Abstract
Objective
To determine the effectiveness of early, routine antioxidant supplementation using α-tocopherol and ascorbic acid in reducing the rate of pulmonary morbidity and organ dysfunction in critically ill surgical patients.
Summary Background Data
Oxidative stress has been associated with the development of the acute respiratory distress syndrome (ARDS) and organ failure through direct tissue injury and activation of genes integral to the inflammatory response. In addition, depletion of endogenous antioxidants has been associated with an increased risk of nosocomial infections. The authors postulated that antioxidant supplementation in critically ill surgical patients may reduce the incidence of ARDS, pneumonia, and organ dysfunction.
Methods
This randomized, prospective study was conducted to compare outcomes in patients receiving antioxidant supplementation (α-tocopherol and ascorbate) versus those receiving standard care. The primary endpoint for analysis was pulmonary morbidity (a composite measure of ARDS and nosocomial pneumonia). Secondary endpoints included the development of multiple organ failure, duration of mechanical ventilation, length of ICU stay, and mortality.
Results
Five hundred ninety-five patients were enrolled and analyzed, 91% of whom were victims of trauma. The relative risk of pulmonary morbidity was 0.81 (95% confidence interval 0.60–1.1) in patients receiving antioxidant supplementation. Multiple organ failure was significantly less likely to occur in patients receiving antioxidants than in patients receiving standard care, with a relative risk of 0.43 (95% confidence interval 0.19–0.96). Patients randomized to antioxidant supplementation also had a shorter duration of mechanical ventilation and length of ICU stay.
Conclusions
The early administration of antioxidant supplementation using α-tocopherol and ascorbic acid reduces the incidence of organ failure and shortens ICU length of stay in this cohort of critically ill surgical patients.
Many critically ill surgical patients survive their initial physiologic insult only to die of infection or organ dysfunction over the ensuing days to weeks. The precise mechanism by which organ dysfunction occurs is unclear; however, there is mounting evidence that tissue injury is mediated at least in part by oxidative metabolites, or reactive oxygen species (ROS), derived from inflammatory cells and/or reperfusion injury. In this setting, ROS induce tissue injury through the peroxidation of plasma membranes, oxidation of critical enzymatic or structural proteins, induction of apoptosis, 1 and activation of NF-κB, leading to the induction of genes critical to the initiation and perpetuation of the systemic inflammatory response. 2 The evidence supporting oxidant-mediated tissue injury and gene activation is greatest for the acute respiratory distress syndrome (ARDS), in which ROS have been detected in the expired breath of patients with the syndrome, and experimental evidence clearly implicates NF-κB activation in the pathogenesis of alveolar inflammation. 3–6
The profound oxidative stress that occurs during critical illness leads to early depletion of many endogenous antioxidants. For example, several investigators have documented lower circulating levels of α-tocopherol and ascorbate in association with increased levels of oxidized glutathione in the plasma of critically ill patients. 7–13 Given the apparent role oxidative stress and oxidant-mediated tissue injury play in the development of ARDS and multiple organ failure, supplementation with antioxidants may augment endogenous antioxidant defenses and serve to prevent the development of organ dysfunction. Further, there is increasing evidence that antioxidants, particularly ascorbic acid and α-tocopherol, may reduce the incidence of infectious complications, putatively by restoring neutrophil function and cell-mediated immunity, respectively. 14–16
To date, prospective randomized trials have demonstrated little benefit from antioxidant supplementation. However, these studies, all carried out in mixed ICU populations, initiated supplementation after the development of organ dysfunction. 17–19 We postulated that patients would derive the greatest benefit if antioxidants were administered prophylactically, prior to the development of secondary organ injury and/or nosocomial infection. To test this hypothesis, we evaluated the efficacy of early, prophylactic antioxidant supplementation using a combination of α-tocopherol and ascorbate in a cohort of critically ill surgical patients admitted to an ICU. Specifically, we postulated that patients receiving antioxidants would have a lower incidence of ARDS, nosocomial pneumonia, and multiple organ failure.
METHODS
Protocol
Subjects were enrolled in this prospective, randomized trial from February 1999 to June 2000. Patients were eligible for the trial if they were 16 to 74 years old, were admitted to the ICU under the general surgery/trauma service, and were available for enrollment within 24 hours of sustaining their injury (in the case of trauma patients) or undergoing an emergency operation. Patients with isolated or severe (Glasgow Coma Scale score = 6 or less) head injury, brain death, anticipated survival less than 48 hours, burns over more than 20% body surface area, sickle cell anemia, need for anticoagulation with coumadin while in the ICU, and chronic renal failure (creatinine > 2.5 mg/dL) were excluded from the trial. Subjects were randomized at the time of ICU admission or shortly thereafter.
Following treatment assignment, patients randomized to antioxidant supplementation received α-tocopherol (dl-α-tocopheryl acetate; Aquasol E, Astra USA, Westborough, MA) 1,000 IU (20 mL) q8h per naso- or orogastric tube and 1,000 mg ascorbic acid given intravenously (American Reagent Labs, Shirley, NY) in 100 mL D5W q8h for the shorter of the duration of admission to the ICU or 28 days. We believed that given the waiver of consent provided to us by our institutional review board (vide infra), we could not administer a dose not clearly known to be safe. Therefore, these doses represent the highest reported doses administered to humans that have no significant side effects. 16–20 Patients randomized to the control group received standard care, with the only restriction that they not receive any antioxidant supplementation while in the ICU. The low levels of antioxidants in our commonly used enteral feeding preparations were permissible as were the standard multivitamin preparation used for patients receiving total parenteral nutrition (TPN). Our most commonly used product for enteral nutrition was Replete with Fiber (Clintec, Deerfield, IL), which contains 340 mg ascorbic acid and approximately 60 IU α-tocopherol per liter. The multivitamin preparation used while on TPN provided 100 mg ascorbic acid and 10 IU α-tocopherol daily.
