Abstract
Objective:
Is skin-sparing mastectomy (SSM) with conservation of the Nipple–Areola Complex (NAC) and immediate autologous reconstruction as safe in oncologic terms as SSM with resection of the NAC as modified radical mastectomy (MRM)?
Summary Background Data:
The originally described technique of SSM included the removal of gland, NAC, and biopsy scar. However, the risk of tumor involvement of NAC in patients with breast cancer has been overestimated.
Patients and Methods:
Between 1994 and 2000, 286 selected patients with an indication for MRM and tumor margins of greater than 2 cm from the nipple were presented with the alternative of a SSM. Regular follow-up data were evaluable of 112 patients with SSM and 134 patients with MRM. Immediate reconstruction was achieved by latissimus dorsi flap or TRAM flap. The mean follow-up time was 59 (18 to 92) months.
Results:
Patients with SSM were significantly younger than those with MRM but were comparable regarding clinical data, tumor parameters, adjuvant treatment, and overall complications. After intraoperative frozen sections of the NAC-ground, the NAC could be conserved in 61 (54.5%) but was resected in 51 (45.5%) of the 112 patients with SSM. The aesthetic results after SSM were evaluated as excellent or good in 91.1% (102/112) patients and were significantly better after preservation of the NAC (P = 0.001). Six (5.4%) recurrences occurred in 112 patients with SSM compared with 11 (8.2%) cases after MRM. Only 1 recurrence in a conserved nipple was treated by wide excision of nipple with conservation of the areola. This patient is still free of disease after 52 months.
Conclusion:
In patients who are candidates for a mastectomy and tumors distant from the nipple, SSM with intraoperative frozen section of the NAC ground offers the opportunity of NAC conservation without increasing the risk of local recurrences.
Skin-sparing mastectomy (SSM) removes the breast, nipple–areola complex (NAC), previous biopsy incisions, and skin overlying superficial tumors.1 Preservation of the native skin envelope facilitates immediate breast reconstruction. The substitution of the mammary gland is performed preferably by autologous tissue from the abdominal wall (TRAM flap) or back (latissimus flap). The most important advantages of SSM are the preservation of the submammary fold, preservation of the breast contour, and avoidance of skin differences. We have shown that the aesthetic results are much better in comparison with secondary breast reconstruction.2 The preservation of the NAC leads to the most perfect result. However, the oncologic safety of this lesser radical procedure compared with the established SSM technique or modified radical mastectomy (MRM) is not proven today. Randomized studies to compare SSM with or without preservation of the NAC to modified radical mastectomy would be ethically debatable and not accepted by the patients. However, there is a great acceptance of SSM by the oncologist as well as by patients. Therefore, follow-up studies are necessary to prove the oncologic safety of the SSM technique, especially in cases of NAC conservation. With the presented study we will supply the first ever results concerning SSM with and without conservation of the NAC.
MATERIAL AND METHODS
During March 1994 and September 2000, a total of 1014 patients with invasive or non-invasive breast cancer were treated at the Department of Obstetrics and Gynecology at the University of Rostock. Of 936 patients with a primary operable breast cancer 524 (56%) patients had breast-conserving therapy. In 412 (44%) patients, an MRM or SSM was recommended. Of these patients, 286 (54.6%) were younger than 75 years, had a body mass index of 21 to 35 kg/m2, absence of skin involvement, showed no central tumor location, presented no contraindication for a flap reconstruction, and therefore met the inclusion criteria according to the study protocol for SSM. In all patients, the distance between the nipple as the center of NAC and per sonography or mammography detectable tumor was at least 2 cm from the NAC. Generally, the diagnosis of breast cancer was histologically proven by core cut biopsy or open biopsy. To these 286 patients, SSM was offered as an alternative to MRM. Patients were not randomized. They were well informed about risks and advantages of SSM. Of these, 134 patients opted for SSM and 152 patients chose MRM. Follow-up