomy. Since that time, 98% of cases have been performed laparoscopically, with planned open surgery reserved for those with prior left upper quadrant surgery, donor obesity, or aberrant anatomy. 16
Use of HCV and Anti-HBc Donor Kidneys
Previous work from our center has shown the safety of using kidneys from HCV-seropositive donors in appropriate recipients. All recipients are tested for antibody to HCV; if positive, the presence of actual viral infection is confirmed by RT-PCR for HCV. Only those recipients with both serologic and virologic evidence of ongoing HCV infection are eligible to receive kidneys from HCV-positive donors.
Although the safety of using anti-HBc seropositive donor kidneys has been demonstrated clinically, evidence in one report revealed asymptomatic serologic conversion in 2 of 37 cases, suggesting that subclinical viral infection may have occurred. This report prompted our program to restrict use of anti-HBc-seropositive kidneys to recipients who have documented evidence of immunity to HBV as a result of immunization with the hepatitis B vaccine. Since the initial vaccine trials suggested that an anti-HBs titer above 10 mIU/mL is protective from infection from HBV, we have restricted transplantation of anti-HBc-seropositive kidneys into recipients with documented anti-HBs titers above 10 mIU/mL. In all cases, recipients are given informed consent regarding the donor’s serologic status. Consent includes discussion of the still relatively short-term experience with the use of seropositive donors, including that the donor’s hepatitis status acts as a surrogate marker for other infectious diseases, and the increased possibility of seronegative HIV infection is discussed beforehand.
Database
Patient demographic and clinical information is recorded prospectively, forming the University of Maryland, Division of Transplantation’s database. This database records patient age, sex, race, human leukocyte antigen (HLA), ABO type, type of transplant, cytomegalovirus status, serologies (hepatitis, HIV, EBV), serum chemistries, type of transplant, date of transplant graft loss, patient death, rejection episodes and dates, and retransplant status. A subgroup of patients whose transplants were performed after August 1995, when mycophenolate mofetil was introduced, was further analyzed to determine the effect of different calcineurin inhibitor therapy on graft survival and the incidence of rejection in African-Americans and non-African-Americans. No patient charts were reviewed and no patients were contacted. This study was submitted to and reviewed by the Institutional Review Board of the University of Maryland School of Medicine and was found to be exempt from a formal review process (Exemption No. CEF-040201).
Patient Selection
All patients are considered for renal transplantation if they are on dialysis for renal failure or have a creatinine clearance of less than 20 mg/mL.
Type 1 diabetic patients are also evaluated for possible simultaneous kidney-pancreas transplantation or simultaneous living donor kidney and cadaver pancreas transplantation. Patients who had simultaneous kidney-pancreas transplantation or simultaneous living donor kidney and cadaver pancreas transplantation are not part of this analysis, as these cases have been previously reported. 17
Immunosuppressive Therapy
Our program has primarily used a triple immunosuppressive therapy program in which the patients are discharged home on a calcineurin inhibitor, steroids, and an antimetabolite. Initially, the calcineurin inhibitor was cyclosporine; however, tacrolimus was introduced in 1994 and eventually replaced cyclosporine. Similarly, the only antimetabolite available during the early years of the program was azathioprine. In August 1995 it was replaced by mycophenolate mofetil 2 g/day in non-African-Americans and 3 g daily in African-Americans. Prednisone is administered at an initial dose of 2 mg/kg/d with a taper over 3 weeks to 0.3 mg/kg/d. The use of antilymphocyte induction has changed considerably with time. Initially, all patients received a course of Minnesota antilymphocyte globulin induction. Subsequently, this was replaced with antithymocyte globulin (ATGAM, Upjohn Pharmaceuticals). Only recipients of cadaveric renal transplants routinely receive induction therapy, initially with ATGAM but now with basiliximab (Simulect, Novartis Corp.) for low-risk cases. High-risk recipients (i.e., panel reactive antibodies > 40%) and those having a retransplant are induced with the antilymphocyte antibody thymoglobulin (Sangstadt Corp.). Current target tacrolimus levels are 10 to 12 ng/mL; however, patients over age 60 are maintained at 6 to 8 ng/mL as well as receiving half of the usual dose of mycophenolate mofetil.
Statistical Analysis
Comparisons between groups of data were made with the t test if data were normally distributed or the Mann-Whitney test if data were skewed. Proportions were compared with the chi-square test. The Kaplan-Meier product limit method was used to calculate transplantation rates and survival rates, and these were compared with the log-rank test. Significance was assigned at P < .05. Multivariate analysis of factors affecting rejection and graft survival was performed for all cases transplanted after August 1995, the “mycophenolate mofetil era.” Statistical calculations were performed with SPSS Graduate Pack 8.0 for Windows (SPSS Inc., Chicago, IL).
Rates of Live Donor Transplantation
To determine how initiation of a formal patient education program and the laparoscopic donation technique affected live donor transplant rates among African-American patients, we divided the population of registrants into three groups. The population of patients for this part of the study consisted of the 1,887 potential kidney recipients referred to the program for transplant evaluation from 1991 through November 2001. We studied three groups within the population, divided according to the time period when patients had their initial evaluation by the transplant team. The first time period (group 1) extended from 1991 until integration of a formal recipient family education program into the evaluation process in October 1994. The second time period (group 2) extended from October 1994 until introduction of laparoscopic donor nephrectomy into the practice in March 1996. The third period (group 3) extended from March 1996 until November 2001. The Kaplan-Meier product limit method was used to calculate live donor transplantation rates, which were compared with the log-rank test.
