Abstract
Objective
To analyze voice function before and after thyroidectomy for patients with normal preoperative voice using a standardized multidimensional voice assessment protocol.
Summary Background Data
The natural history of post-thyroidectomy voice disturbances for patients with preserved laryngeal nerve function has not been systematically studied and characterized with the intent of using the data for postoperative voice rehabilitation.
Methods
During a prospective single-arm study, patients with normal voice underwent functional voice testing using a standardized voice grading scale and a battery of acoustic, aerodynamic, glottographic, and videostroboscopic tests before, 1 week after, and 3 months after thyroidectomy. Differences in observed sample means were evaluated using analysis of covariance or t test; categorical data was analyzed using the Fisher exact or chi-square test.
Results
Fifty-four patients were enrolled; 50 and 46 were evaluable at 1 week and 3 months, respectively. No patient developed recurrent laryngeal nerve injury; one had superior laryngeal nerve injury. Fifteen (30%) patients reported early subjective voice change and seven (14%) reported late (3-month) subjective voice change. Forty-two (84%) patients had significant objective change in at least one voice parameter. Six (12%) had significant alterations in more than three voice measures, of which four (67%) were symptomatic, whereas 25% with three or fewer objective changes had symptoms. Patients with persistent voice change at 3 months had an increased likelihood of multiple (more than three) early objective changes (43% vs. 7%). Early maximum phonational frequency range and vocal jitter changes from baseline were significantly associated with voice symptoms at 3 months.
Conclusions
Early vocal symptoms are common following thyroidectomy and persist in 14% of patients. Multiple (more than three) objective voice changes correlate with early and late postoperative symptoms. Alterations in maximum phonational frequency range and vocal jitter predict late perceived vocal changes. Factors other than laryngeal nerve injury appear to alter post-thyroidectomy voice. The variability of patient symptoms underscores the importance of understanding the physiology of dysphonia.
Operative injury to the recurrent laryngeal nerve is a feared morbidity of thyroid surgery, with attendant serious implications for the patient. More than 50% of patients with unilateral recurrent laryngeal nerve (RLN) palsy may be asymptomatic. 1 The frequency of asymptomatic vocal fold palsy has led some thyroid surgeons to examine the vocal folds preoperatively and to incorporate laryngoscopy as part of postoperative follow-up. Routine identification and preservation of the RLN is not the only factor to consider in the attempt to preserve normal post-thyroidectomy voice.
Injury to the external branch of the superior laryngeal nerve (EBSLN) is another voice-altering complication of thyroid surgery that has significant implications for professional voice users. The symptoms of EBSLN injury can be nonspecific and the subtle laryngoscopic manifestations are often overlooked. 2–4 Advanced diagnostic techniques for evaluating EBSLN have been developed and have documented a significant incidence of this morbidity (5–28%). 2,5,6 The absence of effective treatment for EBSLN palsy and the poor prospects for recovery make prevention crucial.
Preservation of laryngeal nerve integrity is important in sustaining voice function, yet not all voice alterations following thyroidectomy are related to nerve injury. In fact, voice disturbances have been demonstrated in cases where the laryngeal nerves have been preserved; in this situation, voice disturbances have been attributed to surgical trauma and laryngotracheal fixation of the prelaryngeal strap musculature. 7 Vocal fold changes have been observed in 5% of patients following endotracheal intubation alone. 8 Some postoperative voice changes may be attributable to arytenoids trauma sustained during tracheal intubation.
The natural history of post-thyroidectomy voice disturbances for patients with preserved nerve function has not been systematically studied, and its characterization is important for voice rehabilitation. We have conducted a prospective evaluation of patients with normal preoperative voice undergoing thyroid surgery in order to characterize postoperative functional voice changes using a multidimensional voice assessment protocol. The results of early and late postoperative voice analysis are presented.
METHODS
This was a prospective single-arm study of patients with benign and malignant thyroid pathology scheduled to undergo primary thyroid surgery. The study represents a consecutive cohort of patients who met inclusion criteria and underwent thyroidectomy from September 2000 to April 2001. Patients underwent preoperative functional voice testing. The degree of presenting dysphonia was determined using a standardized voice grading scale. Vocal function was assessed further with a battery of acoustic, aerodynamic, glottographic, phonoscopic, and videostroboscopic tests as part of a standardized voice assessment protocol. One to 2 weeks and 3 months following surgery, patients underwent identical voice grading and functional assessment. The Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSKCC) approved this study protocol (MSKCC #00-035). All patients underwent surgery at MSKCC by one of seven surgeons who participated in the trial.
Eligibility Criteria
Patients were eligible if they were at least 18 years of age and had benign thyroid pathology or differentiated thyroid carcinoma and were scheduled to undergo thyroidectomy. Eligible patients had no history of professional voice training, no prior voice pathology requiring therapy, and no previous neck surgery. Patients who met inclusion criteria signed informed consent.
Patients were ineligible if they had previously undergone neck or thyroid surgery or had isthmusectomy only or radical lymphadenectomy at the time of surgery. Patients with known dysphonia, vocal fold paralysis, or a history of speech disorder, those unable to sustain phonation for longer than 10 seconds, or those with hearing impairment were excluded. Patients with anaplastic or medullary carcinoma of the thyroid were also excluded.
