Abstract
Objective:
Novel production and in vitro characterization of tissue engineered colon.
Summary Background Data:
Organoid units, mesenchymal cell cores surrounded by a polarized epithelia derived from full thickness sigmoid colon dissection from neonatal Lewis rats, adult rats, and tissue engineered colon itself, were implanted on a polymer scaffold into the omentum of syngeneic hosts. TEC was either anastomosed at 4 weeks or excised for Üssing chamber studies or histology, immunohistochemistry, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick end labeling assay.
Results:
TEC was generated by 100% of all animals without regard to tissue source, the first instance of engineered intestine from adult cells or an engineered tissue. TEC architecture is identical to native with muscularis propria staining for actin, acetylcholinesterase detected in a linear distribution in the lamina propria, S100-positive cells, ganglion cells, and a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick end labeling assay similar to native colon. Üssing chamber data indicated in vitro function consistent with mature colonocytes, and a positive short circuit current response to theophylline indicating intact ion transfer. TEM showed normal microarchitecture. Colon architecture was maintained in anastomosis with gross visualization of fluid uptake.
Conclusions:
TEC can be successfully produced with fidelity to native architecture and in vitro function from neonatal syngeneic tissue, adult tissue, and TEC itself.
The absence of native large intestine has been linked to significant morbidities, including changes in water and sodium absorption, production of short chain fatty acids, and lack of storage capacity.1 Ileal pouch substitution has a number of serious complications as well, including inflammation and dilation of the pouch, high stool frequency, and pelvic sepsis resulting from pouch leak.2 The cumulative frequency of pouchitis at major referral centers approaches 50% over 10 years.3,4 Therapy directed at relieving pouch morbidity includes revision or conversion to end-ileostomy, transrectal catheter drainage, antibiotics, and steroids.5 Ileal mucosal adaptation in the pouch by colonic metaplasia and the presence of chronic inflammation have been postulated to increase long-term neoplasia.6
Our laboratory first reported making tissue-engineered small intestine by the transplantation of organoid units on a polymer scaffold into the omentum of the Lewis rat.7 Organoid units are multicellular units derived from neonatal rat intestine, containing a mesenchymal core surrounded by a polarized intestinal epithelium, and contain all of the cells of a full-thickness intestinal section.8 We speculated that tissue-engineered colon (TEC) could be produced by adapting the technique of organoid unit transplantation, and that the resulting tissue could be an effective in vivo large intestine substitute with physiologic function that does not require ileal mucosal adaptation.9 We produced TEC in Lewis rats from both neonatal rats and from TEC itself and characterized the resulting tissue by histology, immunohistochemistry, and Üssing chamber studies.
MATERIALS AND METHODS
TEC Formation
Colon organoid units were produced by dissecting the sigmoid colon without mesentery from 4-day-old Lewis rat pups (n = 40), luminally irrigated with cold Hanks’ balanced salt solution (HBSS), then cut into full thickness 2-mm × 2-mm sections after lengthwise opening along the antimesenteric border. These were washed 3 times in 4°C HBSS, sedimenting between washes and digested with dispase 0.25 mg/mL and collagenase 800 U/mL on an orbital shaker at 37°C for 30 minutes. The digestion was immediately stopped with 3 washes at 4°C of a solution of high glucose Dulbecco’s modified Eagle medium, 4% heat-inactivated fetal bovine serum, and 4% sorbitol. The organoid units were centrifuged between washes at 150 g for 5 minutes, and the supernatant removed. Organoid units were reconstituted in high-glucose Dulbecco’s modified Eagle medium with 10% heat-inactivated fetal bovine serum, counted by hemocytometer, loaded 100,000 units per polymer at 4°C, maintained at that temperature until implantation, which occurred in under 1.5 hours.
Scaffold polymers were constructed of 2-mm thick nonwoven polyglycolic acid, formed into 1-cm tubes (outer diameter [OD] = 0.5 cm, inner diameter [ID] = 0.2 cm), and sealed with 5% polylactic acid. Polymer tubes were sterilized in 100% ethanol at room temperature for twenty minutes, then washed with 500 mL of phosphate-buffered saline (PBS), coated with 1:100 collagen Type1: PBS solution for 20 minutes at 4°C and washed again with 500 mL of PBS. Polymer tubes were internally loaded with organoid units by micropipette.
Under pentobarbital anesthesia, 150-g Lewis rats (n = 30) were implanted with TEC. Implantation was achieved through a 1.5-cm upper abdominal incision, through which the greater omentum was externalized and wrapped in a specific manner around the TEC construct, secured with a 6–O prolene suture and returned to the peritoneum before closing the abdomen in layers. Four weeks later, TEC was either harvested for Üssing chamber experiments (n = 10), histology and immunohistochemistry (n = 8), or anastomosed to either small intestine (n = 5) or large intestine (n = 5) in a side-to-side fashion with 6–O Prolene.