The institutional review board waived the need for informed consent due to the minimal risk involved and the inability to carry through with the critical early randomization scheme if direct consent were required. Informational fliers were posted in the ICU and the adjacent waiting rooms to inform the next-of-kin of the study protocol and to allow for withdrawal at their request. Similarly, as enrollment was automatic upon admission to the ICU, the attending surgeon had the right to refuse entry of his or her patient into the study at his or her discretion.
Patients were followed for the shorter of the duration of their hospital admission or 28 days. Baseline characteristics, including demographic information, comorbidities, anatomic (Injury Severity Score [ISS]) and physiologic (APACHE II) markers of disease severity, and admitting diagnoses, were assessed. The primary efficacy endpoint of this study was the proportion of patients who developed either pneumonia or ARDS on or before day 28 (Table 1). 21 This composite endpoint will be referred to as “pulmonary morbidity.” A radiologist not involved with the study and blinded to the treatment arm provided objective assessment of the radiologic criteria to limit bias in the evaluation of chest radiographs for assessment of pneumonia or ARDS. The prospectively defined secondary efficacy endpoints included the development of multiple organ failure, 22 the Multiple Organ Dysfunction Score, 23 ventilator-free days, duration of ICU and hospital length of stay, and 28-day mortality.
Table 1. DEFINITION OF PRIMARY ENDPOINTS

Our prospectively defined primary analysis included all patients who met the inclusion/exclusion criteria and were randomized, with patients analyzed according to the treatment group to which they were assigned at randomization (intent-to-treat analysis). To detect a 25% proportionate decrease in pulmonary morbidity using an α of 0.05 (two-tailed) and β of 0.20, approximately 375 patients per group were necessary, based on an incidence of pulmonary morbidity in the ICU of approximately 40%. Differences between the two groups are expressed as the relative risk (or relative difference for continuous variables) and corresponding 95% confidence intervals (CI) of developing the primary or secondary efficacy endpoint in the treatment group compared to the cohort receiving standard care. Time-to-event curves describing the proportion of patients who reached any endpoint during the 28-day period, according to treatment group, were calculated by the Kaplan-Meier method and compared with the use of log-rank tests. Analyses were conducted using Stata Statistical Software (release 6.0, Stata Corp., College Station, TX, 1999).
One in four of the first 200 patients enrolled were selected randomly for evaluation of circulating plasma levels of ascorbate and α-tocopherol at baseline and on days 1, 3, 5, 7, 14, and 21 following admission. Plasma α-tocopherol levels were assessed using high-performance liquid chromatography. 24 Plasma ascorbic acid levels were measured spectrophotometrically following the conversion of ascorbate to dehydroascorbate by ascorbic acid oxidase and subsequent conversion to the quinoxolone derivative by a fast reaction with o-phenylenediamine at pH 6.5. 25
Trauma patients with an ISS of more than 15 and who remained intubated 72 hours following admission were eligible for assessment of the alveolar inflammatory response by collection of alveolar lining fluid using bronchoalveolar lavage (BAL). After establishing eligibility, informed consent was obtained from the patient’s legal next-of-kin and bronchoscopy was performed on day 3 using a previously established protocol. 26 Briefly, after obtaining adequate anesthesia and sedation, the fiberoptic bronchoscope was inserted through an adapter on the endotracheal tube and guided to the right middle lobe, where it was wedged. Five 30-mL aliquots of 0.9% NaCl were instilled sequentially and manually suctioned each time to recover BAL fluid. Visual inspection of the airways was completed for any evidence of bleeding or infection, and the bronchoscope was removed. The patient was monitored closely and returned to previous ventilator settings, usually within 60 minutes. BAL fluid was processed and evaluated for cell count, concentrations of protein, tumor necrosis factor-α, interleukin-1 (IL-1), IL-6, IL-8, and soluble ICAM-1 (sICAM-1), as previously described. 27,28 As a measure of oxidative stress, concentrations of F2α isoprostanes were assessed in BAL fluid using a commercially available enzyme immunoassay (Cayman Chemical, Ann Arbor, MI). F2α isoprostanes are prostaglandin-like compounds formed from the peroxidation of arachidonic acid and are considered a reliable marker of lipid peroxidation. 29,30
Information on adverse effects specific to ascorbate and α-tocopherol were collected. 20 Specifically, the development of acute renal failure (creatinine > 2.5 mg/dL) was documented, and antioxidant supplementation was discontinued if these patients became oliguric. Since α-tocopherol may exacerbate an underlying coagulopathy due to vitamin K depletion, we recorded the highest INR in the ICU beyond day 2.
Assignment
At the time of ICU admission, patients were randomized in a 1:1 ratio to either antioxidant supplementation or routine care. The pharmacist automatically assigned treatment based on a computer-generated random-number sequence. The investigators were unaware of the treatment allocation prior to randomization. This randomization scheme also allowed for random selection of subjects for sampling of serum vitamin levels in the first 200 patients enrolled. The pharmacy was instructed to randomize all patients fulfilling the inclusion criteria described above. Patients randomized who were later identified by the study coordinator as meeting exclusion criteria based on data available at the time of randomization were withdrawn from the study.