RESULTS
The demographic data comparing of the African-American and non-African-American renal transplant recipients are shown in Table 1. Retransplantations represented 82/686 (11.9%) of cadaver and 17/258 (6.6%) living donor transplants in African-Americans. Retransplantations represented 94/665 (14.1%) of cadaveric and 47/558 (8.4%) of non-African-American transplants. African-American patients were much more likely to have hypertension or diabetes as a cause of ESRD. The fraction of transplants from live donors was significantly higher for non-African-Americans (46% vs. 27.5%) than African-Americans; however, the rate of African-American live donor renal transplantation was twice that of the national average of 13.5%. Cadaver donors ranged in age from 1 to 80 years. The mean cold ischemia time for cadaver kidneys was 26.1 hours (range 1–71).
Table 1. RENAL TRANSPLANTATION—STUDY GROUP DEMOGRAPHICS, APRIL, 1991—NOVEMBER 30, 2001
African-Americans received only 1.5% of their renal allografts from zero-mismatched matched donors; the corresponding figure was 8.6% in non-African-Americans. African-Americans were also less likely to receive renal allografts from donors with five, four, or three HLA matches than non-African-Americans. African-Americans were also more likely to have zero HLA matches (six HLA mismatch) with their cadaver donor.
During the 10.5-year study period, the median waiting time for non-African-American patients was 391 days for a cadaver kidney and 220 days for receipt of a cadaver or living donor kidney. This compares favorably to 734 days nationally, using comparative data from the United Network for Organ Sharing (UNOS). The median waiting time to cadaver transplantation for African-Americans was 681 days for a cadaver transplant and 462 days for receipt of a cadaver or living donor transplant. Nationally, African-American patients have a median waiting time of 1,335 days, indicating that the waiting time for African-Americans at the University of Maryland has been reduced by at least half. 18 Moreover, it appears that African-Americans’ waiting time at the University of Maryland is less than for non-African-American patients nationally. These are only qualitative, nonstatistical comparisons since the methodology used for calculating waiting time from our database is different than that used by UNOS, which takes into account death on the waiting list. Moreover, some transplant programs, the University of Maryland excepted, do not register living donors with UNOS, possibly inflating the overall waiting time.
Graft and Patient Survival
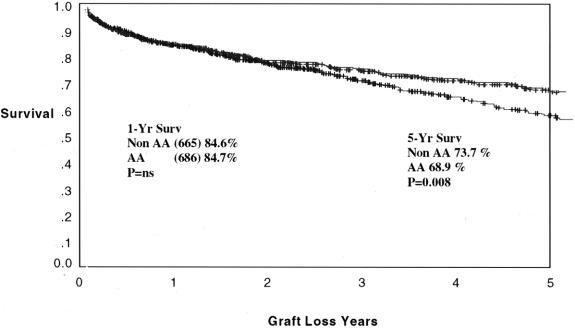
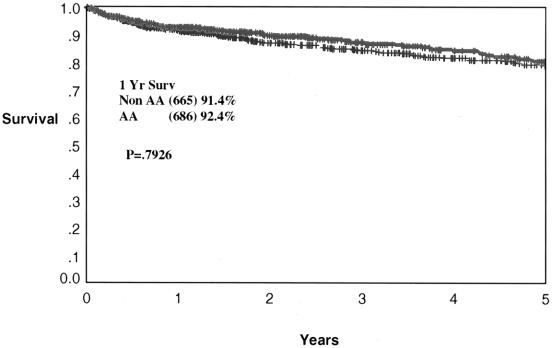
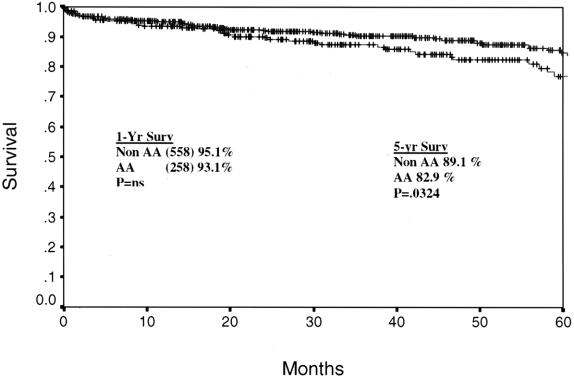
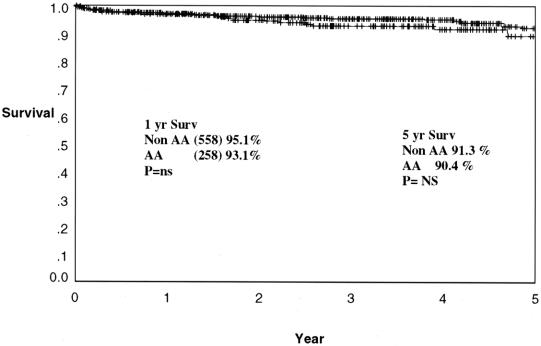
Cadaveric graft survival rates are depicted for the total program in Figure 1. One- and 5-year graft survival rates are 84.7% and 68.9% for African-Americans and 84.6% and 73.7% for non-African-Americans (P = NS, P = .008). The cadaveric kidney recipient patient survival is depicted in Figure 2. One- and 5-year patient survival rates were 92.4% and 80.2% for African-Americans and 91.4% and 78.7% for non-African-Americans (both P = NS). The graft survival of living donor kidney transplants is depicted in Figure 3. One- and 5-year graft survival rates for live donor kidneys were 93.1% and 82.9% for African-Americans and 95.1% and 89.1% for non-African-Americans (P = .032 at 5 years). The patient survival of recipients of living donor kidney transplants is depicted in Figure 4. One- and 5-year patient survival rates for this group were 96.8% and 90.4% for African-Americans and 97.1% and 91.3% for non-African-Americans (both P = NS). The most common cause of graft loss of cadaveric kidney transplants in African-Americans is death with functioning graft, followed by noncompliance and chronic rejection; the most common cause of graft loss for living donor transplants in African-Americans was death with a functioning graft. The most common cause of cadaveric kidney graft loss in non-African-Americans is death with functioning graft followed by chronic rejection; for living donor transplants, the overwhelming cause is death with a functioning graft (Table 2).