The remaining 2 animals were harvested at 4 weeks and organoid units were generated from their TEC, which were generated and implanted as reported above. These constructs were implanted into 8 syngeneic hosts. Similarly, adult colon was harvested from 2400 g 6-month-old Lewis rats and by the same process, constructs were generated and implanted in 8 hosts. Both TEC from TEC and TEC from adult colon experiments were repeated to confirm the results. A blinded pathologist reviewed slides from all 3 sources to describe pathology and to relate differences between the source groups. All animals were allowed free access to laboratory chow and water, consuming approximately equal amounts after postoperative day 1. Pouch size was measured at time of anastomosis and at harvest (day 31 for all anastomosed animals).
Polymer Variation
To establish the optimal polymer configuration for the generation of TEC, before the studies above organoid units were obtained as described and seeded 100,000 units/1.5 cm2. Each construct was implanted in 3 150-g Lewis rats and the resulting cysts were measured, studied with hematoxylin and eosin (H&E), periodic acid Schiff (PAS), and trichrome. The implanted constructs were the following, each group implanted with a 5% coating of poly(L-lactic acid) (PLLA) and without: 0.5-mm thick nonwoven PGA tubes (OD 0.5 mm, length 6 cm); 1-mm thick nonwoven PGA tube (OD 1 cm, lengths 1, 3, 5, 7 cm); the same 5 groups woven into the mesentery rather than the omentum, 2-mm thick nonwoven PGA 1-cm × 1-cm square, and 1-mm thick nonwoven PGA 1-cm × 1-cm square. Polymers woven into the omentum were wrapped between 2 adjacent layers of mesentery without removing the peritoneal layer, and with attention to not disrupt the attached small bowel or to allow the resulting construct to distort the flow of small bowel contents.
TEC Histology and Immunohistochemistry
Formalin-fixed tissue was sectioned and stained with H&E, PAS, and Masson’s Trichrome staining to observe tissue architecture and for evidence of inflammation according to St. Mark’s criteria.10 Immunohistochemistry was performed using the DAKO Envision kit to detect antigens monoclonal alpha antismooth muscle actin (Sigma) and the S100 protein (DAKO). Tissue was snap frozen in frozen section medium and cryosections were collected and stained with Karnovsky’s fluid for detection of Acetylcholinesterase. The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick end labeling (TUNEL) assay was performed using the ApopTag kit (Intergen). Routine processing for transmission electron microscopy (TEM) was employed after 1-mm3 sections were fixed in gluteraldehyde.
Üssing Chamber Experiments
Nonfasted rats were anesthetized and a midline laparotomy was performed; the anastomosed colonic cyst was gently freed from any adhesions and excised off its anastomosis with the native bowel. It was quickly rinsed free of luminal content in cold modified Ringer’s solution. Similarly a 5-cm length of native colon 5 cm distal to the cecum was excised, opened along its mesenteric border, rinsed and placed in cold ringers’ to serve as its control in the Üssing chamber.
The cyst and native colon were mounted on Lucite Üssing chamber block (World Precision Instruments, Sarasota, FL) with an exposed area of 0.64 cm. The native colon was stripped of muscularis under a stereoscopic microscope while the cyst was partially stripped. The tissue was allowed to equilibrate in 10 mL of modified Ringers’ solution containing (in mM) 140 Na+, 5.4 K+, 1.3 Ca2+, 1.2 Mg2+, 2.4 HPO42, 124 Cl−, 0.6 H2 PO42−, 21 HCO32−, 5mM HEPES, and 10 mM fructose on both the mucosal and serosal sides at pH 7.4 ± 0.01. It was gassed with a carboxygen ratio of 5/95% and maintained at 37°C with water-jacketed reservoirs. Transmural potential difference (PD) was measured using calomel electrodes connected to the bathing solution with Ringer–Agar (3%) bridges. Tissues were continuously short-circuited with an automatic voltage clamp (model EVC-4000, World Precision Instruments) except during a 5- to 10-second interval every 5 to 10 minutes when the open-circuit PD (mV) was measured. A transmucosal PD (mV) was read and tissue resistance R (Ohm . cm2) was calculated from Ohm’s law (V = IR), using Isc (Amp/cm2) the short circuit current. Tissues from the same animal were paired. After a 30- to 45-minute equilibration period with modified Ringer’s solution, both the mucosal and serosal sides of the tissue were exposed to a final chamber concentration of 30 mM 3-OMG. Changes in Isc during the following 20 to 40 minutes were recorded. Tissue viability was checked by the addition of 5 mM theophylline to the chambers, after which the maximum change in Isc was recorded.