RESULTS
During the study, 770 patients were randomized at the time of ICU admission. Of these 770, 175 met the predefined exclusion criteria at the time of randomization and were withdrawn from the study shortly after enrollment (Table 2), resulting in a study population of 595 patients: 301 randomized to antioxidant supplementation arm and 294 to standard care. The reasons for exclusion were similar across groups. Forty patients randomized to the treatment group (14%) were not in the ICU long enough to receive any supplementation yet were included in the intent-to-treat analysis. There were no patients in the standard therapy group that received antioxidant supplementation such that overall, 555 (93%) of patients received the treatment to which they were assigned.
Table 2. PATIENTS WITHDRAWN FROM STUDY POST-RANDOMIZATION*
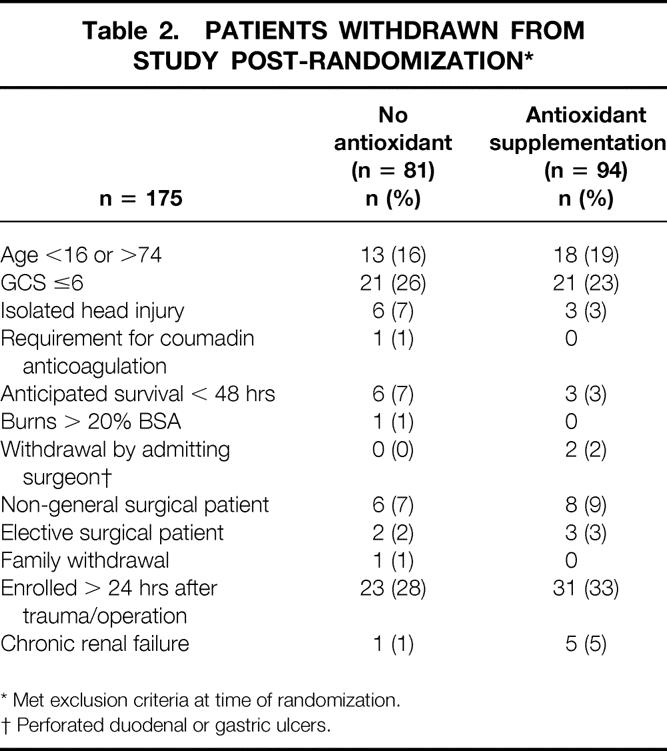
* Met exclusion criteria at time of randomization.
† Perforated duodenal or gastric ulcers.
Baseline Characteristics of Study Population
The study population consisted predominantly of young men, with trauma being the most frequent principal diagnosis (Table 3). On the whole, patients were healthy, with no documented comorbidity in 509 (85%) patients. In patients with a diagnosis of trauma the mean ISS was 19.6 ± 11; the mean APACHE II score in the entire cohort of patients was 14.0 ± 6. Overall, 171 (29%) patients presented with shock (systolic blood pressure < 90 mmHg). There were no differences in any of the baseline characteristics between the two patient groups. The mean time to initiation of antioxidant supplementation was 11.3 ± 6 hours.
Table 3. BASELINE CHARACTERISTICS OF SUBJECTS
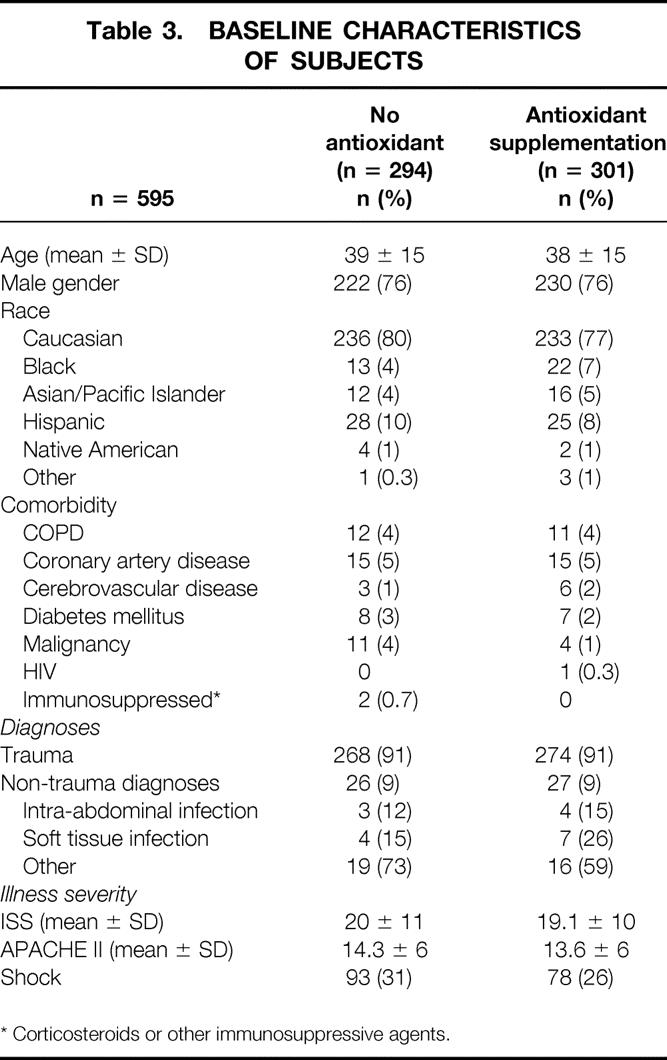
* Corticosteroids or other immunosuppressive agents.