Functional Voice Assessment
All voice data were acquired in the Laryngology Laboratory of the Speech, Hearing and Rehabilitation Center at MSKCC. Voice testing was conducted preoperatively and 1 to 2 weeks and 3 months after thyroidectomy. A multidimensional approach to voice analysis was used to define precisely how the vocal signal was generated, transmitted, and perceived by the listener.
Clinical Examination
Pre- and postoperative assessment of voice included the patient’s and the physician’s appraisal of voice. The degree of dysphonia was evaluated using a modified version of the GRBAS scale that documents perceived grade, roughness, breathiness, and asthenic and strained quality of the voice. 9 Changes in voice pitch, range, intensity, fatigability, and singing quality were assessed during the interview.
Acoustic Testing (Vocal Flexibility and Stability)
The acoustic signal was recorded with a Telex unidirectional microphone maintained at a mouth-to-microphone distance of ∼3 cm. The signal was digitized, analyzed, and stored using a Kay Elemetrics Computer Speech Lab System (model 4300B) and Multi-Dimensional Voice Program (MDVP) software. The assessment of maximum phonational frequency range (MPFR, Hz or semitones) and dynamic intensity range (DynR, dB SPL) were combined using a two-dimensional plot called a voice range profile. 10
Another measure of vocal capacity is the maximum phonation time, a measure of the ability to regulate ventilatory and laryngeal systems for voice production independent of a frequency or intensity target. 11 Maximum phonation time was obtained by having the patient sustain the vowel “a” for as long as possible on a single breath while observing the display of the Kay Elemetrics CSL-4300B computerized oscillogram. The longest of three attempts was recorded as the maximum phonation time.
Detailed appraisal of vocal stability was achieved with frequency-based voice measures including mean vocal fundamental frequency (F0, Hz), F0 variability (%), mean percentage vocal jitter and shimmer, and noise-to-harmonics ratio (dB). Mean percentage vocal jitter and shimmer are indices of the cycle-to-cycle variability of vocal period and amplitude, respectively. Measures of vocal jitter and shimmer are relative average perturbation (RAP, %) and amplitude perturbation quotient (APQ, %), respectively. Vocal stability data were assessed with each patient instructed to sustain three maximally steady phonations of the vowel “a” at a self-selected comfortable pitch and level of loudness. F0, F0 variability, relative average pertubation, amplitude pertubation quotient, and noise-to-harmonics ratio were derived from the middle 2 seconds of each vowel prolongation and averaged across the trials.
Electroglottography
The electroglottographic signal provides an accurate assessment of the vocal fold pattern of vibration, and the regularity and degree of vocal fold contact during phonation. 12 Noninvasive, transcervical impedence plethysmography was used to transduce changes in the vocal fold contact area during the vibratory cycle. The patient’s vocal fold contact pattern was recorded using a two-channel electroglottograph (Glottal Enterprises, model MC2). The contact quotient (CQ) provided a quantitative measure of vocal fold approximation during phonation.
Airflow and Air Pressure Recording
Airflow and air pressure data were digitized, analyzed, and stored using an Aerophone II system (Kay Elemetrics, Lincoln Park, NJ). Airflow was measured with a facemask, sealed over the nose and mouth, connected to a pneumotachograph-based flow system. Mean phonatory airflow was measured during three comfortable prolongations of the vowel “a,” each sustained at a target sound pressure level (SPL) of 75 ± 2 dB. The mean airflow was averaged across 2-second flow samples derived from the midportion of each sustained vowel production. 13 Vocal driving pressure was estimated using an intraoral pressure probe positioned behind the lips resting atop the tongue. The patient repeated the consonant “p” with the mask and catheter in place at a rate of 1.5 syllables/second. The estimated driving pressure was determined by subtracting the mean midvowel pressure from the mean peak intraoral pressure for “p.” The mean laryngeal airway resistance (LAR) was calculated using the mean peak intraoral pressure and midvowel airflow as described by Smitheran and Hixon. 14
Vocal Fold Imaging
Laryngeal videostroboscopy was performed with a Kay Elemetrics model RLS 9100 computer-interfaced unit, triggered by the patient’s electroglottogram using a Fourcin Laryngograph. Videostroboscopic imaging allowed real-time, direct assessment of the symmetry of vocal fold abduction and vibration, the amplitude and regularity of vocal fold movement, traveling (mucosal) wave characteristics, and glottic closure and configuration. Electromyographic testing of the cricothyroid muscle was not performed due to the invasive nature of the procedure.
Surgery
All patients underwent partial (lobe/isthmus), subtotal, or total thyroidectomy, as indicated by the primary pathology, under general endotracheal anesthesia at MSKCC. During surgery the strap musculature was retracted laterally from the midline or divided according to individual surgeon preference. Unilateral or bilateral identification and dissection of the RLN(s) were performed for unilateral thyroid lobectomy and total thyroidectomy, respectively. The surgeon in all cases of thyroid lobectomy and total thyroidectomy identified unilateral and bilateral recurrent nerves, respectively. “Extended” thyroidectomy was defined as total or less-than-total thyroidectomy with central or modified neck dissection. The superior thyroid artery and vein were individually ligated on the thyroid capsule to avoid injury to the EBSLN. When the EBSLN could not be readily identified, no further dissection was pursued to avoid inadvertent nerve injury. The cricothyroid muscle was spared from injury due to electrocoagulation or manual retraction. Operative wound drainage with a closed suction catheter or Penrose drain was at the discretion of the operating surgeon.