RESULTS
The initial studies of polymers resulted in 2 findings; the implanted construct must stent the omentum open, rather than let it collapse around the polymer, and the omentum is the preferred TEC implantation site (Table 1). All animals formed TEC, but constructs that were not tubular, coated with PLLA, and implanted in the omentum formed many small cysts that were not always contiguous along the path of the polymer, whereas any tubular polymer constructed of PGA/PLLA and implanted in the omentum generated a TEC cyst as described above. Increased surface area of the construct did not increase the resulting size of the TEC generated, and there was no statistically significant difference in TEC size once generated. Implantation in the mesentery always resulted in discontinuous multiple small cysts of TEC whether the construct was coated or not.
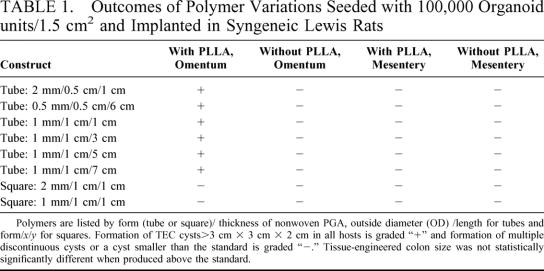
TABLE 1. Outcomes of Polymer Variations Seeded with 100,000 Organoid units/1.5 cm2 and Implanted in Syngeneic Lewis Rats
All animals survived implantation and 100% of all animals generated TEC. Approximately 60,000 OU were harvested from each rat pup colon. This is a variable number as the tissue surface area resected in each case may vary. Because adult colons were far larger, each adult rat colon resulted in about 800,000 OU/resected specimen. These included those generating secondary TEC (TEC from TEC itself) and those implanted with TEC derived from adult tissue. The average size of the TEC was 4 × 5 × 4 cm. No TEC measured less than 3 × 3 × 2 cm, and the largest was 6 × 8 × 7.5 cm. Figure 1 and 2 are photographs of a representative TEC cyst after 4 weeks in the omentum. TEC was first seen as a small cyst with a mucus-filled lumen at 2 weeks. Before that, no clear lumen was identified. Serial growth occurred over the subsequent weeks. TEC that was not harvested at 4 weeks, but was allowed to continue growing in a separate project continued to increase in size each week. A cyst with dimensions of 11 cm × 13 cm × 12 cm was identified in 1 animal at 14 weeks. Results of Üssing chamber studies and histology did not change with regard to the source of the organoid units, and 4-week size was equally distributed between host sources. Therefore, the following data is reported for all groups studied. Differences among the TEC obtained from pup colon, TEC itself, or adult colon, could not be distinguished by a blinded pathologist who reviewed the histology of all specimens. Each TEC specimen included all of the components reported herein and there was no variation in quality of the TEC by its donor source. These findings were confirmed by a second pathologist.
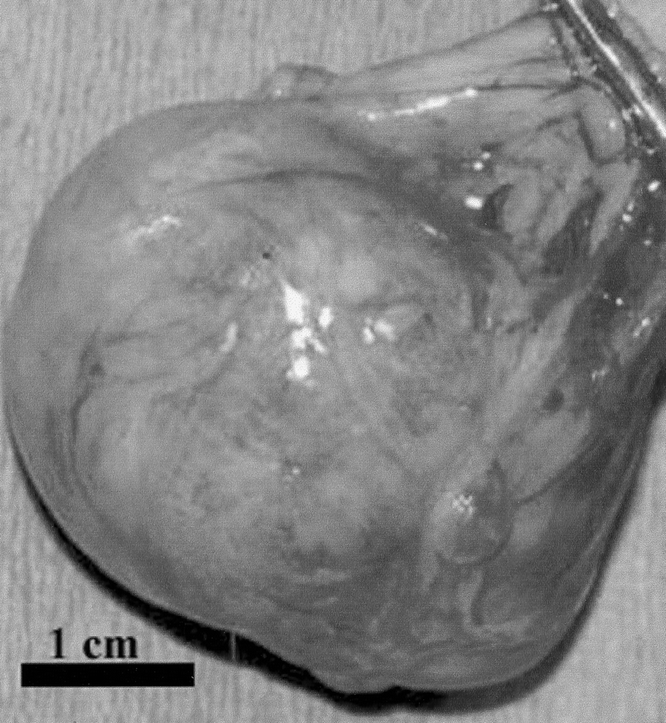
FIGURE 1. Gross picture of a representative TEC cyst at 4 weeks after implantation. Relative size is indicated.