Primary Efficacy Analysis
Twenty-eight days after enrollment, 36 (11.9%) patients receiving antioxidant supplementation had developed pneumonia, compared to 44 patients (15.0%) receiving standard care. The relative risk of pneumonia in the treatment group was 0.79 (95% CI 0.53–1.20) and no statistically significant difference was evident in the time-to-event analysis (P = .3, Fig. 1). Over the same time period, ARDS developed in 47 (15.6%) patients in the treatment group and 53 (18%) patients randomized to standard care. The relative risk of developing ARDS was 0.86 (95% CI 0.60–1.24) in patients receiving antioxidants. Similar to pneumonia, the time-to-event analysis did not demonstrate a statistically significant benefit (P = .4, Fig. 2).
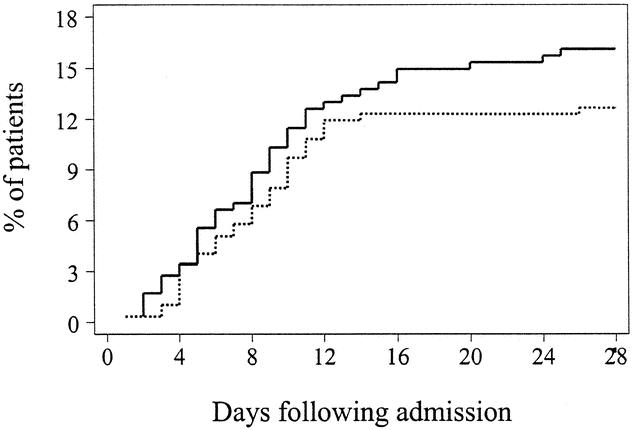
Figure 1. Kaplan-Meier estimates of the risk of pneumonia among 301 patients receiving antioxidant supplementation and 294 patients receiving standard care. There is some suggestion that antioxidant supplementation might be associated with a lower likelihood of pneumonia (P = .3 by the log-rank test). Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
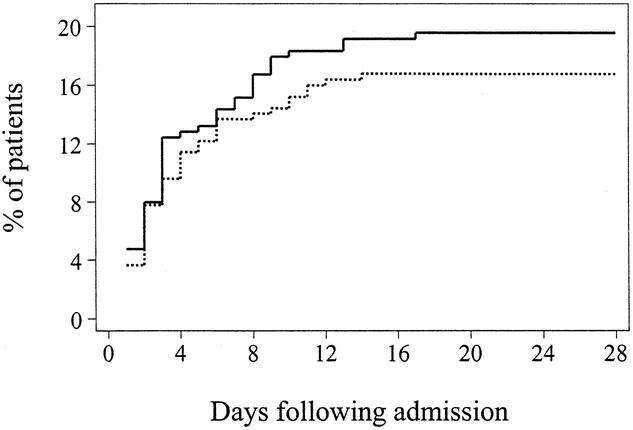
Figure 2. Kaplan-Meier estimates of the risk of ARDS among 301 patients receiving antioxidant supplementation and 294 patients receiving standard care. There is some suggestion that antioxidant supplementation might be associated with a lower likelihood of ARDS (P = .4 by the log-rank test). Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
The composite primary endpoint, ARDS and/or pneumonia, was identified in 60 (19.9%) patients receiving antioxidant supplementation and in 72 (24.5%) patients receiving standard care, for a relative risk of pulmonary morbidity of 0.81 (95% CI 0.60–1.1) in patients receiving antioxidants. The cumulative hazard function in the time-to-event analysis demonstrated no statistically significant difference across the two interventions (P = .2, Fig. 3).
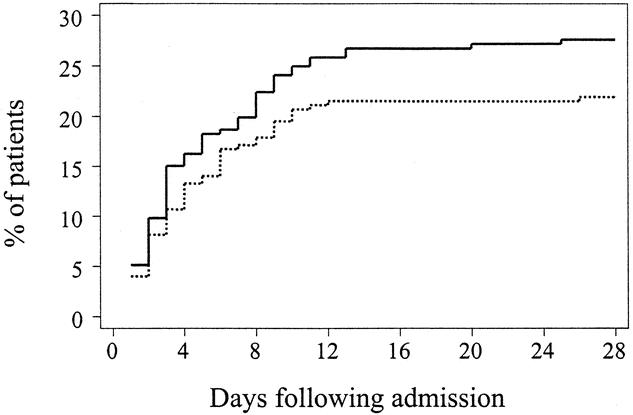
Figure 3. Kaplan-Meier estimates of the risk of pulmonary morbidity (ARDS or pneumonia) among 301 patients receiving antioxidant supplementation and 294 patients receiving standard care. There is a suggestion that antioxidant supplementation might be associated with a lower likelihood of pulmonary morbidity (P = .2 by the log-rank test). Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
Secondary Efficacy Analysis
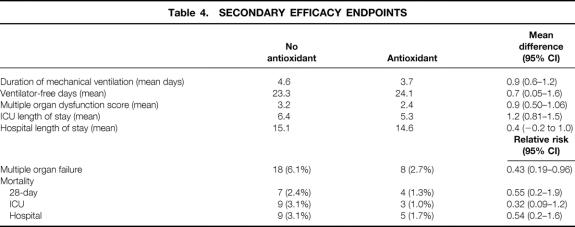
Overall, multiple organ failure occurred in only 26 (4%) patients. However, it was significantly less likely to occur in patients receiving antioxidants than in patients receiving standard care, with a relative risk of 0.43 (95% CI 0.19–0.96, Fig. 4). This effect was underscored by the significantly lower Multiple Organ Dysfunction Score in the treatment group (Table 4). There were also significant benefits in terms of resource utilization. Patients randomized to antioxidant supplementation required nearly 1 less day (0.9, 95% CI 0.6–1.2) of mechanical ventilatory support than patients receiving standard care and had 0.7 (95% CI 0.05–1.63) more ventilator-free days over the 28-day period of analysis (see Table 4). Moreover, patients receiving antioxidants had a 1.2-day (95% CI 0.81–1.5) reduction in their ICU length of stay and a 0.4-day (95% CI: −0.2–1.0) reduction in their hospital length of stay.