Statistics
The dependent variables of interest in the model are changes from baseline in each type of measurement. Differences in observed sample means for single measurements were evaluated using analysis of covariance to adjust for potentially important clinical factors. Correlations were expected both within and between modalities (i.e., acoustic, aerodynamic, glottographic, and videostroboscopic data). Patterns of change from baseline to postoperatively were evaluated using repeated-measures analysis of variance to have each patient serve as his or her own control and to optimize detectability of trends. Changes in voice parameters at each time point were compared between patients with and without voice change using the Wilcoxon rank-sum test. The analysis of categorical data was conducted using the Fisher exact or chi-square test where appropriate. Significance was determined at the P ≤ .05 level. Statistical analyses were carried out using JMP and SPSS statistical software (SAS Institute, Inc., Cary, NC).
RESULTS
Patient, Tumor, and Treatment Characteristics
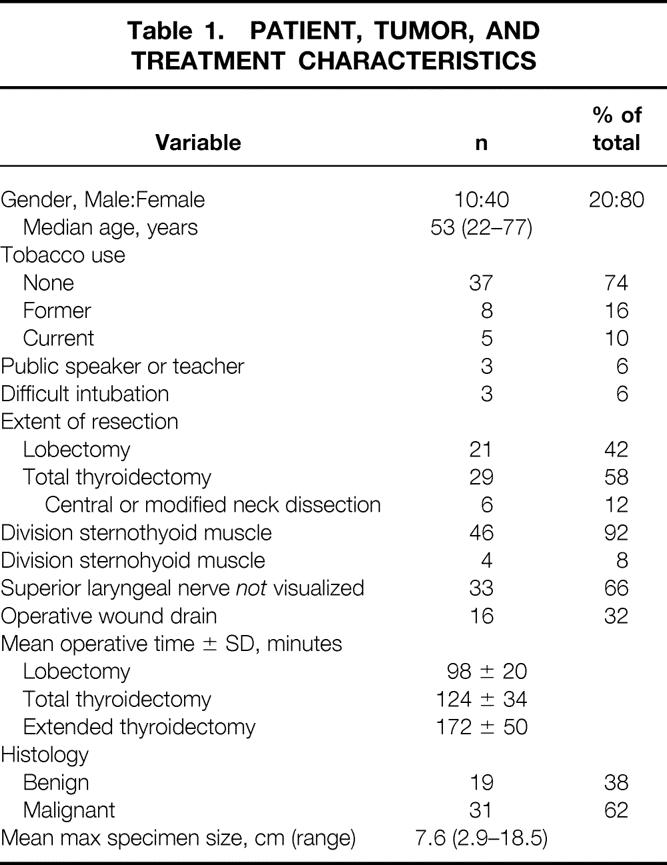
Fifty-four patients were enrolled. Two did not return for early follow-up, one of whom had relocated overseas. Two patients with unilateral vocal fold paralysis diagnosed preoperatively were excluded, leaving 50 evaluable patients at 1 week following thyroidectomy. Four patients did not return for late (3-month) voice testing, leaving 46 evaluable patients at 3-month follow-up. All four patients reported normal voice at 3 and 6 months postoperatively during a telephone interview. The male/female ratio was 1:4. The median patient age was 53 years. Patient demographics and clinical and pathologic data are reported in Table 1.
Table 1. PATIENT, TUMOR, AND TREATMENT CHARACTERISTICS
Time-Related Changes in Objective Voice Parameters
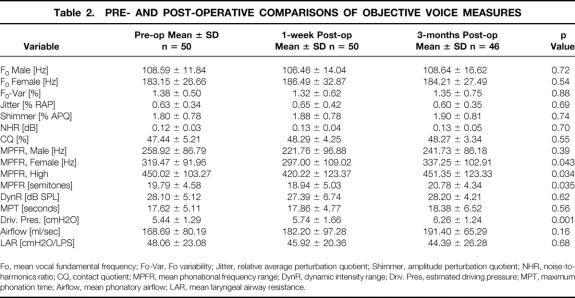
Eighty-four percent (42/50) of patients demonstrated significant change from baseline in at least one objective voice parameter at 1-week follow-up. Twelve percent (6/50) had significant alteration in more than three voice measures. When the study population was analyzed collectively, no statistically significant difference was evident between pre- and postoperative objective voice measures, with the exception of a significant reduction in mean MPFR (Hz) for female patients (P = .043) and MPFR-high (Hz) 1 week following thyroidectomy (P = .034). The MPFR returned to baseline 3 months later. A significant progressive increase in vocal driving pressure was observed at 1 week and 3 months (P = .001, Table 2).
Table 2. PRE- AND POST-OPERATIVE COMPARISONS OF OBJECTIVE VOICE MEASURES
Fo, mean vocal fundamental frequency; Fo-Var, Fo variability; Jitter, relative average perturbation quotient; Shimmer, amplitude perturbation quotient; NHR, noise-to-harmonics ratio; CQ, contact quotient; MPFR, mean phonational frequency range; DynR, dynamic intensity range; Driv. Pres, estimated driving pressure; MPT, maximum phonation time; Airflow, mean phonatory airflow; LAR, mean laryngeal airway resistance.