Figure 1. Actuarial graft survival for all cadaveric renal transplants. One- and 5-year graft survival rates are 84.7% and 68.9% for African-Americans and 84.6% and 73.7% for non-African-Americans (P = .008).
Figure 2. Actuarial patient survival for all cadaveric renal transplants. One- and 5-year patient survival rates are 92.4% and 80.2% for African-Americans and 91.4% and 78.7% for non-African-Americans (P = .793).
Figure 3. Actuarial graft survival for all living donor renal transplants. One- and 5-year graft survival rates are 93.1% and 82.9% for African-Americans and 95.1% and 89.1% for non-African-Americans (P = .032).
Figure 4. Actuarial patient survival for all living donor renal transplants. One- and 5-year patient survival rates are 96.8% and 90.4% for African-Americans and 97.1% and 91.3% for non-African-Americans (P = .230).
Table 2. CAUSES OF GRAFT LOSS: RENAL TRANSPLANTS
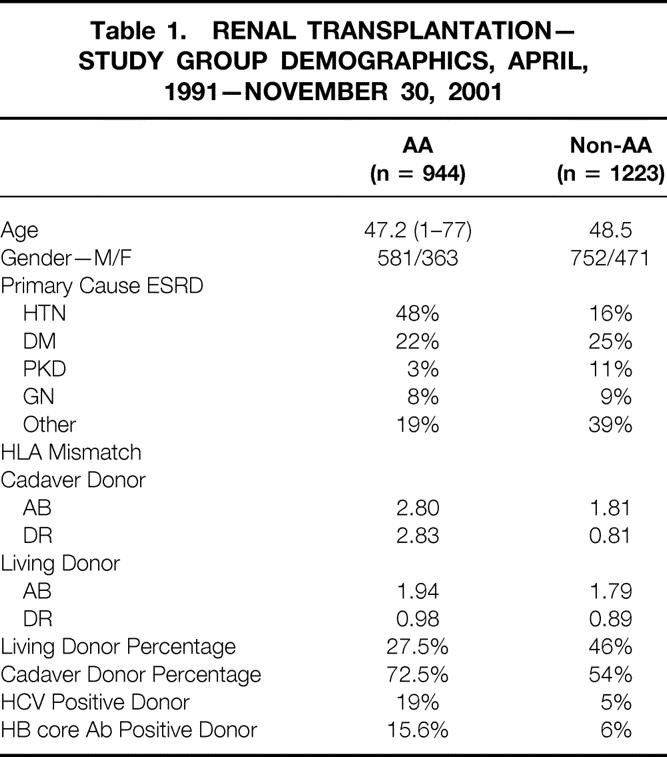
A total of 107/686 (15.6%) of African-American recipients and 40/665 non-African-American recipients (6.0%) of cadaveric kidney transplants used donors with positive anti-HBc serology. One- and 5-year graft survival rates for recipients of anti-HBc-seropositive cadaveric recipients is shown in Figure 5. One- and 5-year graft survival rates were 87% and 62% in African-Americans and 90% and 73% for non-African-Americans. There was no difference in the outcome for African-American and non-African-American patient (P = .762). Moreover, the outcome of recipients of anti-HBc-positive and anti-HBc-negative kidneys was nearly identical at 5 years for non-African-American and African-American patients. These results confirm the safety of using anti-HBc donor kidneys in fully vaccinated recipients with demonstrable anti-HBs.
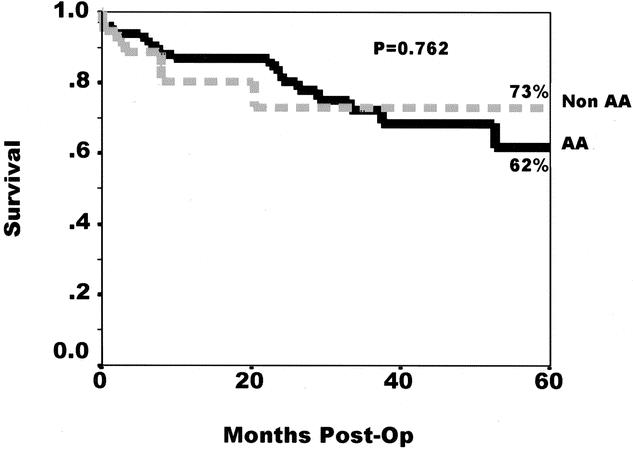
Figure 5. Actuarial graft survival for all cadaveric renal transplants from donors positive for anti-HBc. One- and 5-year graft survival rates are 87% and 62% in African-Americans and 90% and 73% for non-African-Americans. There is no difference in the outcome for African-American and non-African-American patients (P = .762). The outcome of recipients of anti-HBc-positive and anti-HBc-negative kidneys is nearly identical at 5 years for non-African-American and African-American patients.
A total of 131/686 (19%) of African-American recipients and 33/665 (5.0%) of cadaveric kidney transplants were from HCV-seropositive donors into HCV- seropositive and HCV RT-PCR-positive recipients. Of the 131 HCV-seropositive kidneys transplanted into African-American recipients, 20 were lost to noncompliance (15%). This accounted for 39% (20/52) of the graft losses in this population. In contrast, only 1 of the 33 HCV-seropositive kidneys (3%) transplanted into non-African-American recipients was lost to noncompliance, which was 9% (1/11) of the graft losses in this group. The 5-year actuarial graft survival rate for African-American recipients of HCV-seropositive kidneys was only 47.3%, compared to 71.9% for non-African-American recipients (P = .22, log-rank). This difference was predominantly due to higher rates of graft loss due to noncompliance among the African-American recipients. When graft survival is censored for losses due to noncompliance, the 5-year graft survival rate for African-American recipients of HCV-seropositive kidneys is very similar to that of non-African-Americans (Fig. 6). The second most common cause of graft loss in this subgroup was chronic rejection. Importantly, neither acceleration of hepatitis nor sepsis played a role in graft losses. This finding is note-worthy, as others have observed higher rates of septic causes of death in HCV-positive recipients independent of the donor status. 19 Since many of the African-American and non-African-American patients in this last group originally developed HCV infection secondary to remote intravenous drug abuse, the risk of late graft loss secondary to noncompliance may be increased, although this cannot be determined from our data.