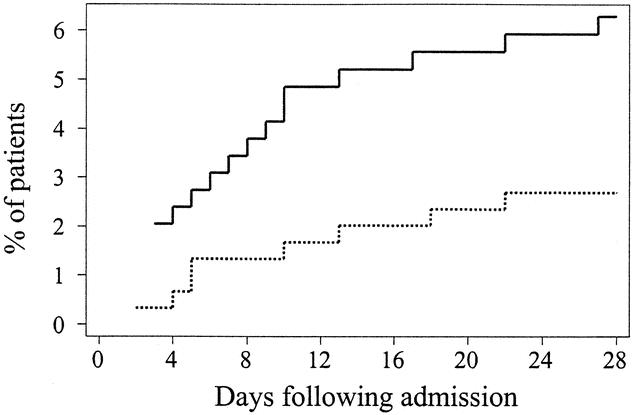
Figure 4. Kaplan-Meier estimates of the risk of multiple organ failure among 301 patients receiving antioxidant supplementation and 294 patients receiving standard care. Treatment with antioxidant supplementation was associated with a significant reduction in the risk of developing multiple organ failure (P = .04 by the log-rank test). Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
Table 4. SECONDARY EFFICACY ENDPOINTS
In this study, 28-day mortality was only 1.8% (11/595) in the entire cohort of patients. Mortality tended to be lower in the antioxidant-supplemented patients: four (1.3%) patients died in the supplemented group and 7 (2.3%) patients died in the cohort receiving standard care (see Table 4). The relative risk of death in the treatment group was 0.55 (95% CI 0.17–1.88). Similar benefits were also evident when ICU mortality and hospital mortality were examined.
Plasma Antioxidant Concentrations
Plasma ascorbate concentrations were similar in both cohorts at baseline and approached the lower limit of normal (Fig. 5). Ascorbate concentrations remained at or below this level for as long as 3 weeks in the group receiving standard care. In contrast, plasma ascorbate concentrations normalized within 1 day of admission and met or exceeded the upper limit of normal in the supplemented group. Similarly, plasma α-tocopherol concentrations were comparable in both groups at baseline and diverged by 3 days of treatment, reaching supranormal levels by day 5 (Fig. 6).
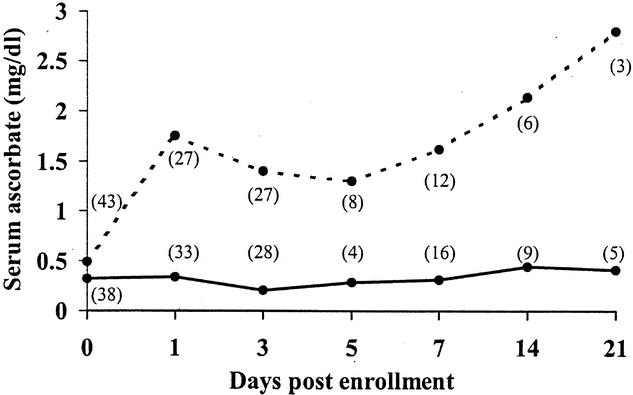
Figure 5. Plasma concentrations of ascorbate in a subset of patients receiving antioxidant supplementation and those receiving standard care. Shaded area represents normal range for healthy individuals. Numbers in parentheses indicate number of patients available for sampling. Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
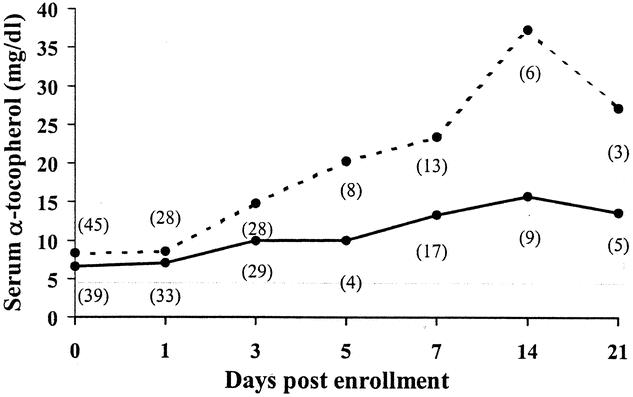
Figure 6. Plasma concentrations of α-tocopherol in a subset of patients receiving antioxidant supplementation and those receiving standard care. Shaded area represents normal range for healthy individuals. Numbers in parentheses indicate number of patients available for sampling. Solid line: no antioxidant supplementation; dashed line: antioxidant supplementation.