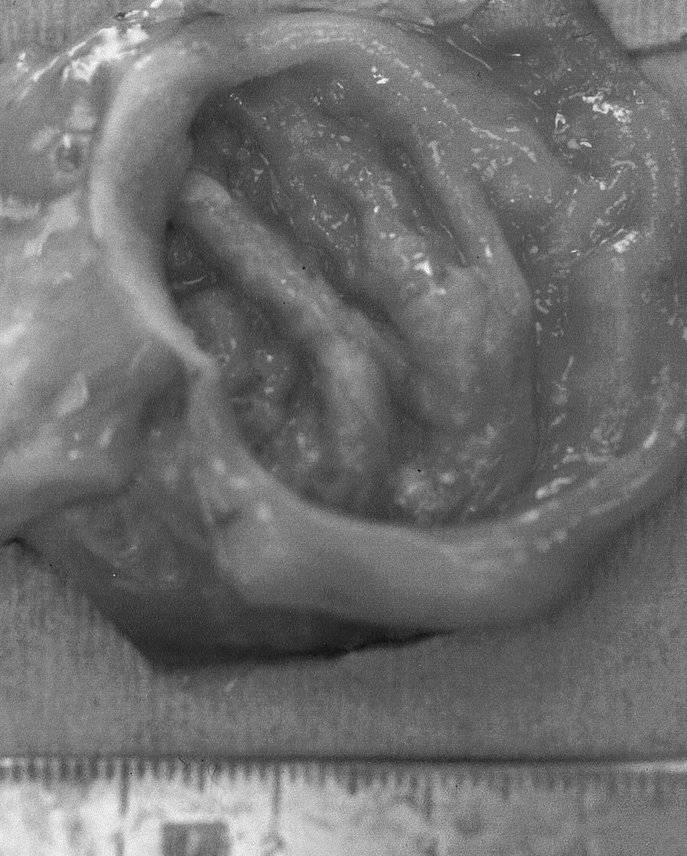
FIGURE 2. Gross picture of the same cyst, opened with scalpel handle in foreground.
The same observations except for TEM, which was not repeated, were made of TEC after anastomosis, and whether anastomosed to small intestine or large intestine, the architecture of the TEC was preserved. At harvest, the stool contents above the TEC whether anastomosed to small intestine or large intestine were grossly more liquid than the contents that exited the anastomosed area, reflecting overall fluid absorption by the TEC segment. There was no statistically significant difference in pouch size after anastomosis at the time of harvest. Animals were deeply anesthetized at the time of harvest, so observations of peristalsis or its absence may be skewed. Indirect evidence of some motility included the mixing of contents noted, with no rim of static material close to the mucosa, and absence of obstruction in any of the anastomosed animals.
H&E TEC staining revealed an uninterrupted uniform large intestinal mucosa oriented toward the lumen of the cysts. This included straight tubular shaped crypts of Lieberkuhn with numerous goblet cells, and a loose lamina propria bearing lymphoid cells. There was an absence of villi or Paneth cells and an outer longitudinal layer of smooth muscle, normal vascularization, present ganglion cells, and no evidence of inflammation (Fig. 3). PAS staining showed a normal distribution of goblet cells. Trichrome staining revealed a normal collagen rich blue submucosa with a basement membrane <7 μm (Fig. 4). Immunohistochemical staining for the antigen smooth muscle actin revealed smooth muscle fibers in the muscularis propria exactly recapitulating staining in native colon (Fig. 5). Immunohistochemical staining to the S100 antigen revealed dense staining in the areas of Auerbach’s and Meissner’s plexi with less density in the TEC but appropriate positioning (Fig. 6). In the midst of the S100-positive cells, large lucent ganglion cells were identified in both native and TEC (Fig. 7). The TUNEL assay identified identical numbers of positive cells in native and TEC, 4/hpf. Cryosections stained with antiacetylcholinesterase demonstrated a normal contiguous distribution of nerve fibers in the lamina propria without tufting (Fig. 8).
FIGURE 3. 10× H&E section of TEC.
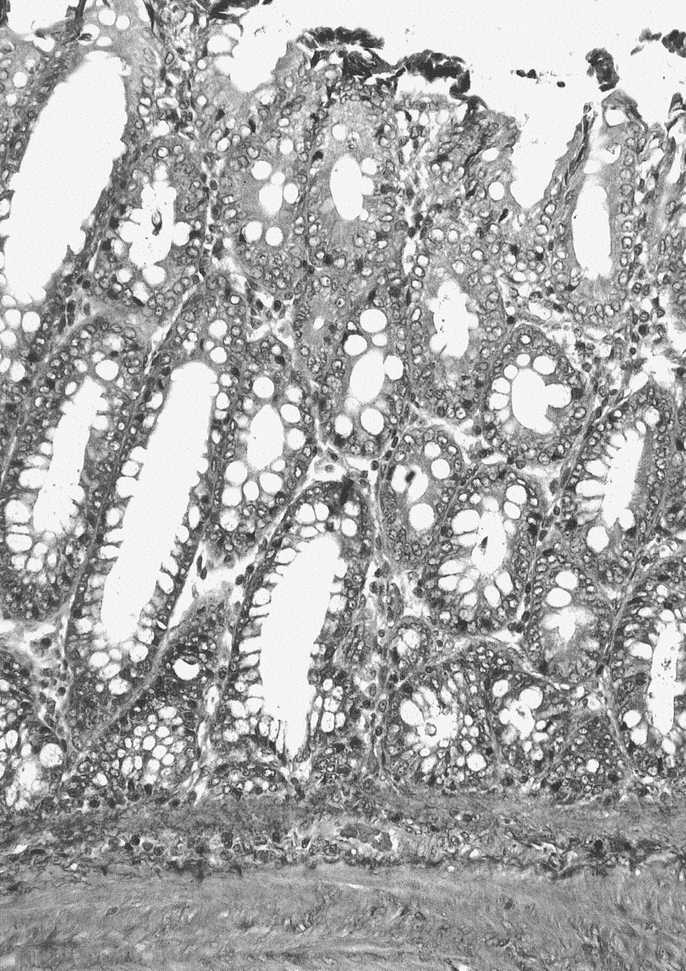
FIGURE 4. Trichrome 20× section of TEC, collagen-rich submucosa stains in pattern similar to native.