Changes in Objective Voice Parameters According to Extent of Thyroidectomy
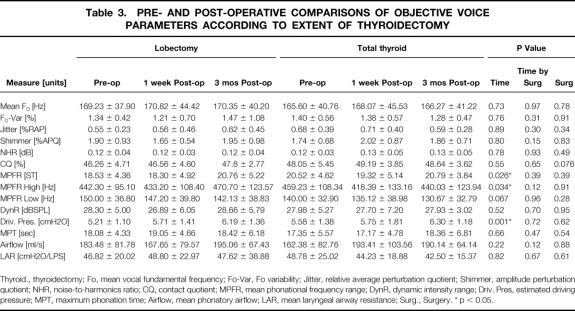
When the same objective voice parameters were evaluated according to extent of resection, a significant increase at 1-week and 3-month follow-up in vocal driving pressure was identified in both the lobectomy and total thyroidectomy groups, although there was no difference in driving pressure between groups (Table 3). The significant decrement in MPFR-high (Hz) observed for both groups at 1 week returned to baseline at the 3-month follow-up.
Table 3. PRE- AND POST-OPERATIVE COMPARISONS OF OBJECTIVE VOICE PARAMETERS ACCORDING TO EXTENT OF THYROIDECTOMY
Thyroid., thyroidectomy; Fo, mean vocal fundamental frequency; Fo-Var, Fo variability; Jitter, relative average perturbation quotient; Shimmer, amplitude perturbation quotient; NHR, noise-to-harmonics ratio; CQ, contact quotient; MPFR, mean phonational frequency range; DynR, dynamic intensity range; Driv. Pres, estimated driving pressure; MPT, maximum phonation time; Airflow, mean phonatory airflow; LAR, mean laryngeal airway resistance; Surg., Surgery. * p < 0.05.
Perceived Vocal Changes 1 Week and 3 Months Following Thyroidectomy
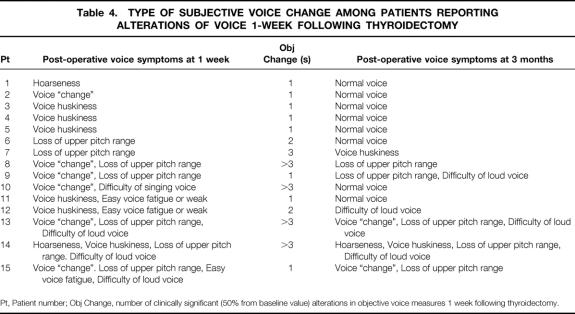
Fifteen (30%) patients reported voice symptoms 1 week following surgery; seven (14%) patients had persistent symptoms at 3-month follow-up (Table 4). All asymptomatic patients at 1-week follow-up remained asymptomatic 3 months later.
Table 4. TYPE OF SUBJECTIVE VOICE CHANGE AMONG PATIENTS REPORTING ALTERATIONS OF VOICE 1-WEEK FOLLOWING THYROIDECTOMY
Pt, Patient number; Obj Change, number of clinically significant (50% from baseline value) alterations in objective voice measures 1 week following thyroidectomy.
Seven of the 15 symptomatic patients described loss of upper voice pitch 1 week postoperatively; one of these (2% overall incidence) had videostroboscopic signs of EBSLN palsy, including ipsilateral posterior glottal rotation, bowing, and inferior displacement of the affected vocal fold with decreased mucosal traveling wave. Although two patients described postoperative voice hoarseness, objective findings of RLN palsy were not identified. No patient in this study had identifiable injury to the RLN. Four of seven patients who reported abnormal voice at 3 months had different symptoms than those described at 1-week follow-up. There were no significant differences in any investigated clinical, operative, or pathologic factors between the 15 patients with and 35 without subjective voice changes 1 week following thyroidectomy (data not shown).
Changes in Objective Voice Measures According to Perceived Voice Changes
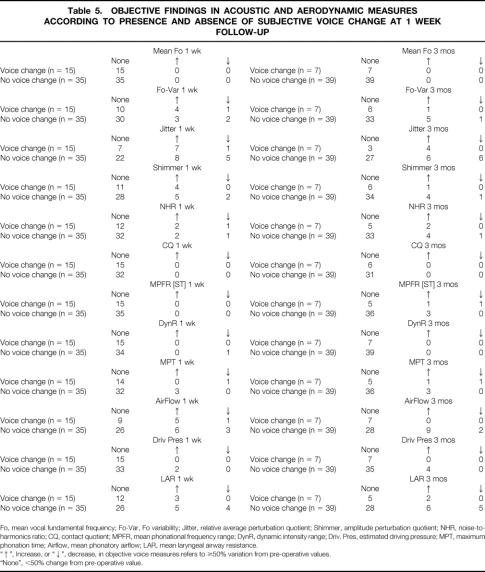
The objective changes in voice parameters were correlated with voice symptoms (Table 5). A variation of at least 50% from preoperative objective voice measure was considered clinically significant. The mean fundamental frequency (Fo), contact quotient (CQ), and dynamic intensity range (DynR) did not differ from baseline among patients with and without postoperative voice symptoms. Changes in objective parameters were more frequent among symptomatic than asymptomatic patients.