data of at least 18 months were evaluable for 114 (85.1%) patients who had chosen SSM and 132 (86.8%) patients who had decided on MRM. In 2 of 114 patients with planned SSM, an MRM was performed because of intraoperative detection of unexpectedly involved margins (less than 5 mm of uninvolved tissue). Both patients were included in the MRM group. In all SSM cases, there was the intention to preserve the NAC. According to the distance between tumor and NAC the underlying breast parenchyma was more or less reduced and examined by intraoperative frozen section. The base of the NAC was marked with thread and black pen. After macroscopic evaluation of the opened mammary gland, tissue samples for frozen section were taken from the base of NAC and from the vicinity of the tumor towards the NAC. Corresponding samples were processed according to hematoxylin and eosin technique. The NAC was resected in 51 patients with an extensive intraductal component defined as more than 25% of tumor cells in ducts, with tumor growth closer than 2 cm from the NAC and with suspicious cells in the base of the NAC not clearly identifiable as tumor or benign cells during frozen section technique. In 61 patients with SSM, the NAC could be conserved. Axillary lymph node evaluation was done by hematoxylin and eosin technique and simultaneous immunohistochemistry.3 Nine (3.7%) of the 246 patients were administered a preoperative chemotherapy according to study protocols. For breast reconstruction with autologous tissue, we used TRAM or latissimus dorsi flap, the latter with complete transsection of its humerus insertion and complete mobilization.4 In smokers we preferred the latissimus dorsi flap or delayed TRAM flap.5
Prophylactic application of perioperative antibiotics (1 × 3 g Unacid i.v., Ampicillin and Sulbactam) and low-molecular heparins (Clexane 40 mg, Enoxaparin) postoperatively up to complete mobilization were generally applied. Adjuvant systemic treatment was performed according to the contemporary recommendations of the St. Gallen consensus meetings. After mastectomy, the thoracic wall, supraclavicular, and parasternal region were irradiated in cases of medial tumor location, tumors larger than 5 cm, and those with more than 3 involved axillary lymph nodes.
All patients were regularly examined and questioned about their satisfaction with the aesthetic result (subscales: excellent, good, fair, and poor). The final postoperative aesthetic results were evaluated by 2 independent physicians reviewing the photographs or seeing the patients themselves. The aesthetic result was stratified by subscales according to Lowery et al6 that have also been used by others7 and allow a comparison. Briefly, volume, contour, placement of breast mound, and inframammary fold were evaluated with 0 to 2 points. Results were defined as excellent: 7 to 8 points, good: 6 to 6.9 points, fair: 5 to 5.9 points, and poor: <5 points.
Mammography and sonography of the healthy breast and sonography alone of the reconstructed breast were done at least once a year. Because of doubtful findings in patients with SSM, 37 (33%) MR mammograms and 11 (9.8%) biopsies were conducted. The mean follow up time was 59 (range, 18 to 92) months.
Differences were tested for significance by χ2 test. The analysis of variance was used and post hoc comparisons by Scheffe’s procedure were performed to compare mean values of independent samples. All tests were two-sided. The level of significance was set at P < 0.05.
RESULTS
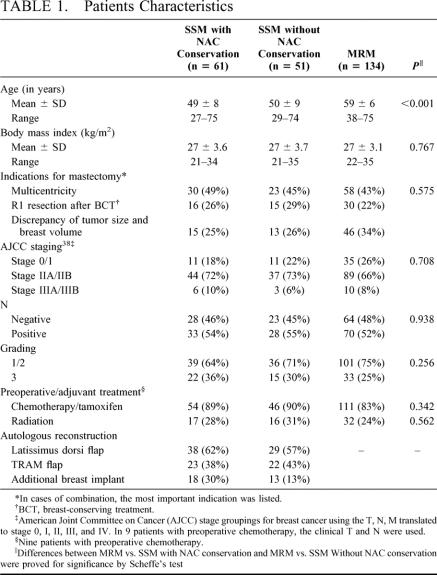
The mean age of patients with SSM and NAC conservation was 49 ± 8 and 50 ± 9 years for patients without NAC conservation, respectively (Table 1). Patients who decided for MRM were significantly older (59 ± 6, P < 0.001). There were no significant differences between the SSM subgroups themselves as well as compared with the MRM patients regarding body mass index, indications for mastectomy, involvement of axillary lymph nodes and grading. Systemic preoperative or adjuvant therapy was performed in 82.8% (MRM), 88.5% (SSM with NAC conservation), and 90.2% (SSM without NAC conservation; P = 0.342). Radiotherapy was performed in 23.9%, 27.9%, or 31.4% of the patients, respectively (P = 0.562). For autologous tissue reconstruction after SSM, we preferred the latissimus dorsi flap (n = 67, 59.8%). In 31 (27.7%) patients, a breast implant was used in addition to a flap (Table 1).