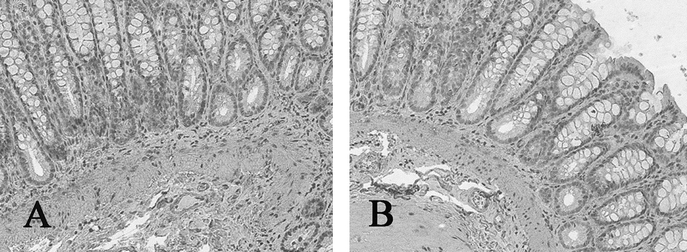
FIGURE 5. Actin immunohistochemistry of native (A) and tissue-engineered (B) colon, positive in the muscularis propria (arrow), 10×.
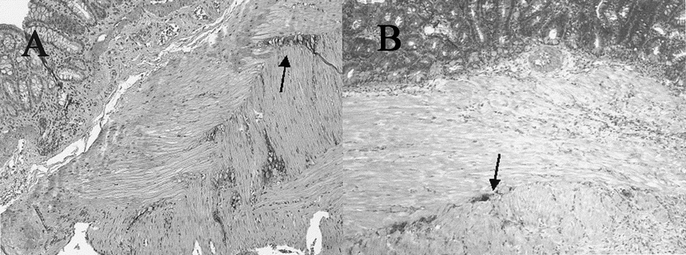
FIGURE 6. S100 immunohistochemistry of native (A) and tissue-engineered (B) colon, positive in the area of Auerbach’s plexus, 20×.
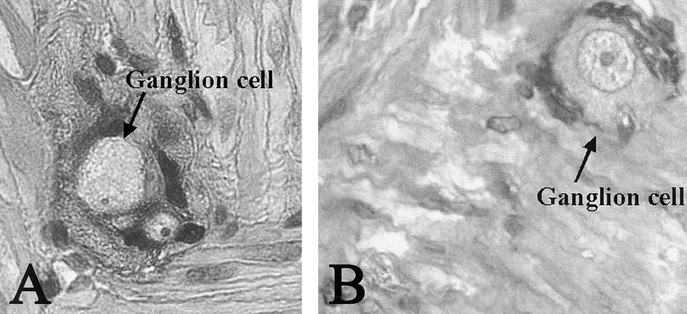
FIGURE 7. Ganglion cells seen as large lucent cells surrounded by S100-positive cells in of native (A) and tissue-engineered (B) colon, 40×.
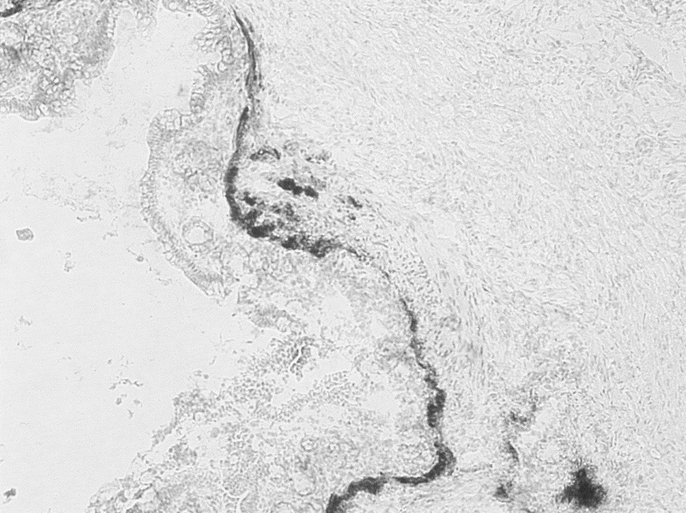
FIGURE 8. Detection of acetylcholinesterase by frozen section staining with Karnovsky’s fluid, 10×.
