Abstract
Objective:
To evaluate the postoperative cytology of drained fluid from the pancreatic bed as a predictive indicator of local recurrence after curative (R0) resection of pancreatic cancer.
Summary Background Data:
The pancreatic bed offers a common site of cancer recurrence (local recurrence), even after curative (R0) resection is performed for pancreatic cancer. If local recurrence is thereby predicted precisely, soon after surgery, we have a chance to treat it by adding radiation or some other locoregional therapy before it can grow or spread beyond the pancreatic bed. However, there have been no previous reports of cytology performed on the drained fluid after pancreatectomy.
Methods:
This study includes 94 patients who had shown negative results in the peritoneal washing cytology before resection and subsequently received pancreatectomies for pancreatic tumors. They consisted of 12 benign tumors, 17 noninvasive or minimally invasive carcinomas and 65 invasive ductal carcinomas (R0 = 58; R1/2 = 7). Postoperatively, the drained fluid from the pancreatic bed was collected for 24 hours and used for cytologic examination. The cytologic results were examined in association with the histopathology of the resected tumor, patient’s survival, and mode of cancer recurrence, including local recurrence.
Results:
Patients with benign tumors or noninvasive/minimally invasive carcinomas had negative result in cytology, and none of them have died of local recurrence (limited to the pancreatic bed) to date. However, patients with invasive ductal carcinoma revealed higher cytology-positive rates: 28% (16/58) in curative (R0) resection; and 71% (5/7) in noncurative (R1/2) resection. Among 58 patients with R0 resection, the 3-year survival rate was 14% in 16 cytology-positive patients and 55% in 42 cytology-negative patients (P < 0.05). The 3-year cumulative rate of local recurrence was 85% and 23%, respectively (P < 0.05). Compared with other histopathologic parameters obtained from the resected specimens, the drain cytology was more specific in predicting the subsequent development of local recurrence.
Conclusions:
Drain-cytology was a quick examination that enabled us to specifically indicate both minute residual cancer and subsequent development of local recurrence even after R0 resection of pancreatic cancer.
To date, surgical resection has offered the only chance for complete cure in the treatment of invasive ductal adenocarcinoma of the pancreas. However, the 5-year survival rates after resection of this cancer have been reported to be as low as 10–30%,1,2 and more than half of patients die of cancer relapse within 2 postoperative years. Such a poor result is largely attributed to a high incidence of local recurrence3,4 after curative (R0) resection had been performed without macroscopic or microscopic cancer residual. In accounting for this fact, we can easily speculate that a minute and occult focus of the cancer might have been left behind in the pancreatic bed because pancreatic cancer cells are likely to infiltrate into the surrounding soft tissues. If occult cancer residual could be predicted correctly and quickly, even after resection, we may have a chance to treat it immediately before it can grow, form an obvious tumor mass, and spread beyond the pancreatic bed, by adding some locoregional therapy. For instance, the GITSG5 succeeded in improving the patient’s survival by combining postoperative radiation therapy on the pancreatic bed with an intravenous administration of 5-fluorouracil.
Either peritoneal or pleural lavage cytology has been widely performed to strictly select the operative indication for cancer patients.6,7 However, in addition to preoperative pleural lavage cytology, Higashiyama8 performed postoperative cytology after lung cancer resection to confirm the operative curability. He described that the patients’ survival periods were reduced because of a high incidence of local recurrence among the patients whose cytologic results had shifted from negative to positive following resection, suggesting the need for adjuvant locoregional therapy. Additionally, Doki9 performed a similar analysis after resecting squamous cell carcinomas of the esophagus and showed that patients with positive postoperative cytology had a short survival based on a high incidence of distant metastasis. He recommended systemic chemotherapy rather than radiation therapy for positive patients. Such knowledge is essential in selecting the most suitable adjuvant therapies for each postoperative patient. However, there have been no previous reports of postoperative cytology after resecting cancers of the intra-abdominal organs, including pancreatic cancer. Thus, this study is conducted to investigate whether the postoperative cytology of drained fluid from the pancreatic bed can correctly predict both the patient’s prognosis and the site of disease relapse after a macroscopically curative resection of pancreatic cancer.