TABLE 1. Patients Characteristics
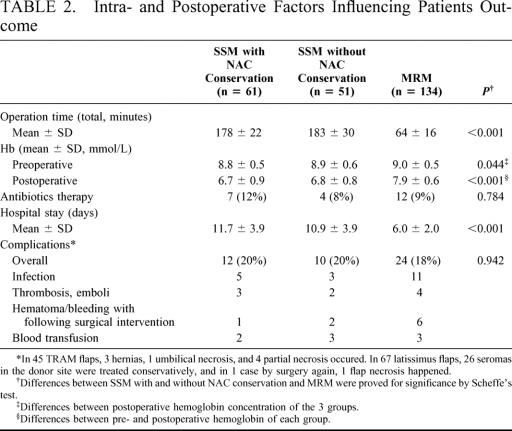
As shown in Table 2, the operation time (178 ± 22 and 185 ± 30 minutes; P < 0.001) and hospitalization length (11.7 ± 3.9 and 10.9 ± 3.9 days; P < 0.001) in SSM patients were significantly longer and the blood loss expressed by postoperative hemoglobin concentration (6.7 ± 0.9 and 6.8 ± 0.8 mmol/L; P < 0.001) higher than in MRM patients (64 ± 16 minutes, 6.0 ± 2.0 days; 7.9 ± 0.6 mmol/L). Differences in hospital stay were not caused by medical reasons but were caused by economic specifics of the German healthcare system. However, there were no significant differences with respect to antibiotics therapy (P = 0.784) and total number of complications (P = 0.942). Flap-related complications were 3 hernias, 1 umbilical necrosis, and 4 partial necrosis in 45 TRAM flaps. In 67 patients with latissimus flap, 26 seromas in the donor site were treated conservatively, and in 1 case by surgery, 1 flap necrosis happened. In our opinion a seroma after latissimus-flaps is not a complication. In no case a recommended systemic treatment was postponed after surgery due to complications, the treatment starting 4 weeks after the operation.
TABLE 2. Intra- and Postoperative Factors Influencing Patients Outcome
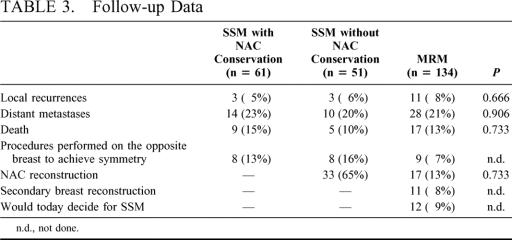
During a mean follow-up of 59 months, 6 (5.4%) recurrences were detected in 112 patients with SSM (Table 3). Two recurrences were located at the thoracic wall, 2 in the skin of the upper breast parts, and 1 in the submammary fold. In 1 patient with NAC conservation after invasive breast cancer and an intraductal component of 20 to 25% but free margins, a non invasive recurrence in the NAC was detected after 27 months. It was treated by wide excision of the nipple with conservation of the areola and is still recurrence free after a further 52 months. Both patients with thoracic wall recurrence underwent “salvage mastectomy” but died as a result of distant metastases. The other 3 patients with invasive recurrence were treated by breast conserving surgery and have now been free of disease for 19 to 54 months. After modified radical mastectomy, a local recurrence occurred in 11 (8.2%) of 134 patients. The local recurrence rates between SSM with or without NAC conservation and MRM patients revealed no significant differences (P = 0.666). Also with respect to distant metastases (P = 0.906) and death (P = 0.733) no significant differences existed between the 3 groups (Table 3).
TABLE 3. Follow-up Data
After MRM 11 (8.2%) patients agreed to secondary breast reconstruction. In 16 of 112 (14.3%) patients with SSM and in 9 of 134 (6.7%) with MRM, a secondary operation (lifting, reduction plasty) for achievement of symmetry was performed. In total, 12 (9%) of the patients would decide for SSM today, if they had the opportunity to reverse their decision (Table 3).