Table 5. OBJECTIVE FINDINGS IN ACOUSTIC AND AERODYNAMIC MEASURES ACCORDING TO PRESENCE AND ABSENCE OF SUBJECTIVE VOICE CHANGE AT 1 WEEK FOLLOW-UP
Fo, mean vocal fundamental frequency; Fo-Var, Fo variability; Jitter, relative average perturbation quotient; Shimmer, amplitude perturbation quotient; NHR, noise-to-harmonics ratio; CQ, contact quotient; MPFR, mean phonational frequency range; DynR, dynamic intensity range; Driv. Pres, estimated driving pressure; MPT, maximum phonation time; Airflow, mean phonatory airflow; LAR, mean laryngeal airway resistance.
“↑”, Increase, or “↓”, decrease, in objective voice measures refers to ≥50% variation from pre-operative values.
“None”, <50% change from pre-operative value.
At 1-week follow-up, significant differences in the percentage change from baseline were observed for mean vocal jitter (P = .019), MPFR-high (Hz) (P = .034), and maximum phonation time (P = .032) between patients with and without perceived early (1-week) voice change. At 3-month follow-up significant differences in the percentage change from baseline were demonstrated for mean Fo variability (P = .045), MPFR (Hz) (P = .025), MPFR-high (Hz) (P = .023), and maximum phonation time (P = .021) between the 7 patients with persistent voice symptoms and the remaining 39 asymptomatic patients.
Predictive Value of 1-Week Objective Voice Data
Eight (23%) asymptomatic patients had no significant alteration in objective voice measures at 1-week follow-up; however, all symptomatic patients had at least one objective voice parameter with at least 50% variation from baseline. Overall, 12% (n = 6) of all study patients had significant alterations in more than three objective voice parameters (Table 6). Six percent of asymptomatic patients and 27% of symptomatic patients at 1 week had significant variation from baseline in more than three objective voice measures (P = .058). Extent of thyroidectomy did not correlate significantly with degree of subjective or objective voice changes.
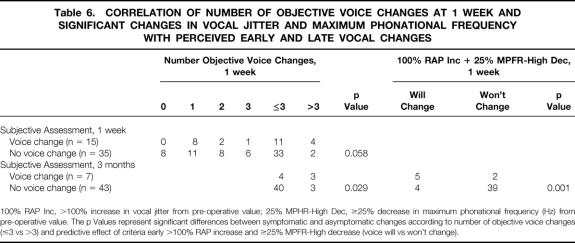
Table 6. CORRELATION OF NUMBER OF OBJECTIVE VOICE CHANGES AT 1 WEEK AND SIGNIFICANT CHANGES IN VOCAL JITTER AND MAXIMUM PHONATIONAL FREQUENCY WITH PERCEIVED EARLY AND LATE VOCAL CHANGES
100% RAP Inc, >100% increase in vocal jitter from pre-operative value; 25% MPHR-High Dec, ≥25% decrease in maximum phonational frequency (Hz) from pre-operative value. The p Values represent significant differences between symptomatic and asymptomatic changes according to number of objective voice changes (≤3 vs >3) and predictive effect of criteria early >100% RAP increase and ≥25% MPFR-High decrease (voice will vs won’t change).
The number of abnormal voice parameters at 1 week correlated with voice symptoms at 3 months. Nine percent (4/44) of patients with three or fewer and 50% (3/6) with more than three significantly abnormal voice measures at 1 week reported abnormal voice at 3 months (P = .029, see Table 6). The comparison of 1-week objective voice data in symptomatic (n = 7) to asymptomatic (n = 43) patients at 3 months identified clinically significant changes in early (1-week) mean vocal jitter (P = .006), MPFR (Hz) (P = .011), MPFR (semitones) (P = .039), and MPFR-High (Hz) (P = .004). These early objective changes correlated significantly with voice symptoms at 3-month follow-up. Maximum phonation time at 1 week did not correlate significantly with late voice symptoms (P = .060).
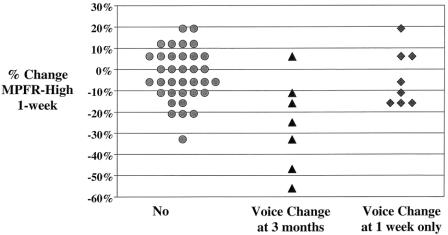
MPFR-high (Hz) was a sensitive predictor of perceived voice change at 3 months: five of six patients with a 25% or greater decrease from baseline in MPFR-high (Hz) at 1 week reported abnormal voice at 3-month follow-up (Fig. 1). An early (1-week) increase in vocal jitter greater than 100% from baseline also correlated significantly with persistent voice symptoms at 3 months (P = .006). The criterion of at least a 25% decrease in MPFR-high (Hz) and more than a 100% increase in vocal jitter (% relative average perturbation) had a sensitivity for long-term voice symptoms of 71% compared to 43% when using more than three significant objective voice changes (P = .001). Corresponding specificities for the two objective criteria were 91% and 93%. The criterion of at least a 25% decrease in MPFR-high (Hz) and more than a 100% increase in vocal jitter (% relative average perturbation) had a positive predictive value for long-term perceived voice changes of 56% and a negative predictive value of 95%. The positive and negative predictive values of at least three significant objective voice changes for persistent voice symptoms were 50% and 91%, respectively.