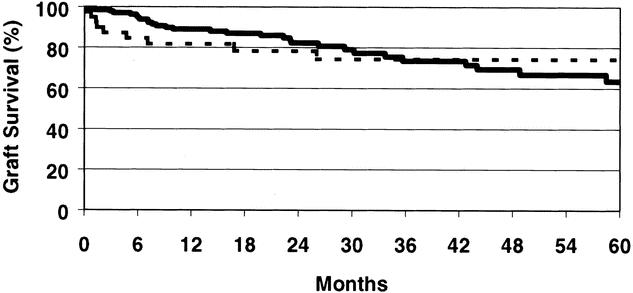
Figure 6. Five-year, non-compliance-censored, Kaplan-Meier graft survival rates for African-American (solid line) and non-African-American recipients of HCV-positive kidneys. At 5 years the graft survival rate among African-Americans was not different from that of non-African-Americans (63.5% vs. 74.3%, P = .98, log-rank).
The rate of living donor kidney transplantation among African-American patients before initiation of either the family education program or laparoscopic donation (group 1) was 9.4% at 3 years after the patient’s initial transplant evaluation (Fig. 7). Introduction of the family education program (group 2) increased the transplant rate to 16.6%, although this change did not quite achieve statistical significance (P = .10). Transplant rates among African-Americans who were exposed to the family education program and also had the laparoscopic donation technique available (group 3) was 17.0%; this rate was significantly higher than that for group 1 (P = .008). These data indicate that the family education program had a much more dramatic effect on transplantation rates among African-Americans than the availability of laparoscopic donation. This pattern is distinct from what we observed for our program overall, where the laparoscopic donation technique had a greater impact than education on the living donor transplant rates.
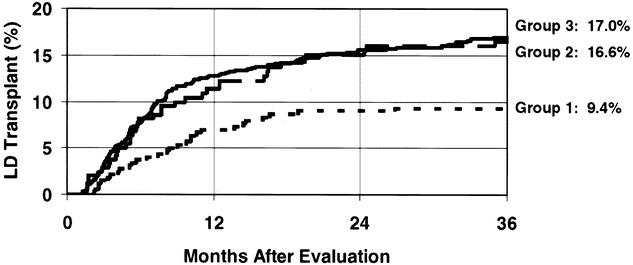
Figure 7. Kaplan-Meier rates of living donor transplantation among African-American kidney registrants. The lowest rate was for group 1 patients (short dashes); these patients were registered before initiation of the live donor kidney transplant formal education program or laparoscopic live donor nephrectomy. The transplant rate was higher after introduction of the formal family education program (group 2, long dashes) (P = .10). The transplant rate after initiation of the laparoscopic donation technique (group 3, solid line) was similar to that after introduction of the formal education program, and higher than the transplant rate of group 1 patients (P = .008).
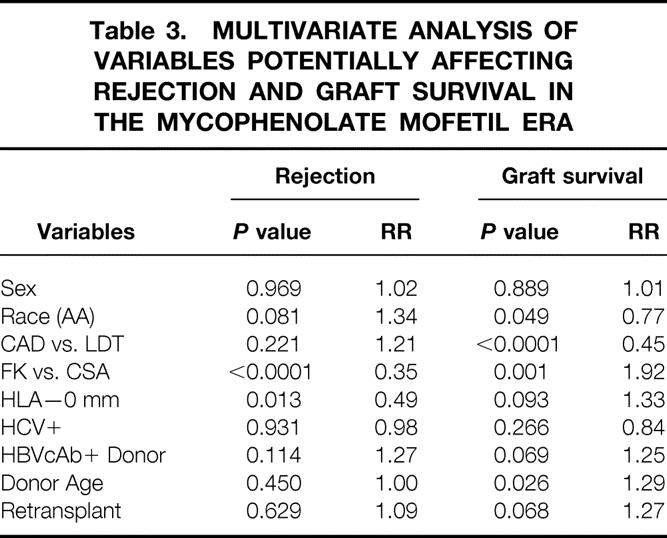
A previous study has suggested an advantage of tacrolimus for African-American recipients. To determine if this effect was present in our patients, the result of all kidney transplants, cadaveric and living donor cases combined, was analyzed for patients transplanted after August 1995. During this period all patients received prednisone, mycophenolate, and either cyclosporine or tacrolimus. The combined living donor and cadaveric 1- and 5-year graft survival rates for African-Americans in the mycophenolate mofetil era were 89% and 79% with tacrolimus-based therapy and 85% and 60% with cyclosporine-based therapy (P = .006) (Fig. 8). The incidence of rejection is depicted in Figure 9 for all transplants. At 1 year the rate in African-American patients was 36% with cyclosporine-based therapy but only 9% with tacrolimus-based therapy (P < .0001). Finally, multivariate analysis of factors that might affect rejection demonstrates a strong beneficial effect of tacrolimus over cyclosporine (RR = 0.35, P < .0001) and the receipt of a zero antigen-mismatched kidney (RR = 0.49, P = .013). Graft survival was influenced by four factors by multivariate analysis: African-American race (RR = 0.77, P = .049), cadaver donor (RR = 0.45, P < .0001), tacrolimus use (RR = 1.92, P = .001), and younger donor age (RR = 1.29, P = .026) (Table 3).