TEM revealed the same ultramicroscopic findings as that in normal colonic mucosa: abundant mitochondria, apical microvilli, tight junctions, desmosomes with keratin filaments, neuroendocrine cells, and goblet cells. (Fig. 9, Fig. 10, and Fig. 11).
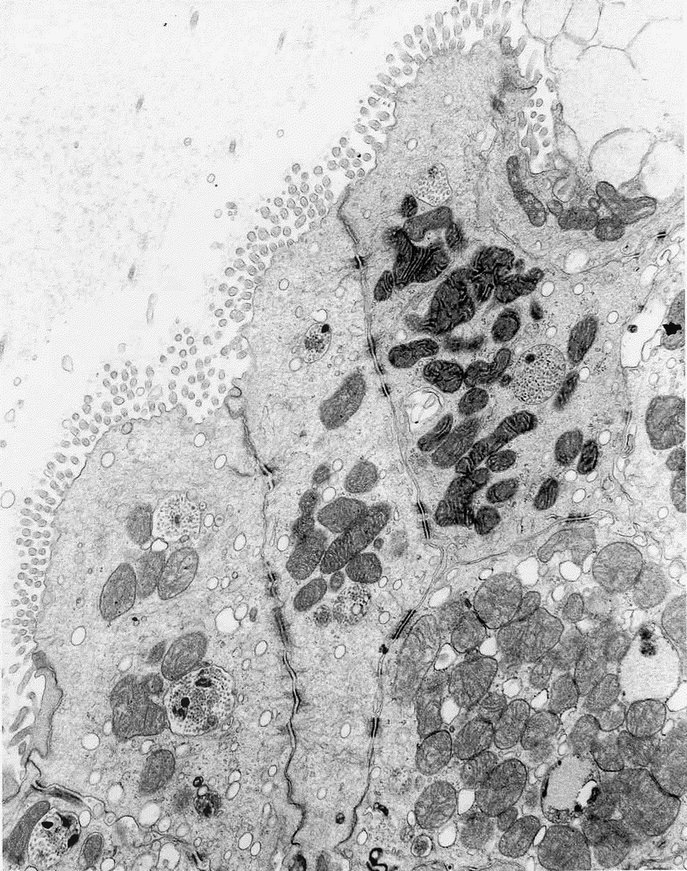
FIGURE 9. TEM of TEC showing tight junctions and abundant mitochondria as well as desmosomes with keratin filaments.
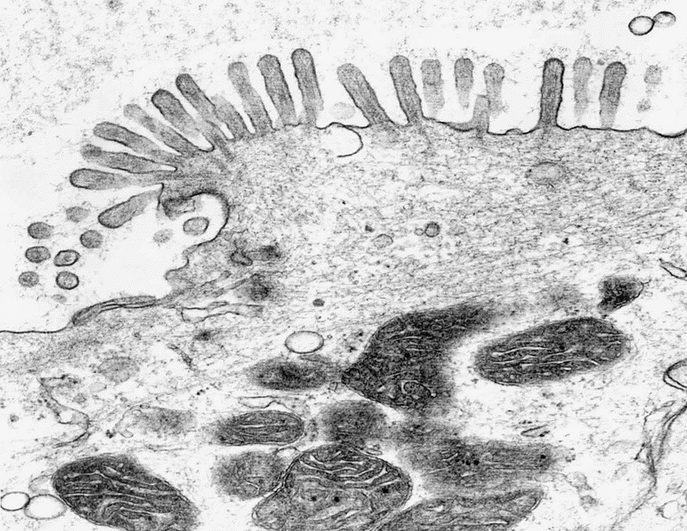
FIGURE 10. TEM of TEC showing apical microvilli as seen in mature native colon.
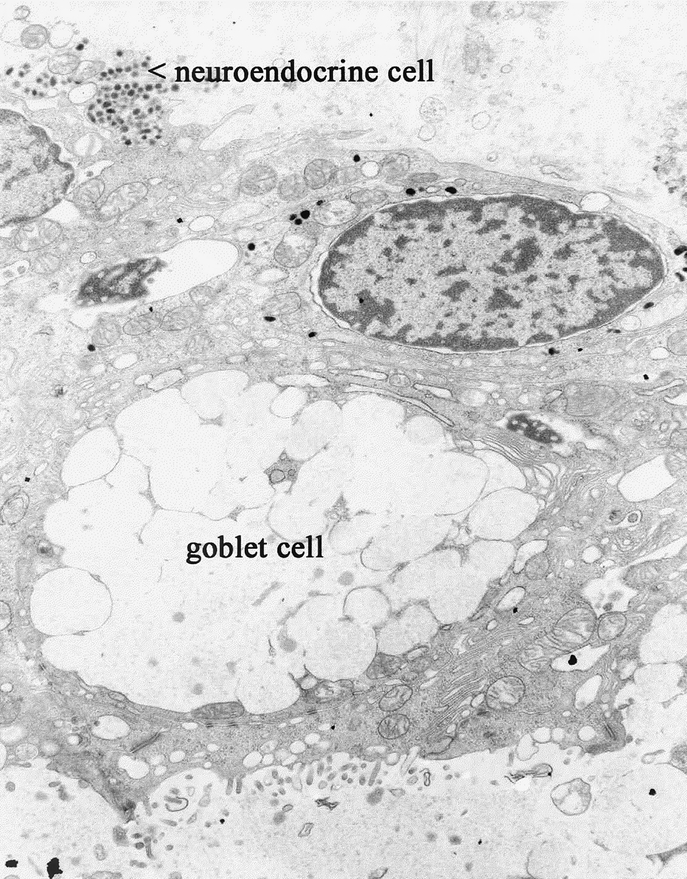
FIGURE 11. TEM of TEC showing characteristic neuroendocrine and goblet cells.