The aesthetic results were evaluated for at least 12 months after the end of primary treatment (operation, chemotherapy, radiation). All 61 patients with SSM and NAC preservation and 50 of 51 without NAC preservation evaluated the aesthetic results as excellent or good (Fig. 1). The evaluation by the surgeons was more critical. Altogether in 102 (91.1%) of all 112 SSM cases, an excellent or good result was confirmed by the judges. Only in 10 (19.6%) of 51 cases without NAC preservation the cosmetic results were evaluated as fair, but none in the NAC preserved group (P = 0.001; Fig. 2). Forty-six (75.4%) of the 61 patients with preserved NAC reported sensitivity of the NAC. A depigmentation of the preserved NAC was seen in 26 (42.6%) and a necrosis of the nipple-top in 6 (9.8%) patients. Figures 3 and 4 show examples for SSM, and it should be stated that the periareolar approach was preferred.
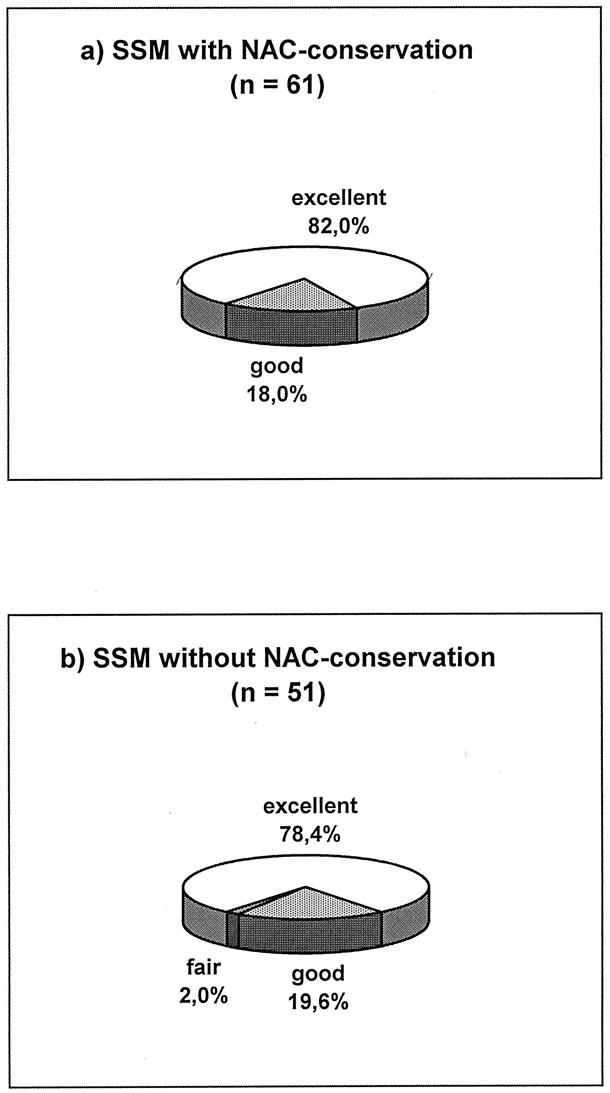
FIGURE 1. Aesthetic grading results of breast reconstruction (evaluation by patients).
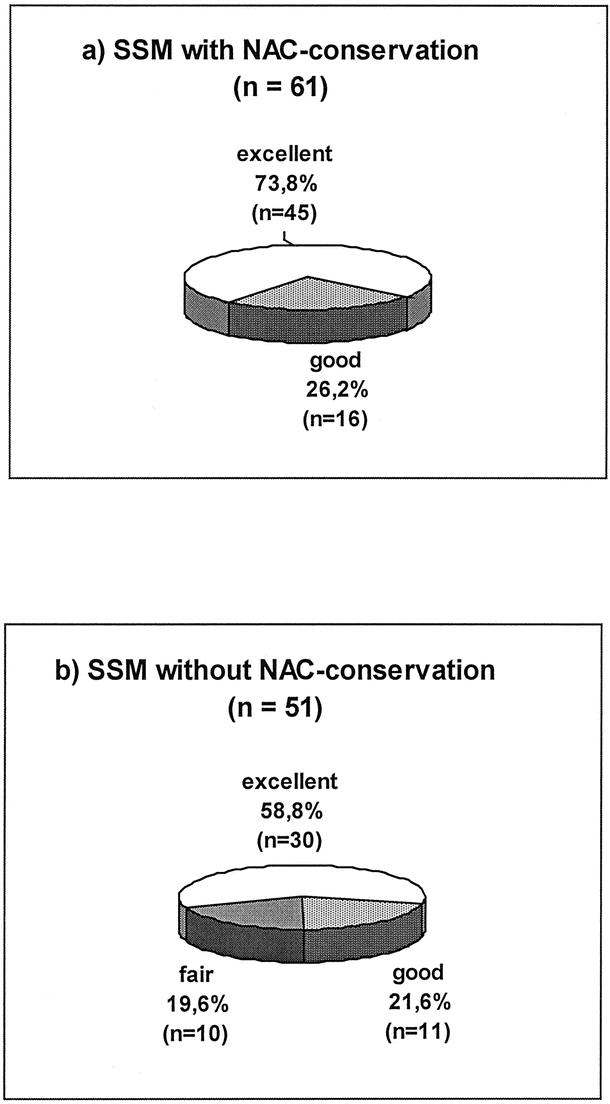
FIGURE 2. Aesthetic grading results of breast reconstruction (evaluation by surgeons).