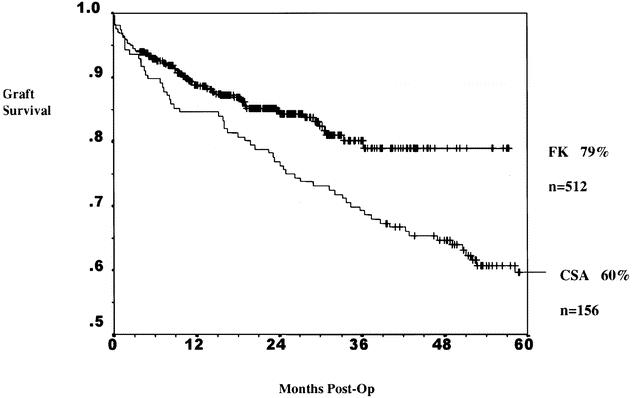
Figure 8. Actuarial graft survival in all African-American kidney transplant recipients in the mycophenolate mofetil era. Comparison of tacrolimus (FK)- and cyclosporin (CSA)-based immunosuppression. The combined living donor and cadaveric 1- and 5-year graft survival rates for African-Americans in the mycophenolate mofetil era were 89% and 79% with tacrolimus-based therapy and 85% and 60% with cyclosporine-based therapy (P = .006).
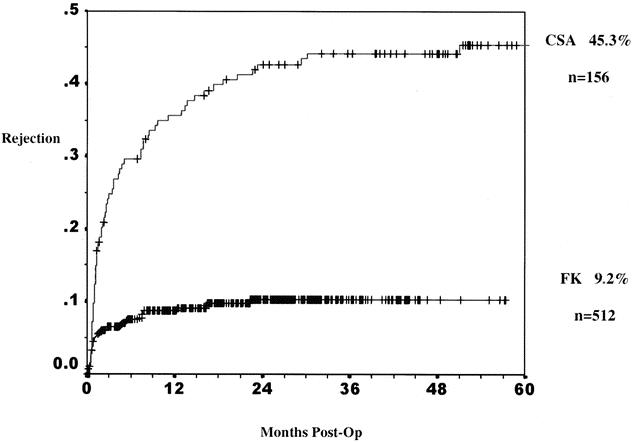
Figure 9. Cumulative rate of at least one rejection in all African-American kidney transplant recipients in the mycophenolate mofetil era. Comparison of tacrolimus (FK)- and cyclosporin (CSA)-based immunosuppression. At 1 year the rate in African-American patients was 36% with cyclosporine-based therapy but only 9% with tacrolimus-based therapy (P < .0001).
Table 3. MULTIVARIATE ANALYSIS OF VARIABLES POTENTIALLY AFFECTING REJECTION AND GRAFT SURVIVAL IN THE MYCOPHENOLATE MOFETIL ERA
DISCUSSION
These data give strong support to our premise that a multifactorial effort was successful in improving transplantation rates in African-Americans. Four strategies (improved patient education and off-site evaluation, ethnically appropriate audio-video teaching, and selective use of kidneys from donors who are serologically positive for evidence of past HBV infection and past or current HCV infection) combined to significantly improve transplant rates for African-American patients. These strategies led to excellent patient and graft survival rates, particularly in a modern immunosuppression era with the availability of the potent immunosuppressive agents tacrolimus and mycophenolate mofetil. It is likely that no one of these strategies was, by itself, pivotal in the improved transplantation rates. Each factor is worthy of further discussion since further refinements may be possible, such as improved psychosocial evaluation of HCV-positive recipients to reduce the risk of transplanting an individual who could have been predicted to lose graft function from noncompliance.
Of the various barriers to renal transplantation identified by previous investigators, all have nonparametric components making exact definition of the impact of a specific intervention difficult to test. For example, the original intent of off-site patient evaluation was to give patients with transportation issues an opportunity to learn about transplantation from an experienced team of nurses and physicians. The concept was that while inability to obtain transportation for evaluation might make it less likely that the transportation needed to come for the actual transplant or follow-up visits. In reality, though, transportation can almost always be arranged; the real barrier is that the overwhelmed dialysis unit social worker cannot complete this time-consuming task for every patient. Some are excluded, perhaps those less strident in vocalizing demands for assistance or those with language barriers. Such is the way with complex steps to start and complete a transplant evaluation: seemingly trivial factors can have profound effects on access to care and the long-term health status of a single individual. Since seemingly minor obstacles vary from patient to patient, a broad-based multifaceted strategy is needed to help those least able to obtain medical care. We have been impressed by the value of off-site evaluation of patients. The chance to discuss the social and medical issues with the dialysis unit staff face-to-face is invaluable. Second, since medical records are rarely forwarded to a transplant center, the chance to inspect medical records, with the patient’s permission, in many cases eliminates duplicate testing. Unfortunately, this type of evaluation is extremely popular with patients, and efforts should be made to limit this approach to those with real obstacles to traditional in-house evaluation. It is difficult to measure precisely the impact of this grassroots effort on access to transplantation for African-Americans. Structured surveys of groups of patients having each type of evaluation, off-site versus in-house, might provide information not available in this study.
The effect of focused educational programs at hospitals and dialysis units is similarly difficult to measure. In contrast, our structured education program surrounding live donor transplantation and laparoscopic nephrectomy has been validated to be effective in African-Americans, non-African-Americans, and elderly recipients. Whether the inclusion of ethnically appropriate subjects in the teaching video is noticed could be determined with survey data. Thus, while it is clear that our teaching program has significantly progressed, it is not clear how we could further improve it beyond ensuring that it reflects current ideas about live donor kidney transplantation. One refinement instituted is to include clear statements, currently given in a PowerPoint presentation regarding the risks. We have found that making clear statements about donor risk increases the chance a potential recipient will feel informed enough to approach his or her loved ones.