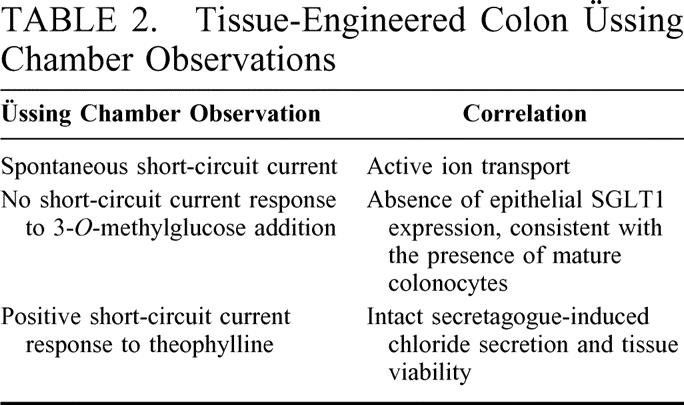
The electrophysiological parameters of native colon and anastomosed tissue-engineered cyst were evaluated in the Üssing chamber (Summarized in Table 2). The following values were obtained at equilibrium PD (mV), Isc (ΩAmp/cm2) and R (Ωcm2) for native colon and anastomosed cyst 4.85 ± 1.22 mV, 47.0 ± 6.38 ΩAmp/cm2, 63.0 ± 8.011 Ωcm2 versus –1.60 ± 0.44 mV, 13.25 ± 3.20 ΩAmp/cm2 and 82.95 ± 19.70 Ωcm2 respectively (n = 4). There was no change in Isc to 30 mM 3-OMG from both tissues (n = 4). The maximum change in Isc after theophylline was 72.5 ± 13.96 versus 11.67 ± 3.96 ΩAmp/cm2 for native colon and anastomosed cyst respectively (n = 3). Neither native nor engineered colon exhibited any change in Isc in response to the addition of 30 mM 3-OMG. Both native and engineered colon exhibited increases in Isc following the addition of theophylline 270 ± 50% and 220 ± 50% respectively.
TABLE 2. Tissue-Engineered Colon Üssing Chamber Observations
DISCUSSION
There are many instances in which total colectomy is necessary and the most common surgical correction after colectomy to replicate storage and absorption properties of the lost native tissue is the formation of an ileal pouch. Although ileal pouch replacement for a resected native large intestine is fairly durable, pouchitis is a common complication after total proctocolectomy, and there are postcolectomy morbidities associated with lack of physiologic large intestinal function. The symptoms associated with pouchitis are not trivial, including reoperation, stool frequency, abdominal cramping, fever, and extraintestinal manifestations.11 Although pouchitis is certainly related in part to host factors, which accounts for an increased incidence of pouchitis in ulcerative colitis as compared with familial adenomatous polyposis,11 the adaptation of small intestine to large intestinal function must also be important. This reported technique of tissue engineering colon from syngeneic tissue may currently exclude its use for the treatment of patients with abnormal autologous donor cells if those cells grow into abnormal TEC, which has not yet been undertaken. However, in patients with the ability to donate autologous cells, this may be an improvement over current practices of surgical substitution.
The underlying principle of tissue engineering is exact replacement of tissue function and architecture that have been removed rather than replacement by proxy. In this case, we report tissue-engineered large intestine that is produced in a reasonable quantity with high efficiency and from a number of sources, including TEC itself and adult sources. This is the first instance of intestine engineered from adult cells and from an engineered tissue itself, an observation with interesting prospects both for reducing animal experimentation as well as for eventually developing phenotypes of engineered intestine. As well, growth of a tissue-engineered organ from adult cells will be necessary for the majority of autologous recipients toward which tissue engineering is directed. The relative hardiness of the TEC is further reflected in the 100% growth rate, which continues to date in further experiments, compared with rates around 60 to 80% for tissue-engineered small intestine.7
The histology with adjunct immunohistochemical studies of this novel tissue reflect intact epithelial, muscular, vascular, and nerve components including an appropriate distribution of acetylcholinesterase. This is the first demonstration of ganglion cells and S100-positive groupings in tissue-engineered intestine, and the failure of anastomosed pouches to distend makes future peristalsis studies imperative. TEM demonstrated correct microarchitecture, including apical microvilli with anchoring filaments and tight junctions as well as abundant mitochondria.