Alveolar Inflammatory Response
BAL fluid F2α isoprostane levels are shown in Figure 7. Median levels appeared to be lower in the patients receiving antioxidant supplementation (14.8 pg/mL vs. 70.7 pg/mL, P = .5). Associated with lower levels of oxidative stress in the antioxidant group was a tendency for an attenuated alveolar inflammatory response, as demonstrated by a lower alveolar white blood cell and protein concentration as well as reduced concentrations of TNF-α, IL-1β, and IL-6 (Table 5). No consistent response was evident upon assessment of IL-8 or sICAM-1 concentrations.
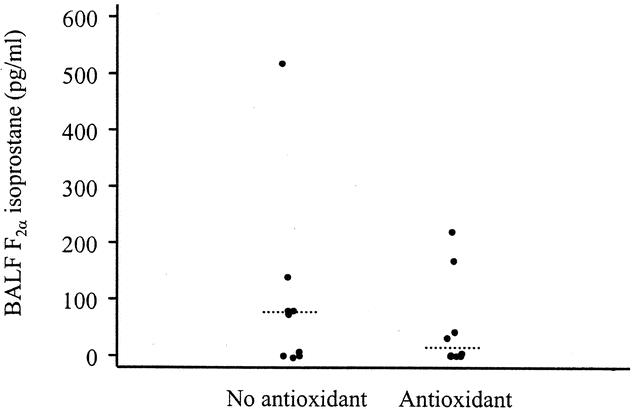
Figure 7. Concentrations of alveolar lavage fluid F2α isoprostanes on day 3 as a measure of oxidative stress in patients undergoing BAL in the antioxidant group (n = 9) and those receiving standard care (n = 9). Median values are demonstrated as a dashed line (P = .5, Wilcoxon rank-sum test).
Table 5. ALVEOLAR INFLAMMATORY RESPONSE*
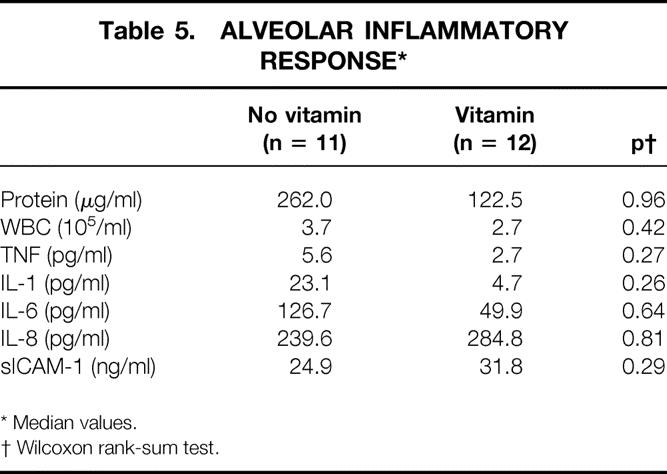
* Median values.
† Wilcoxon rank-sum test.
Complications
The incidence of acute renal failure was no different between the two groups: three (1%) patients in the treatment group and four (1.3%) patients in the control group developed acute renal failure (creatinine > 2.5 mg/dL). There was no difference in the highest INR over the course of ICU stay: 1.4 in patients receiving antioxidants and 1.5 in patients receiving standard care.
DISCUSSION
A significant body of evidence suggests that oxidative stress plays an important role in the manifestations of critical illness. ROS induce direct oxidative tissue injury by means of peroxidation of cellular membranes, oxidation of critical enzymatic and structural proteins, and induction of apoptosis. Additionally, extensive in vitro data support the relationship between oxidative stress and the induction of genes integral to the systemic inflammatory response, including TNF-α, IL-1, IL-8, and ICAM-1, putatively mediated through activation of the nuclear transcription factor NF-κB. 2,31,32 Several in vivo studies document a clear benefit to antioxidant supplementation in animal models of endotoxemia, ARDS, and reperfusion injury 4,5,33–37 and have confirmed that there is a relationship between oxidative stress and NF-κB activation in vivo. 4
The importance of oxidative stress in the early phases of critical illness is underscored by the relative antioxidant depletion reported in many observational studies of this population. For example, patients admitted to the ICU have reduced total antioxidant capacity, 38,39 with lower circulating levels of α-tocopherol 9–11 and serum ascorbate 11–13 and increased alveolar fluid levels of oxidized glutathione. 7 The relative decrease in antioxidant capacity appears to correlate with the severity of illness and suggests a causal relationship between antioxidant depletion and increasing levels of organ dysfunction. 8,12,13,38,39 This relationship is strengthened by evidence of oxidative stress, manifest in these patients by a substantial increase in products of lipid peroxidation. 8,11,40 The evidence for oxidative stress in the critically ill patient, coupled with the potential for direct oxidative tissue injury and induction of the systemic inflammatory response, provides a sound biologic rationale for antioxidant supplementation. However, in contrast to animal studies, clinical trials using the cysteine-based moiety N-acetylcysteine have demonstrated either no or marginal benefit. 17,18,41 In all of these studies the antioxidant was initiated after the development of acute lung injury. By contrast, in a small prospective randomized controlled trial in high-risk trauma patients in which a combination of α-tocopherol, ascorbate, selenium, and N-acetylcysteine was administered early during the resuscitation phase, there was a dramatic but not statistically significant reduction in the incidence of multiple organ failure and infectious complications. 42 These data suggest the potential for benefit if antioxidants are administered prophylactically, before the onset of significant organ dysfunction and infection.