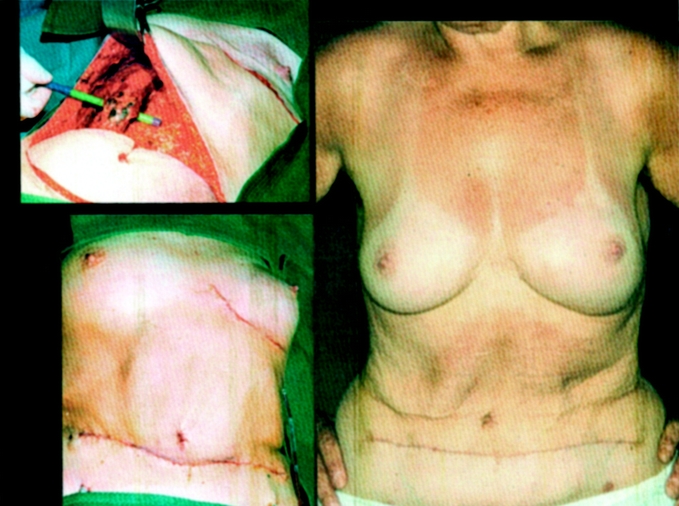
FIGURE 3. A 57-year-old patient with breast cancer pT2 (26 mm, m) N0(0/16)M0, R0, and SSM with NAC conservation and TRAM flap reconstruction 2 years after surgery.

FIGURE 4. A 40-year-old patient with breast cancer pT2 (21 mm, m) N1 (3/22) M0, R0, and SSM with NAC conservation and latissimus dorsi flap flap reconstruction 6 months after surgery.
DISCUSSION
The most impressive advantages of skin sparing mastectomy are avoidance of mastectomy trauma and subsequent operations for secondary breast reconstruction. As a result of skin and submammary fold conservation, the cosmetic results are better in comparison to secondary breast reconstruction. Differences in the color and quality of inserted skin are avoided or limited to the NAC area.
In the presented study 102 (91.1%) of 112 patients were well satisfied (excellent/good) with the aesthetics results. In other studies, excellent and good results have been reported in 63 % (7), 71 % (8), 75 % (9), 87 % (10), and 94 % (11) of patients. The better results in the presented study may be the result of patient selection (age, body mass index, breast size, and tumor localization), consideration of risk factors, and 1 surgeon performing the whole operation. In cases of mammary hyperplasia or ptosis, a simultaneous reduction plasty with replacement of the glandular tissue by autologous tissue is recommended. Best results will also be obtained by periareolar incision,12 which we extended arch-shaped to the axilla. By doing this, we have an excellent view for SSM, low blood loss, and optimal access to axillary dissection and cutting of the latissimus dorsi tendinous.4 In no SSM patients was the beginning of systemic treatment delayed because of to complications. Generally, complications were not significantly more frequent after the more extensive SSM technique than after MRM.