The above-mentioned discussion underscores the need to establish standards for patient education. This is a difficult task, given the broad range of ethnic backgrounds, languages, mores, and past educational experiences. Nevertheless, it is clear that many patients continue to follow a dated approach of preparation for dialysis, a period of adjustment to dialysis, followed by eventual transplant evaluation for some. Pre-emptive transplantation was employed in only 2,002 of 77,325 (2.6%) incident cases in 1998, which suggests great room for improvement in establishing standards of education for both patients and physicians. The recently released National Kidney Foundation guidelines for the management of ESRD patients have provided a sound structure for optimal dialytic management of ESRD. The nephrologist’s role in pretransplant preparation and management could be expanded in a later iteration. 20
The strategy of using hepatitis-seropositive donor kidneys worked more effectively in favor of African-Americans than was originally anticipated when these strategies were devised. Transplant rates for African-Americans exceeded those for non-African-Americans by almost threefold. Several factors may play a role in this positive finding. Separate waiting lists for the safe application of hepatitis-seropositive kidneys must be maintained by the transplant center. Inclusion on such a list requires that the patient have the appropriate serologic profile; in the case of HCV, the patient must also have a positive RT-PCR. This precaution is employed to avoid transplanting an individual who has a false-positive serologic test for HCV with an HCV-seropositive donor kidney. Second, acceptance of a kidney from a seropositive donor requires informed consent. Our experience shows that patients with past histories of intravenous drug abuse are best able to grasp the nuances of a discussion of the increased risk of seronegative HIV infection presented by a donor with positive HCV or anti-HBc serologies. These patients quickly grasp the concept of surrogate markers and their connection to the increased risk of seronegative HIV infection. After careful explanation of the known and unknown risks of use of seropositive donors, many but not all patients will accept a seropositive transplant. Whether this factor played a role in the increased transplantation rates in African-Americans cannot be determined from the limited data in our database.
Our group established the safety of transplantation of anti-HBc-positive donor kidneys into vaccinated immune recipients. 21 In that report, 2 cases out of 40 recipients of anti-HBc-positive donor kidneys developed new HBc antibodies, suggesting exposure to viral antigen or noninfectious particles. These recipients were seropositive for anti-HBs only before transplantation, and none developed active disease with detectable HBs antigen. PCR testing, however, was not performed in that study. Even naive recipients of anti-HBc-seropositive kidneys did not develop clinical disease in 37 cases reported by Wachs et al from UCSF. 14 It is noteworthy that two patients developed new anti-HBc antibody, again suggesting noninfectious or subclinical exposure to the virus. 14 These findings are consistent with observations in high-risk blood donors with positive anti-HBc that the rate of HBV DNA hybridization positivity was 4%. In low-risk groups the rate of positivity was zero. 22,23 This 4% positive viral DNA rate in high-risk populations reinforces the need to use these kidneys only in recipients who have demonstrable immunity to HBV. As in our previous series, no cases of clinical hepatitis developed as a consequence of receipt of an anti-HBc-seropositive kidney. Our results show no difference in outcome whether a recipient receives an anti-HBc-positive or -negative cadaver donor kidney.
A major concern associated with the use of HCV-positive donor kidneys is the possibility that a less virulent strain might be replaced by one that is more virulent. In a previous study of 61 cases between 1991 and 1996, we demonstrated the safety of use of HCV-positive kidneys. Patient sera in five of these cases (8%) displayed the new appearance of the donor genotype in addition to the recipient genotype. These five instances were not associated with episodes of increased transaminases or clinical hepatitis. Clinical hepatitis flares did not characterize the current group of patients. Second, we were concerned that the risk of late hepatic dysfunction or death from accelerated liver disease could make this approach problematic. We did not observe either accelerated disease or development of clinical cirrhosis within 5 years of transplantation. This is supported by others’ short-term results. 24 In addition, one report suggests an increased incidence of septic causes of death in HCV-seropositive recipients of kidney transplants. This concern was not noted in our patients.
The major drawback to our approach was the high rate of noncompliance leading to graft loss. While it cannot be determined from our data, some of these recipients may have acquired HCV infection from past intravenous drug abuse. If that was the case, this may have represented a group at risk for noncompliance. Our data match those of Meier-Kriesche, who showed excellent early and significantly lower late graft survival rates in HCV-seropositive recipients in a study of the USRDS database. 25 Our results suggest the need for enhanced psychosocial assessment of these candidates pretransplant and, more importantly, increased psychosocial support in the late transplant phase. Our data do not suggest that the use of HCV-seropositive kidneys per se leads to an increased risk of liver disease or septic events.
Our data support Neylan’s finding that tacrolimus is superior to cyclosporine for immunosuppression of African-American patients. 26 We found that among four variables affecting late graft function in African-American patients, the use of tacrolimus had the strongest beneficial effect. Interestingly, this benefit was not seen in non-African-American recipients. Moreover, the 9% 1-year rate of rejection is predictive of optimal long-term graft survival. Other critical factors for success in African-American patients were found to be younger donors or living donors, factors under partial control of the transplant center.
If the donor hepatitis serology strategy we employed becomes widely accepted in most transplant centers, the advantage our African-American patients experienced will be partially lost, although other strategies left in place will continue to play a role. This fact underscores the need for continued examination and evolution of UNOS allocation policies to eliminate ethnic and geographic disparities in transplant rates. Centers should look for strategies that enable entry of minority patients into the transplant process. Once wait-listed, centers should attempt to use strategies such as patient education that are fair to all individuals.