The present study examined the effect of α-tocopherol and ascorbate in preventing the development of pulmonary morbidity (ARDS, pneumonia) and organ failure in a cohort of severely ill surgical patients, the majority of whom were victims of trauma. At ICU admission, patients receiving standard care and those randomized to antioxidant supplementation were comparable based on reason for ICU admission and severity of illness and anatomic injury. Patients receiving antioxidant supplementation demonstrated a 19% (95% CI −10%–40%) reduction in incidence of pulmonary morbidity. As a result of a lower-than-expected incidence of the primary endpoint, the power to detect a difference across the two groups was only 24%. This study would have required 1,300 patients per group to have 80% power to detect a difference at an α of 0.05.
The treatment group had a 57% (95% CI 4–81%) lower incidence of multiple organ failure and a trend toward a reduction in 28-day mortality. These benefits translated into a reduction in resource utilization, as measured by a shorter duration of mechanical ventilation and ICU length of stay. Further, there were no adverse effects attributable to the antioxidants. Specifically, administration of high-dose ascorbate or α-tocopherol did not increase the risk of renal failure or coagulopathy. 20
The mechanisms by which antioxidant supplementation exerted its beneficial effects in this cohort of patients are unclear. Alveolar fluid levels of F2α isoprostane tended to be lower in patients receiving antioxidants, as were concentrations of TNF-α, IL-1β, and IL-6. These data imply that antioxidants are reducing both the amount of oxidative tissue injury and the early inflammatory response, possibly through effects on gene activation. Paradoxically, there appears to be no effect on alveolar fluid concentrations of sICAM-1 and IL-8, two other proteins whose genes have previously been reported to be sensitive to redox alterations. 31,32 The small number of patients in whom biochemical measures of oxidative stress and alveolar inflammation were available and the intrinsic biologic variability preclude making any definitive conclusions from a mechanistic standpoint.
This trial has a relatively homogenous population as one of its strengths. This homogeneity reduces the intrinsic noise and variability and thus increases the likelihood of observing a measurable effect. However, this homogeneity reduces the generalizability of the intervention. For example, trauma patients tend to be male and younger and have little comorbidity. Further, the time of the physiologic insult is well established. It is unclear whether there might be any observable benefit in a mixed cohort of patients with significant comorbidity in whom the time of onset is less clear (e.g., sepsis) and in whom there are a variety of different diagnoses.
One additional limitation is the lack of a placebo and blinding on the part of the investigators, potentially introducing bias into the evaluation of the endpoints. However, the definitions for all of the endpoints had objective criteria. In addition, a radiologist not involved with the study and blinded to the treatment arm provided objective assessment of the radiologic criteria to limit bias in the evaluation of chest radiographs for assessment of pneumonia or ARDS. There exists the possibility that knowledge of the treatment group might alter care, and this alone may have an impact on outcome independent of any effect of treatment; however, management was not influenced by the knowledge that a patient was or was not receiving antioxidant therapy.
This large, randomized prospective trial in a cohort of critically ill surgical patients suggests benefit from the routine early, prophylactic administration of α-tocopherol and ascorbate. Potentially greater benefit may be obtained if an intravenous formulation of α-tocopherol were available and higher circulating levels were achieved early in the course of illness; however, no such agent is currently approved for use in humans. The lack of adverse effects, coupled with the minimal expense, supports that this combination is a reasonable therapeutic intervention in critically surgical patients.
Footnotes
Supported by grant R49/CCR002570 from the Centers for Disease Control and Prevention and a Surgical Infection Society Fellowship in Evaluative Sciences, supported by Wyeth-Ayerst Laboratories. Presented in part at the Annual Meeting of the Surgical Infection Society, Snowbird, Utah, May 2001.
Correspondence: Avery B. Nathens, MD, MPH, Harborview Medical Center, Box 359796, 325 9th Ave., Seattle, WA 98104-2499.
E-mail: anathens@u.washington.edu
Accepted for publication June 24, 2002.