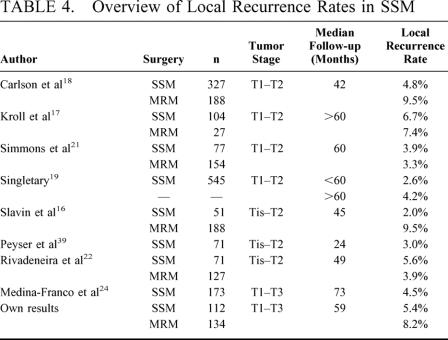
The originally described technique involves the removement of NAC, biopsy scar, and an optimal incision for axillary access.1 The technique was used in risk patients,13 noninvasive lesions,14 and small invasive tumors.15,16 There is still only a limited number of patients treated in nonrandomized studies of SSM in T1-2 tumors, all of which support the hypothesis that SSM does not increase the local recurrence rate in these patients (Table 4).16–23 However, a mastectomy is mostly indicated in patients with large or multicentric tumors. Factors associated with higher local recurrence rate were tumor stage 2 or 3, tumor size larger than 2 cm, node-positive disease, and poor tumor differentiation.24 Today, patients with large breast tumors are candidates for primary chemotherapy. Also, in cases of large tumors and clinical complete remission after primary systemic treatment, we remove the tumor area in its original size, as it is currently not proven that a local tumor control can be achieved by reduction in local radicality.
TABLE 4. Overview of Local Recurrence Rates in SSM
The SSM with or without NAC conservation is quite different from the subcutaneous mastectomy with prostheses reconstruction. For a good and long-lasting aesthetic result, the surgeon has to find a compromise between radical gland tissue removal and conservation of gland tissue along with the NAC. The first is burdened with unsatisfactory aesthetic results and an increased number of complications whereas by the latter 10% of the gland and in 7% occult tumor cells would be retained.25 In a series of 133 patients with subcutaneous mastectomy and 910 patients with modified radical mastectomy, local recurrence occurred significantly more frequently after subcutaneous mastectomy.26 Carlson et al7 have shown an increased local failure rate after expander reconstruction (10%) compared with autologous tissue reconstruction (4.9 to 5.6%). There has been an ongoing discussion as to how much skin and subcutaneous tissue should be resected to perform an adequate mastectomy while leaving viable skin flaps. One of the common recommendations is to dissect just superficial to the superficial layer of the superficial fascia of the breast. Histological evaluation revealed that the superficial layer is not present in all breasts and, if present, is often too thin. Therefore, a dissection superficial to the superficial layer would not leave viable skin flaps in skin-sparing mastectomies.27 However, after SSM histologically, no gland tissue was retained.16
As yet, there is no study comparing the oncologic safety of SSM with and without conservation of the NAC. Reconstruction by autologous tissue allows thinning-out the NAC like a free NAC transplant. This would result in a maximal oncologic safety. In a small series of only 7 patients with 1-stage immediate breast reconstruction by SSM and TRAM flap, the areola reconstruction was performed by harvesting the areola as a full-thickness graft from the mastectomy specimen. However, the nipple itself was reconstructed.8 Later, the same author reported the use of the entire NAC as a full-thickness graft.28 Overall, this technique is not very different from the presented SSM with NAC conservation, the difference being we preserve the nerves of the NAC. Advantages of a single breast reconstruction are better aesthetic results, minimal scars, lower costs, and no psychological trauma of mastectomy.
NAC tumor involvement depends predominantly on the distance of the tumor to the NAC, and the risk seems overestimated in the past. It was reported in 5.6% (16/286 patients),29 6.4% (11/173),30 16% (22/140),31 0% (0/26),32 and 0% (0/7)8 for NAC tumor distances greater than 2.0 to 2.5 cm. In a series of 217 patients with mastectomy a nipple involvement was reported in 9.75% for tumors smaller than 1 cm, and in 11.9% (1 to <2 cm tumor size), 4.5% (2 to <4 cm), and 18.2% (≥4 cm).30 The only variable that reliably predicted nipple involvement was the location of the breast cancer. In centrally located tumors a nipple involvement was found in 27.3% (12/44)30 and 54.1% (19/37)33 compared with 6.4%30 in patients with more distant tumors. Otherwise, a central tumor location nowadays does not represent a contraindication for breast conserving treatment if the margins are clear.34,35 By radiation and systemic therapy, the risk of recurrence was further decreased.36
Our data justify a preservation of the NAC at least in patients with preoperatively evaluated tumors at least 2 cm from the nipple, no extensive intraductal component (>25%), and intraoperatively estimated clear margins. SSM with preservation of the NAC offers no disadvantage regarding local recurrences and distant metastases as well as overall survival. Recently, Jensen37 dealt with the question: When can the nipple-areola complex safely be spared during mastectomy? He also concluded that the NAC could be conserved in patients with free margins without disadvantage for survival. Furthermore he appealed to the surgeons’ responsibility of avoiding mutilation of the female patients.