Discussion
Dr. Clive O. Callender (Washington, D.C.): Thank you, Dr. Foster, for the opportunity to read your paper. Certainly it is quite clear that we have unequivocal data that demonstrates that African-Americans after transplantation nationally have a 10% to 20% poorer graft survival for living related and cadaver kidney transplantation, and they wait twice as long for transplants as the white population. In your study, it is clear that they didn’t wait quite as long, and I noted they have the great degree of graft survival improvement, although they did have a poorer graft survival after 5 years. I value the study and learned a lot from reading your paper. I have four comments.
One, I was thrilled at the educational program that you used and was jealous that I hadn’t used it myself for the purpose that you did. But I wondered, since this is something that could be a model program for other programs, how to quantify its effectiveness and how to use surveys or questionnaires pre and post to further quantify what you have done here, because it has potential for application as a national model.
Second, the hepatitis C policy, which was so effective, and surprisingly so. I wonder if you have any hypotheses that could help us understand it and also use this concept which you have used so effectively here, perhaps nationally.
The third question was relative to the mycophenolate mofetil (Cellcept) dose, which is the 3-g dose which was formerly recommended. I know, however, that I have a lot of trouble with my African-American patients getting them to tolerate even 2 g. And I wonder if you had the same problem. Or maybe you didn’t.
Then the fourth issue has relevance to the noncompliance. Many of the recent studies, when they have looked at it from a larger perspective, have not been able to identify the noncompliance problem. Did you think it related to the lack of long-term reimbursement for immunosuppressants? Or how did you account for it?
Finally, I wondered if you did use at all any alteration of the HLA allocation policy that UNOS has used in order to help accomplish the fact that you had such a similarity between your waiting time for African-Americans and others.
I thank you for the opportunity to read the paper. I enjoyed it and learned much from it.
Presenter Dr. Clarence E. Foster, III (Baltimore, MD): The first part, in terms of our graft and patient survival, as good as they are in our study, I didn’t really talk much about immunosuppression. But certainly as our program has evolved we have also tailored our immunosuppressions to this target population that we know has a higher graft acute rejection. They have a lower graft survival rate. So we have used certainly the 3 g of mycophenolate mofetil in our African-American patient population. And I, like you, have found that it is very difficult to have the patient tolerate such a large dose. But we have certainly tried to do that.
In terms of any of the new protocols that are being espoused, the steroid-sparing protocol, we try not to do that in African-Americans particularly.
In terms of a national model for living donation, I think that is an excellent idea. We have never really thought about that. Certainly we were using it for our own intents and purposes to improve our program. We have videos in which a variety of patients with different ethnic backgrounds present their experience of going through the donation process. And then of course, as I said, we have people specifically knowledgeable of some of the risk factors that are seen in African-Americans in terms of diabetes and hypertension. But I think it is an excellent idea for a national model.
In terms of hepatitis C, in looking at other centers’ experience I agree with you that it is not as clear-cut as maybe I have made it today. Certainly you can get pretty good results in this patient population. But I think particularly in African-Americans, sort of tying into your noncompliance question, that hepatitis C patients who are on dialysis may be a special subset of patients that may have some of the more difficult problems. I wasn’t really talking about noncompliance in all African-Americans; I was really talking about noncompliance in this subset of patients that have hepatitis C. And I think obviously, as you know, these patients probably have a higher incidence of drug abuse and other things that may affect their long-term compliance.
Dr. Clyde F. Barker (Philadelphia, PA): It is an excellent paper and you are to be congratulated on increasing the likelihood of transplant in this special group.
With regard to the hepatitis C, there are several strains of hepatitis C, two major ones that I am aware of, one of which is much more virulent than the other. And we have been reluctant to utilize donors who carry the more virulent strain even in recipients who though CMV-positive were infected with the less virulent strain, fearing that they might still be susceptible to the more virulent strain. Is this a valid concern? Do you genotype your patients to distinguish the different strains of CMV?
Dr. Clarence E. Foster, III (Baltimore, MD): This is true, Dr. Barker. Dr. Oldach, one of our Infectious Disease attendings at the University of Maryland, actually looked at this very question, looking at the various hepatitis C serotypes in patients who have undergone renal transplants. And he did find that there was a conversion rate but did not find any increasing morbidity if it converted to more malignant varieties of hepatitis C.
Also, before doing any of these patients, we make sure, obviously, there is no liver disease. We oftentimes do a biopsy and we do RNA studies to make sure there isn’t any active hepatitis C. And I think that also helped us in terms of some of our long-term outcomes.
Dr. Paul S. Russell (Boston, MA): This is a welcome contribution. We have had a lot of anguish about what goes on in the African-American community. And it has been difficult, I think, for most of us to know what to do about it. You have described for us that it is complicated in many things. And I am sure that is absolutely correct. And I wonder if you would be willing to say a couple of words more about two things.
One of them was that you talked about the education program. I presume that means education of patients who are in the end-stage renal disease state. Have you extended it beyond that? Because we have the donors to think about, and we have the whole African-American community and their opinions to think about. And I would be very interested in what your thoughts are about that for now or for the future.
The other thing you mentioned was that you have improved a good deal in the waiting time. What are the numbers on that? Can you explain to us a little bit? Are the African-American people now becoming a little bit more similar to the other people on your waiting list? What type of progress have you actually made? Because that is important to all of us, too.
Dr. Clarence E. Foster, III (Baltimore, MD): To answer your first question, no, we have not extended that education program beyond educating patients about, one, undergoing renal transplantation as recipients, and also the living donor donation process. We do try to have nurses as well as physicians who are interacting with all of our patients treated are aware of the risk of diabetes and hypertension in this patient population and make sure that they are screened adequately. But we haven’t really applied this to some of the education programs that Dr. Callender has espoused in terms of outreach to not only educate about renal transplantation but also educate about the risk factors of renal disease to prevent disease from recurring.
In terms of mean waiting times, in our patient population, the African-American mean waiting time is roughly 680 days, which was basically the same as non-African-American waiting time nationally. And African-American mean waiting time nationally is 1,300 days. So we are easily able to decrease the time they have to wait pretty significantly.