References
- 1.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 2000; 29: 323–333. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler-Heitbrock HW, Sternsdorf T, Liese J, et al. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol 1993; 151: 6986–6993. [PubMed] [Google Scholar]
- 3.Sznajder JI, Fraiman A, Hall JB, et al. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest 1989; 96: 606–612. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell TS, Blackwell TR, Holden EP, et al. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol 1996; 157: 1630–1637. [PubMed] [Google Scholar]
- 5.Nathens AB, Bitar R, Davreux C, et al. Pyrrolidine dithiocarbamate attenuates endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol 1997; 17: 608–616. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MD, Moore EE, Moore FA, et al. Nuclear factor-kappa B is activated in alveolar macrophages from patients with the acute respiratory distress syndrome. Crit Care Med 1996; 24: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1993; 148: 1174–1178. [DOI] [PubMed] [Google Scholar]
- 8.Goode HF, Cowley HC, Walker BE, et al. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med 1995; 23: 646–651. [DOI] [PubMed] [Google Scholar]
- 9.Richard C, Lemonnier F, Thibault M, et al. Vitamin E deficiency and lipid peroxidation during adult respiratory distress syndrome. Crit Care Med 1990; 18: 4–9. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand Y, Pincemail J, Hanique G, et al. Differences in tocopherol-lipid ratios in ARDS and non-ARDS patients. Intensive Care Med 1989; 15: 87–93. [DOI] [PubMed] [Google Scholar]
- 11.Metnitz PG, Bartens C, Fischer M, et al. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med 1999; 25: 180–185. [DOI] [PubMed] [Google Scholar]
- 12.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996; 24: 392–397. [DOI] [PubMed] [Google Scholar]
- 13.Schorah CJ, Downing C, Piripitsi A, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr 1996; 63: 760–765. [DOI] [PubMed] [Google Scholar]
- 14.Hemila H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J 1997; 16: 836–837. [DOI] [PubMed] [Google Scholar]
- 15.Maderazo EG, Woronick CL, Hickingotham N, et al. A randomized trial of replacement antioxidant vitamin therapy for neutrophil locomotory dysfunction in blunt trauma. J Trauma 1991; 31: 1142–1150. [PubMed] [Google Scholar]
- 16.Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: a randomized controlled trial. JAMA 1997; 277: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 17.Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997; 112: 164–172. [DOI] [PubMed] [Google Scholar]
- 18.Suter PM, Domenighetti G, Schaller M-D, et al. N-acetylcysteine enhances recovery from acute lung injury in man. Chest 1994; 105: 190–194. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen S, Herlevsen P, Knudsen P, et al. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo controlled study. Crit Care Med 1992; 20: 918–923. [DOI] [PubMed] [Google Scholar]
- 20.Diplock AT. Safety of antioxidant vitamins and beta-carotene. Am J Clin Nutr 1995; 62: 1510S–1516S. [DOI] [PubMed] [Google Scholar]
- 21.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS. Am J Respir Crit Care Med 1994; 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 22.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg 1994; 129: 39–45. [DOI] [PubMed] [Google Scholar]
- 23.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23: 1638–1652. [DOI] [PubMed] [Google Scholar]
- 24.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of α-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr 1979; 32: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 25.Tulley RT. New enzymatic method for vitamin C in plasma on CX5. Clin Chem 1992; 38: 1070. [Google Scholar]
- 26.Steinberg KP, Mitchell DR, Maunder RJ, et al. Safety of bronchoalveolar lavage in patients with adult respiratory distress syndrome. Am Rev Resp Dis 1993; 148: 556–561. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg KP, Milberg JA, Martin TR, et al. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 150: 113–122. [DOI] [PubMed] [Google Scholar]
- 28.Goodman RB, Strieter RM, Martin DP, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Resp Crit Care Med 1996; 154: 601–611. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 1998; 114: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 30.Roberts LJ, Morrow JD. Isoprostanes. Novel markers of endogenous lipid peroxidation and potential mediators of oxidant injury. Ann NY Acad Sci 1994; 744: 237–242. [DOI] [PubMed] [Google Scholar]
- 31.DeForge LE, Preston AM, Takeuchi E, et al. Regulation of IL-8 gene expression by oxidant stress. J Biol Chem 1993; 268: 25568–25576. [PubMed] [Google Scholar]
- 32.Lo SK, Janakidevi K, Lai L, et al. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am J Physiol 1993; 264: L406–L412. [DOI] [PubMed] [Google Scholar]
- 33.Davreux CJ, Soric I, Nathens AB, et al. N-acetyl cysteine attenuates acute lung injury in the rat. Shock 1997; 8: 432–438. [PubMed] [Google Scholar]
- 34.Hybertson BM, Leff JA, Beehler CJ, et al. Effect of vitamin E deficiency and supercritical fluid aerosolized vitamin E supplementation on interleukin-1 induced oxidative lung injury in rats. Free Radic Biol Med 1995; 18: 537–542. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MD, Repine JE, Abraham E. Xanthine oxidase-derived oxygen radicals increase lung cytokine expression in mice subjected to hemorrhagic shock. Am J Respir Cell Mol Biol 1995; 12: 434–440. [DOI] [PubMed] [Google Scholar]
- 36.Terada LS, Dormish JJ, Shanley PF, et al. Circulating xanthine oxidase mediates lung neutrophil sequestration after intestinal ischemia-reperfusion. Am J Physiol 1992; 263: L394–L401. [DOI] [PubMed] [Google Scholar]
- 37.Bulger EM, Helton WS, Clinton CM, et al. Enteral vitamin E supplementation inhibits the cytokine response to endotoxin. Arch Surg 1997; 132: 1337–1341. [DOI] [PubMed] [Google Scholar]
- 38.Alonso DV, Diaz J, Serrano E, et al. Plasma redox status relates to severity in critically ill patients. Crit Care Med 2000; 28: 1812–1814. [DOI] [PubMed] [Google Scholar]
- 39.Cowley HC, Bacon PJ, Goode HF, et al. Plasma antioxidant potential in severe sepsis: a comparison of survivors and nonsurvivors. Crit Care Med 1996; 24: 1179–1183. [DOI] [PubMed] [Google Scholar]
- 40.Quinlan GJ, Lamb NJ, Evans TW, et al. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med 1996; 24: 241–246. [DOI] [PubMed] [Google Scholar]
- 41.Molnar Z, MacKinnon KL, Shearer E, et al. The effect of N-acetylcysteine on total serum anti-oxidant potential and urinary albumin excretion in critically ill patients. Intensive Care Med 1998; 24: 230–235. [DOI] [PubMed] [Google Scholar]
- 42.Porter JM, Ivatury RR, Azimuddin K, et al. Antioxidant therapy in the prevention of organ dysfunction syndrome and infectious complications after trauma: early results of a prospective randomized study. Am Surg 1999; 65: 478–483. [PubMed] [Google Scholar]