The initial Üssing chamber studies indicate appropriate large intestinal physiologic function, which were additionally seen in a preliminary way in vivo. Üssing chamber values show that native PD is 3 times larger than that of the anastomosed neo-intestinal colon cyst and its Isc is also 3.5 times greater. The resistance however was 1.3 greater in the anastomosed cyst. These values could be explained as the cyst is less mature or a has a less electrogenic property than the native colon but because of the thicker partially stripped wall of TEC, it has a greater resistance value (Table 2). The Ussing chamber studies suggest appropriate colonic transport parameters, including vectorial ion transport, barrier function, and viability. Adult rat colon does not exhibit sodium-dependent glucose absorption although neonatal rat colon does. The failure of TEC to exhibit sodium-dependent glucose absorption suggests a mature transporter phenotype, at least with respect to glucose.
Although the TEC grows orders of magnitude beyond the tissue mass originally implanted, TUNEL assays do not indicate an increase in apoptosis that might indicate a future risk for the development of unregulated growth. After anastomosis, TEC retained its size but did not continue to grow in size although it does so without anastomosis. Furthermore, TEC retains its histology when anastomosed to small intestine, demonstrating the donor source of the cells rather than a repopulation of a graft by adjacent tissue.
We demonstrated that a large intestine substitute can be generated in a novel fashion by the transplantation of organoid units on a polymer scaffold, and that this TEC functions in vitro, meeting basic physiologic demands. We speculate that this technique could be a successful way to replace large intestinal function when its lack is a source of morbidity, and to avoid the morbidity of treating complications related to other large intestinal replacement techniques. The growth of TEC from various sources including adult syngeneic tissue and TEC itself are important for planning the extension of these studies to therapy for postcolectomy morbidity.
Footnotes
Supported by the Center for the Integration of Medicine and Innovation in Technology, Department of Defense #DAMDIT-99–2–9001.
Reprints: Joseph P. Vacanti, MD, Department of Pediatric Surgery, Massachusetts General Hospital, Warren 1157, 55 Fruit Street, Boston, MA 02114. E-mail: jvacanti@partners.org.
REFERENCES
- 1.Papa MZ, Karni T, Koller M, et al. Avoiding diarrhea after subtotal colectomy with primary anastomosis in the treatment of colon cancer. J Am Coll Surg. 1997;184:269–272. [PubMed] [Google Scholar]
- 2.Fonkalsrud EW, Bustorff-Silva J. Reconstruction for chronic dysfunction of ileoanal pouches. Ann. Surg. 1999;229:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahlberg D, Gullberg K, Liljeqvist L, et al. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum. 1996;39:1012–1018. [DOI] [PubMed] [Google Scholar]
- 4.Meagher AP, Farouk R, Dozois RR, et al. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800–803. [DOI] [PubMed] [Google Scholar]
- 5.Dayton MT. Redo ileal pouch-anal anastomosis for malfunctioning pouches—acceptable alternative to permanent ileostomy? Am. J Surg. 2000;180:561–565. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd NA. The pelvic ileal reservoir: apocalypse later? BMJ. 1990;301:886–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi RS, Vacanti JP. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Trans Proc. 1997;848–851. [DOI] [PubMed] [Google Scholar]
- 8.Evans GS, Flint N, Somers AS, et al. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101:219. [DOI] [PubMed] [Google Scholar]
- 9.Grikscheit TC, Ochoa ER, Ramsanahie A, et al. Tissue engineered colon, characterization and comparison to native colon. Owen Wangensteen Surgical Forum Abstracts of the American College of Surgeons 87th Annual Clinical Congress 2001;LII:55–56.
- 10.Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal reservoir. Int J Colorectal Dis. 1986;1:167–174. [DOI] [PubMed] [Google Scholar]
- 11.Shen B, Achkar JP, Lashner BA, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121:261–267. [DOI] [PubMed] [Google Scholar]
- 12.Stein J, Zores M, Schroder O. Short chain fatty acid uptake in Caco-2 cells by a ph-dependent and carrier mediated transport mechanism. Eur J Nutr. 2000;39:121–125. [DOI] [PubMed] [Google Scholar]
- 13.Zaharia V, Varzescu M, Djavadi I, et al. Effects of short chain fatty acids on colonic Na+ absorption and enzyme activity. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:335–347. [DOI] [PubMed] [Google Scholar]