With respect to cosmetics and increasing importance of radiation therapy even after mastectomy the autologous tissue reconstruction is preferable to prostheses reconstruction.19 According to our data, SSM with NAC preservation represents a favorable method in suited cases that were candidates for mastectomy. Local recurrences due to conserving NAC are not increased compared to SSM with excision of the NAC and also to modified radical mastectomy. However, in choosing NAC-conserving surgery strict criteria for selecting patients must be honored, and careful postoperative follow-up is necessary.
Footnotes
Reprints: Bernd Gerber, PhD, LMU Munich, Department of Obstetrics and Gynecology, Maistraβe 11, 80337 Munich, Germany. E-mail: bernd.gerber@fk-i.med.uni-muenchen.de.
REFERENCES
- 1.Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87:1048–1053. [PubMed] [Google Scholar]
- 2.Gerber B, Krause A, Kuchenmeister I, et al. Skin sparing mastectomy with autologous immediate reconstruction: oncological risks and aesthetic results. Zentralbl Gynakol. 2000;122:476–482. [DOI] [PubMed] [Google Scholar]
- 3.Gerber B, Krause A, Muller H, et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol. 2001;19:960–971. [DOI] [PubMed] [Google Scholar]
- 4.Gerber B, Krause A, Reimer T, et al. Breast reconstruction with latissimus dorsi flap: improved aesthetic results after transection of its humeral insertion. Plast Reconstr Surg. 1999;103:1876–1881. [DOI] [PubMed] [Google Scholar]
- 5.Jensen JA, Handel N, Silverstein MJ, et al. Extended skin island delay of the unipedicle TRAM flap: experience in 35 patients. Plast Reconstr Surg. 1995;96:1341–1345. [DOI] [PubMed] [Google Scholar]
- 6.Lowery JC, Wilkins EG, Kuzon WM, et al. Evaluations of aesthetic results in breast reconstruction: an analysis of reliability. Ann Plast Surg. 1996;36:601–606. [DOI] [PubMed] [Google Scholar]
- 7.Carlson GW, Losken A, Moore B, et al. Results of immediate breast reconstruction after skin-sparing mastectomy. Ann Plast Surg. 2001;46:222–228. [DOI] [PubMed] [Google Scholar]
- 8.Hudson DA, Dent DM, Lazarus D. One-stage immediate breast and nipple-areolar reconstruction with autologous tissue I: a preliminary report. Ann Plast Surg. 2000;45:471–476. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo DA, Borgen PJ, Petrek JA, et al. Immediate reconstruction after complete skin-sparing mastectomy with autologous tissue. J Am Coll Surg. 1998;187:17–21. [DOI] [PubMed] [Google Scholar]
- 10.Delay E, Gounot N, Bouillot A, et al. Autologous latissimus breast reconstruction: a 3-year clinical experience with 100 patients. Plast Reconstr Surg. 1998;102:1461–1478. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre JI, Fix RJ, Gardner PM, et al. Reconstruction with the latissimus dorsi flap after skin-sparing mastectomy. Ann Plast Surg. 2001;46:229–233. [DOI] [PubMed] [Google Scholar]
- 12.Shrotria S. The peri-areolar incision—gateway to the breast. Eur J Surg Oncol. 2001;27:601–603. [DOI] [PubMed] [Google Scholar]
- 13.Lopez MJ, Porter KA. The current role of prophylactic mastectomy. Surg Clin North Am. 1996;76:231–242. [DOI] [PubMed] [Google Scholar]
- 14.Verheyden CN. Nipple-sparing total mastectomy of large breasts: the role of tissue expansion. Plast Reconstr Surg. 1998;101:1494–1500. [DOI] [PubMed] [Google Scholar]
- 15.Cady B. Risk of recurrence after treatment of early breast cancer with skin- sparing mastectomy: another editorial perspective [editorial; comment]. Ann Surg Oncol. 1998;5:103–104. [DOI] [PubMed] [Google Scholar]
- 16.Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102:49–62. [DOI] [PubMed] [Google Scholar]