Figure 1. Association of MPFR-High (semitones) at 1 week with voice change at 1-week and 3-month follow-up. At 1-week follow-up significant differences in the percentage change from baseline were observed for MPFR-high ([Hz], P = .034) between patients with voice change at 1 week only and those without perceived voice change following thyroidectomy, as well between the seven patients with persistent voice symptoms at 3 months and the remaining asymptomatic patients (P = .004).
Videostroboscopic Findings
Two of the 15 (13%) symptomatic patients had postoperative videostroboscopic changes. One patient (described above) manifested classic findings of EBSLN injury after total thyroidectomy. The other had findings of unilateral reduction in mucosal traveling wave and vocal fold vibratory amplitude with incomplete contact following extended thyroidectomy. The patient with the EBSLN injury had persistent symptoms and unchanged videostroboscopic abnormalities at 3-month follow-up. The second patient’s symptoms (“voice change and difficulty of singing voice”) and videostroboscopic abnormalities resolved by the 3-month clinic visit.
Two of 30 (7%) asymptomatic patients had abnormal videostroboscopy. One patient had irregular vocal fold contact 1 week after thyroid lobectomy that normalized 3 months later. The other patient had unilateral reduction in mucosal traveling wave and vocal fold vibratory amplitude following total thyroidectomy for locally advanced papillary cancer. These abnormal findings persisted in this patient despite reportedly normal voice at 3-month follow-up.
Summary
Thirty percent of patients with normal voice preoperatively reported early voice symptoms and 14% reported late voice symptoms following thyroidectomy. No patient had recurrent laryngeal injury and one (2%) had EBSLN injury evident postoperatively. Only two asymptomatic (6%) patients had more than three objective voice changes at 1 week, whereas four (27%) patients with early voice change had significant variation from baseline in more than three parameters. Multiple (more than three) early alterations in objective voice parameters significantly correlated with persistent postoperative symptoms at 3 months. The criteria of at least a 25% decrease in MPFR-high and more than a 100% increase in vocal jitter had a sensitivity for long-term voice symptoms of 71% and a specificity of 91%.
DISCUSSION
This prospective single-arm diagnostic trial of patients with benign and malignant thyroid pathology and normal voice characterizes the alterations in voice that occur following thyroidectomy. All patients were evaluated using a standardized multidimensional functional voice assessment protocol. Patients served as their own controls, having undergone preoperative voice testing. This study defines the extent and range of variability of quantifiably altered voice function in the early (1 week) and late (3 months) postoperative period following thyroidectomy.
Although 84% of patients had significant alteration of at least one objective voice parameter, only 30% of patients reported voice symptoms early after thyroidectomy and 14% were symptomatic at 3-month follow-up. Two recently published prospective studies of voice function after thyroidectomy found that early postoperative voice changes occur commonly (41–47%). 15,16 Eight percent of patients in the present study had abnormal videostroboscopy postoperatively, two each in the symptomatic and asymptomatic groups. The degree and number of alterations in voice function did not correlate with the extent of thyroidectomy.
The present data suggest that clinically significant postoperative changes in more than three objective acoustic and aerodynamic voice measures from baseline are associated with early postoperative voice symptoms. The number of abnormal voice parameters at 1 week correlated significantly with persistent voice symptoms at 3 months post-thyroidectomy. Early (1-week) maximum phonational frequency and vocal jitter changes from baseline were significantly associated with both early and late perceived alterations in voice. The criterion of at least a 25% decrease in MPFR-high and more than a 100% increase in vocal jitter had a higher sensitivity for long-term voice symptoms than that of more than three significant objective voice changes.
A recent study found objective deterioration in voice function in 23% of patients with and without preoperative dysphonia undergoing thyroidectomy; prelaryngeal strap muscle division during surgery did not significantly influence this outcome. 16 The rate of concordance between subjective and objective voice appraisal was 64% in that study. Applying the criteria of more than three objective voice parameter changes among a standardized multidimensional voice testing protocol, we have found a similar concordance rate of 67%. We find that patients tend to interpret unfamiliar laryngeal sensations following endotracheal intubation as changes in voice. All patients found to be asymptomatic 1 week following thyroidectomy remained symptom-free at 3-month follow-up. The present data suggest that one, two, or even three acoustic or aerodynamic changes in the absence of videostroboscopic abnormalities may not have clinical relevance. These findings may account for the 33% rate of discordance observed between subjective and objective voice data.
Despite the multidimensional acoustic and aerodynamic analysis employed in this study, only a few objective parameters appear to significantly correlate with early and late voice symptoms. Although the criteria of more than three significant postoperative alterations in objective voice measures may be useful in identifying patients who may benefit from voice rehabilitation early in the postoperative period, specific acoustic measures such as vocal jitter, maximum phonational frequency, and phonation time appear to be more predictive of persistent vocal symptoms. These data must be interpreted cautiously as the overall study population was small, voice parameters were measured in different quantifiable units, and the comparisons conducted between them were multiple. There was greater variability in some voice measures than others, and employing one criteria of clinically significant change (i.e., ≥50% change from baseline) for every voice measure may not be the optimal method to distinguish normal from abnormal. A prospective study with greater statistical power is warranted to validate our preliminary findings. It is interesting that over half of patients with persistent symptoms at the 3-month clinic visit perceived voice changes that differed from those experienced during the early postoperative period; however, a voice-specific outcome measure to assess degree of voice handicap was not used in the present study.