Dr. Ashok Kumar B. Jain (Pittsburgh, PA): I just have one small question. The response to interferon alpha for HCV infection in renal failure patients is better than other individuals. Did you use interferon in your HCV patients while waiting for kidney transplantation?
Dr. Clarence E. Foster, iii (Baltimore, MD): For the most part, no, we don’t do that. We check them for active hepatitis, we check their viral loads, and then anyone that has any abnormalities of their liver function test gets a liver biopsy before being transplanted. But in terms of being treated with interferon before transplantation, we don’t have that as a program specific part of the protocol.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Clarence E. Foster, III, MD, Division of Transplantation, Department of Surgery, University of Maryland, School of Medicine, 29 S. Greene Street, Suite 200, Baltimore, Md. 210201.
E-mail: cfoster@smail.umaryland.edu
Accepted for publication April 24, 2002.
References
- 1.Rettig RA, Levinsky NG, eds. Kidney failure and the federal government. Washington, DC: National Academy Press, 1991. [PubMed]
- 2.Brown JG. Inspector General. Racial and geographic disparity in the distribution of organs for transplantation. Department of Health and Human Services, Office of the Inspector General, June 1998;OEI-01-98-00360.
- 3.Wolfe RA, Ashby BA, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Hanson JA, Meier-Kriesche H-U, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 2001; 12: 589–597. [DOI] [PubMed] [Google Scholar]
- 5.Schweitzer EJ, Wiland A, Evans D, et al. The shrinking renal replacement therapy “break-even” point. Transplantation 1998; 66: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 6.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Intl 1996; 50: 235–240. [DOI] [PubMed] [Google Scholar]
- 7.Ayanian JZ, Cleary PD, Weissman JS, et al. The effect of patient preferences on racial differences in access to renal transplanation. N Engl J Med 1999; 341: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation. N Engl J Med 2000; 343: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe RA, Ashby BA, Milford EL, et al. Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 2000; 36: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 10.Schweitzer EJ, Wilson J, Jacobs SC, et al. Increased rates of donation with laparoscopic donor nephrectomy. Ann Surg 2000; 232: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander GC, Sehgal AR. Barriers to cadaveric transplantation among blacks, women, and the poor. JAMA 1998; 280: 1148–1152. [DOI] [PubMed] [Google Scholar]
- 12.Callender CO, Hall LE, Yeager CY, et al. Special report: Organ donation and blacks, a critical frontier. N Engl J Med 1991; 325: 442–444. [DOI] [PubMed] [Google Scholar]
- 13.Callender CO, Hall MB, Branch D. An assessment of the effectiveness of the Mottep model for increasing donation rates and preventing the need for transplantation—adult findings: program years 1998 and 1999. Semin Nephrol 2001; 21: 419–428. [DOI] [PubMed] [Google Scholar]
- 14.Wachs ME, Amend WJ, Ascher NL, et al. The risk of transmission of hepatitis B from HbsAg(-), HbcAb(+), HBIgM(-) organ donors. Transplantation 1995; 59: 230. [PubMed] [Google Scholar]
- 15.Miller J, Roth D, Schiff ER. Letter to the Editor. N Engl J Med 1993; 328: 512. [PubMed] [Google Scholar]
- 16.Flowers J, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997; 226: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farney AC, Cho E, Schweitzer EJ, et al. Simultaneous cadaver pancreas living-donor kidney transplantation: a new approach for the type 1 diabetic uremic patient. Ann Surg 2000; 232: 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2000 OPTN/SR. 1990–1999. HHS/HRSA/OSP/DOT; UNOS. Median waiting time, kidney, by ethnicity, year 1996.
- 19.Meier-Kriesche H-U, Ojo AO, Hanson JA, et al. Hepatitis C antibody status and outcomes in renal transplant recipients. Transplantation 2001; 72: 241–244. [DOI] [PubMed] [Google Scholar]
- 20.Keane W, Eknoyan G, et al. et al. K/DOQI clinical practice guidelines on chronic kidney disease work group and evidence review team memberships. Am J Kidney Dis 2002; Suppl 1, 39: S11–S12. [Google Scholar]
- 21.Madayag RM, Johnson LB, Bartlett ST, et al. Use of renal allografts from donors positive for hepatitis B core antibody confers minimal risk for subsequent development of clinical hepatitis B virus disease. Transplantation 1997; 64: 1781–1786. [DOI] [PubMed] [Google Scholar]
- 22.Douglas DD, Rakela TJ, Rabe D. Absence of hepatitis B virus DNA detected by polymerase chain reaction in blood donors who are hepatitis B surface antigen negative and antibody to hepatitis B core antigen positive from a United States population with a low prevalence of hepatitis B serologic markers. Transfusion 1993; 33: 212–216. [DOI] [PubMed] [Google Scholar]
- 23.Wang JT, Wang TH, Sheu JC, et al. Detection of hepatitis B virus DNA by polymerase chain reaction in plasma volunteer blood donors negative for hepatitis B surface antigen. J Infect Dis 1991; 163: 397–399. [DOI] [PubMed] [Google Scholar]
- 24.Mandal AK, Kraus ES, Samaniego M, et al. Shorter waiting times for hepatitis C virus seropositive recipients of cadaveric renal allografts from hepatitis C virus seropositive donors. Clin Transplant 2000; 14: 391–396. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Kresche H, Ojo AO, Hanson JA, et al. Hepatitis C antibody status and outcomes in renal transplant recipients. Transplantation 2001; 72: 241–244. [DOI] [PubMed] [Google Scholar]
- 26.Neylan JF, for the FK506 Kidney Transplant Study Group. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. Transplantation 1998; 65: 515–523. [DOI] [PubMed] [Google Scholar]