- 17.Kroll SS, Schusterman MA, Tadjalli HE, et al. Risk of recurrence after treatment of early breast cancer with skin- sparing mastectomy. Ann Surg Oncol. 1997;4:193–197. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GW, Bostwick J, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singletary SE, Kroll SS. Skin-sparing mastectomy with immediate breast reconstruction. Adv Surg. 1996;30:39–52. [PubMed] [Google Scholar]
- 20.Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg. 1999;104:77–84. [PubMed] [Google Scholar]
- 21.Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol 1999;6:676–681. [DOI] [PubMed] [Google Scholar]
- 22.Rivadeneira DE, Simmons RM, Fish SK, et al. Skin-sparing mastectomy with immediate breast reconstruction: a critical analysis of local recurrence. Cancer. 2000;6:331–335. [PubMed] [Google Scholar]
- 23.Foster RD, Esserman LJ, Anthony JP, et al. Skin-sparing mastectomy and immediate breast reconstruction: a prospective cohort study for the treatment of advanced stages of breast carcinoma. Ann Surg Oncol. 2002;9:462–466. [DOI] [PubMed] [Google Scholar]
- 24.Medina-Franco H, Vasconez LO, Fix RJ, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santini D, Taffurelli M, Gelli MC, et al. Neoplastic involvement of nipple-areolar complex in invasive breast cancer. Am J Surg. 1989;158:399–403. [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi J, Iino JHY, Takei H, et al. A comparative study of subcutaneous mastectomy with radical mastectomy. Anticancer Res. 2001;21:2963–2967. [PubMed] [Google Scholar]
- 27.Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer. 2002;94:1619–1625. [DOI] [PubMed] [Google Scholar]
- 28.Hudson DA, Skoll PJ. Single-stage, autologous breast restoration. Plast Reconstr Surg. 2001;108:1163–1171. [DOI] [PubMed] [Google Scholar]
- 29.Laronga C, KempB, Johnston D, et al. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6:609–613. [DOI] [PubMed] [Google Scholar]
- 30.Simmons RM, Brennan M, Christos P, et al. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol. 2002;9:165–168. [DOI] [PubMed] [Google Scholar]
- 31.Vyas JJ, Chinoy RF, Vaidya JS. Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol. 1998;24:15–16. [DOI] [PubMed] [Google Scholar]
- 32.Verma GR, Kumar A, Joshi K. Nipple involvement in peripheral breast carcinoma: a prospective study. Indian J Cancer. 1997;34:1–5. [PubMed] [Google Scholar]
- 33.Galimberti V, Zurrida S, Zanini V, et al. Central small size breast cancer: how to overcome the problem of nipple and areola involvement. Eur J Cancer. 1993;29A:1093–1096. [DOI] [PubMed] [Google Scholar]
- 34.Canadian Association of Radiation Oncologists. Mastectomy or lumpectomy? The choice of operation for clinical stages I and II breast cancer. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. Car Med Assoc J. 1998;158(Suppl 3) S15–S21. [PubMed] [Google Scholar]
- 35.Dale PS, Giuliano AE. Nipple-areolar preservation during breast-conserving therapy for subareolar breast carcinomas. Arch Surg. 1996;131:430–433. [DOI] [PubMed] [Google Scholar]
- 36.Gajdos C, Tartter PI, Bleiweiss IJ. Subareolar breast cancers. Am J Surg. 2000;180:167–170. [DOI] [PubMed] [Google Scholar]
- 37.Jensen JA. When can the nipple-areola complex safely be spared during mastectomy? Plast Reconstr Surg. 2002;109:805–807. [DOI] [PubMed] [Google Scholar]
- 38.AJCC. American Joint Committee on Cancer. http://www.jasper-web.com/texascanceronline/staging.htm 2002.
- 39.Peyser PM, Abel JA, Straker VF, et al. Ultra-conservative skin-sparing “keyhole” mastectomy and immediate breast and areola reconstruction. Ann R Coll Surg Engl. 2000;82:227–235. [PMC free article] [PubMed] [Google Scholar]