The often-elusive symptoms and laryngoscopic findings of EBSLN palsy necessitate the use of laryngeal videostroboscopy and/or cricothyroid electromyography to better detect this recognized morbidity of thyroid surgery. Short of the invasive cricothyroid electromyography, videostroboscopy is considered to be an important noninvasive diagnostic tool for evaluating signs of cricothyroid muscle dysfunction. 7,15,17,18 Loss of upper pitch range following thyroidectomy has been attributed to EBSLN injury. Of seven patients reporting loss of upper pitch, two had significant reduction in maximum phonational frequency, of which one had videostroboscopic findings of EBSLN palsy. It is impossible to prove whether this patient truly had EBSLN injury or arytenoid trauma sustained during endotracheal intubation, as cricothyroid electromyography was not performed. A prospective study evaluating post-thyroidectomy voice function employing acoustic, videostroboscopic, and laryngeal electromyographic analysis found EBSLN palsy in 3 of 21 (14%) patients with postoperative voice symptoms. 15 We discussed the potential of employing electromyography with our patients prior to initiating this trial and noticed reluctance on their part to participate with the invasive component as part of the voice testing. Despite symptoms of hoarseness in two patients, no one in this study had objective evidence of RLN palsy.
In this study, the superior thyroid vasculature was individually ligated on the thyroid capsule and no further dissection in search of the EBSLN was undertaken when it could not be readily identified in order to avoid injury to the nerve. The incidence of EBSLN injury in this study (2%) on the basis of this surgical technique compares favorably with other modern published series (0–14%). 15,18,19–21 The relationship of the EBSLN to the vasculature and its course to the inferior constrictor muscle was variable, and it could not be identified in 66% of our patients. There are several technical approaches to preserving the integrity of the EBSLN, including isolation and individual ligation of the superior pole vessels adjacent to the thyroid capsule, identification of the EBSLN prior to securing the vasculature in the same manner, and neuromonitoring of the EBSLN during thyroidectomy. 19,20 A recently published prospective randomized trial compared skeletonization and individual ligation of the superior pole vessels very close to the thyroid capsule, and identification of the EBSLN before ligating the superior thyroid vessels. 19 It demonstrated that careful distal ligation of the superior thyroid vessels is a safe technique to preserve the EBSLN, making routine exposure unnecessary during thyroidectomy.
It is conceivable that in situations where the RLN arborizes prior to its entrance into the larynx at the cricothyroid joint, injury to a small branch may contribute to the changes in voice identified in this patient cohort without significant changes in vocal fold motion. Post-thyroidectomy voice symptoms are common despite careful preservation of the EBSLN and RLN and avoidance of trauma to the cricothyroid muscle. Mechanisms other than laryngeal nerve injury may account for postoperative voice changes as well. Endotracheal intubation alone is associated with a 5% risk of voice impairment. 8 Another possible explanation for the disparity in the patient’s perception of voice quality and objective findings is that videostroboscopy lacks sensitivity to detect subtle changes of partial and temporary EBSLN injury or permanent nerve disruption. Excluding the single patient with EBSLN injury, the absence of significant deterioration in mean vocal fundamental frequency (Fo) or dynamic voice range among patients reporting voice changes in the current study provides indirect evidence of preserved superior laryngeal nerve integrity and function. 7
Objective voice measures are meant to characterize vocal function as a consequence of pathology. They rarely provide the capability to diagnose specific tissue or neuropathology. For that reason an acoustic or aerodynamic profile of recurrent laryngeal nerve or EBSLN cannot be defined, specifically. In general, however, these lesions involve a varying degree of glottal incompetence and at least a unilateral restriction of mass/tension regulation. Thus, we would expect a higher airflow (or at least lower laryngeal airway resistance) and a restriction of the dynamic range of the voice. If unilateral involvement results in unequal vocal fold mass, length, and tension, then we would expect higher perturbation values (vocal jitter, shimmer, and noise-to-harmonics ratio).
The degree to which sternohyoid or sternothyroid muscular division influences voice function is unknown. One group of investigators applied videostroboscopy and electromyography to patients whose strap musculature was not divided during thyroidectomy, and who had normal laryngeal nerve function postoperatively. 7 Their hypothesis is that laryngotracheal fixation of the extralaryngeal strap musculature results in post-thyroidectomy voice alterations. The impact of strap muscle division on postoperative voice in the present study could not be determined as most (92%) patients had the sternothyroid muscle(s) divided at the time of surgery.
Preservation of the voice is an important consideration for patients undergoing thyroid surgery, particularly for the majority who use their voice professionally. Vocal and laryngeal symptoms appear to be common following thyroidectomy. This multidimensional analysis of vocal physiology and vocal signal quality characterizes the functional implications of thyroid surgery for patients with normal preoperative voice. The natural history of these documented changes in voice remains to be defined fully as longer follow-up is warranted. The existence of factors other than laryngeal nerve injury contributing to voice changes following thyroidectomy and the considerable variability of patient symptomatology underscore the importance of comprehensive voice analysis before and after thyroid surgery. Future studies may include cricothyroid electromyography to further define the physiology of post-thyroidectomy dysphonia, pending approval of patients and treating physicians. A comprehensive functional voice assessment will allow early diagnosis of vocal disturbance and facilitate timely voice rehabilitation.
Acknowledgements
The authors thank Robin Howard and Denis Leung, PhD, for their valuable contributions. Their support of the statistical analysis of this work was indispensable. The authors also appreciate the administrative and technical assistance provided by Hilary Cathcart and Margaret Ho, MA, CCC/SLP.
Footnotes
Presented at the American Head and Neck Society Annual Meeting at COSM, Boca Raton, Florida, May 11, 2002.
Correspondence: Dennis H. Kraus, MD, Attending Surgeon, Head and Neck Service, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: krausd@mskcc.org
References
- 1.Rueger R. Benign disease of the thyroid gland and vocal cord paralysis. Laryngoscope 1974; 84: 897–907. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum B, Wenig B. Superior laryngeal nerve injury from thyroid surgery. Head and Neck 1995; 17: 36–40. [DOI] [PubMed] [Google Scholar]
- 3.Ward PH, Berci G, Calcaterra TC. Superior laryngeal nerve paralysis: an often-overlooked entity. Trans Am Acad Opthalmol Otolaryngol 1977; 84: 77–89. [PubMed] [Google Scholar]
- 4.Abelson TI, Tucker HM. Laryngeal findings in superior laryngeal nerve paralysis. Otolaryngol Head Neck Surg 198; 89:463–470. [DOI] [PubMed]
- 5.Cernea C, Ferraz A, Fulani J, et al. Identification of the external branch of the superior laryngeal nerve during thyroidectomy. Am J Surg 1992; 164: 634–638. [DOI] [PubMed] [Google Scholar]
- 6.Jansson S, Tisell L, Hagne I, et al. Partial superior laryngeal nerve lesions before and after thyroid surgery. World J Surg 1988; 12: 522–527. [DOI] [PubMed] [Google Scholar]
- 7.Hong K, Kim Y. Phonatory characteristics of patients undergoing thyroidectomy without laryngeal nerve injury. Otolaryngol Head Neck Surg 1997; 117: 399–404. [DOI] [PubMed] [Google Scholar]
- 8.Kark A, Kissin M, Auerbach R, et al. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br J Med 1984; 289: 1412–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano M. Clinical examination of the voice. New York: Spinger-Verlag, 1981.
- 10.Gramming P. The phoneotogram: An experimental and clinical study. Malmo, Sweden: University of Lund, 1988.
- 11.Kent R, Kent J, Rosenbeck J. Maximum performance tests of speech production. J Speech Hear Dis 1987; 52: 367–387. [DOI] [PubMed] [Google Scholar]
- 12.Orlikoff RF. Assessment of the dynamics of vocal fold contact from the electroglottogram: Data from normal male subjects. J Speech Hear Res 1991; 34: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 13.Orlikoff RF, Kraus DH, Budnick AS, et al. Vocal function following successful chemoradiation treatment for advanced laryngeal cancer: preliminary results. Phonoscope 1999; 2: 67–77. [Google Scholar]
- 14.Smitheran JR, Hixon TJ. A clinical method for estimating laryngeal airway resistance during vowel production. J Speech Hear Res 1981; 46: 138–146. [DOI] [PubMed] [Google Scholar]
- 15.Aluffi P, Policarpo M, Cherovac C, et al. Post-thyroidectomy superior laryngeal nerve injury. Eur Arch Otorhinolaryngol 2001; 258: 451–454. [DOI] [PubMed] [Google Scholar]
- 16.McIvor NP, Flint DJ, Gillibrand J, et al. Thyroid surgery and voice-related outcomes. Aust NZ J Surg 2000; 70: 179–183. [DOI] [PubMed] [Google Scholar]
- 17.Sercarz JA, Berke GS, Ming Y, et al. Videostroboscopy of human vocal cord paralysis. Ann Otol Rhinol Laryngol 1992; 101: 567–577. [DOI] [PubMed] [Google Scholar]
- 18.Dursun G, Sataloff R, Spiegal J, et al. Superior laryngeal nerve paresis and paralysis. J Voice 1996; 10: 206–211. [DOI] [PubMed] [Google Scholar]
- 19.Bellantone R, Boscherini M, Lombardi CP, et al. Is the identification of the external branch of the superior laryngeal nerve mandatory in thyroid operation? Results of a prospective randomized study. Surgery 2001; 130: 1055–1059. [DOI] [PubMed] [Google Scholar]
- 20.Yeung P, Erskine C, Mathews P, et al. Voice changes and thyroid surgery: Is pre-operative indirect laryngoscopy necessary? Aust NZ J Surg 1999; 69: 632–634. [DOI] [PubMed] [Google Scholar]
- 21.Jonas J, Bahr R. Neuromonitoring of the external branch of the superior laryngeal nerve during thyroid surgery. Am J Surg 2000; 179: 234–236. [DOI] [PubMed] [Google Scholar]