PATIENTS AND METHODS
During the period 1996–2001, 94 patients with cancer or benign tumors of the pancreas received pancreatectomies (pancreatoduodenectomy, caudal pancreatectomy, or total pancreatectomy) at Osaka Medical Center for Cancer and Cardiovascular Diseases. According to the postoperative histopathologic diagnosis, they were classified into the following 3 groups: 12 benign tumors (intraductal papillary mucinous adenoma = 4, solid and papillary neoplasm = 3, pancreatitis = 3, benign islet cell tumor = 2); 17 noninvasive/minimally invasive carcinomas (intraductal papillary-mucinous carcinoma = 15 [noninvasive = 6; invasive but limited in the pancreas = 9], and noninvasive cystadenocarcinoma = 2); and 65 invasive ductal adenocarcinomas (Table 1). Immediately after laparotomy, peritoneal washing cytology was performed before pancreatic resection and none revealed a positive result. For patients with a benign tumor or noninvasive/minimally invasive carcinomas, the pancreatic tumor was completely removed without macroscopic residual tumor. All patients with invasive ductal carcinoma received lymphatic and connective tissue clearance in addition to the pancreatectomy,3 but 3 of them showed a macroscopic residual cancer (R2 resection; UICC classification10) in the pancreatic bed.
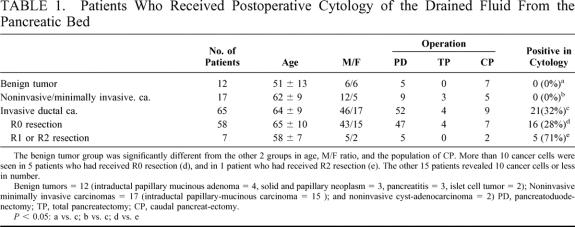
TABLE 1. Patients Who Received Postoperative Cytology of the Drained Fluid From the Pancreatic Bed
Before closing the abdomen, the abdominal cavity was washed carefully with more than 4 L of physiologic saline until it became clear, and 2 drainage tubes were placed in the pancreatic bed. For all 94 patients, on the second or third postoperative day, the drained fluid usually cleared (serous) without blood clots or debris, and it was collected for 24 hours in a storage bag which containing 5000 units of heparin. For 8 patients with invasive ductal carcinoma, the drained fluid also was collected on the first postoperative day. Three patients were excluded from this study because they still had discharges of thick blood or clots at this time. The collected fluid was then centrifuged at 2000 rpm (900 ∼ 1000 × g) for 3 minutes, and the sediment was placed on a slide glass. It was stained using Papanicolaou’s method, and microscopically observed by 2 or 3 cytologists who were blind to the patient’s data. The cytodiagnosis was made based on the ratio of nucleus/ cytoplasm, shape of nucleus, amount and distribution of nuclear chromatin, status of nucleoli and cytoplasmic mucin,11 and the result was classified as either negative and positive. If positive, the number of cancer cells per patient was also counted. Patients with borderline positive results, in whom only a small number (range, 1 to 3) of cells with highly severe atypia or possible cancer cells were detected in the sample, were also classified as positive. Cells were excluded from the evaluation, when they were so degenerated that diagnosis was difficult.
The resected specimens were fixed in 10% formaldehyde, sliced into 5-mm sections, embedded in paraffin blocks, sliced into 4-μm sections, and stained with hematoxylin and eosin. They were used for microscopic observation to determine the histology of the tumor, the status of nodal involvement, and the extent of cancer invasion into the peripancreatic tissues. The surgical margin was determined microscopically, and a positive diagnosis (R1 resection) was made when cancer cells were detected on the resected line. No positive results were seen for patients with benign or low-grade malignant cancer, but were noted in 4 patients with invasive ductal carcinoma. Also, for each of the negative cases (R0 resection: n = 58), the shortest distance was measured between the cut lines and cancer tissues. Patients were classified into 2 subgroups according to whether or not cancer cells were seen in the area within 5 mm of the cut lines. No patients received postoperative radiation therapy, and all patients have been followed monthly or bimonthly at our outpatient clinic until the present or until death. Follow-up included the serial determination of plasma carcinoembryonic antigen, carbohydrate 19–9, ultrasonography, magnetic resonance imaging, and computed tomography to determine whether and where any cancer recurrence developed. During the 2 postoperative years, blood sampling was performed at 2- to 3-month intervals and imaging diagnosis at 3- to 6-month intervals. At the macroscopic level, the sites of cancer recurrence were classified into local recurrence, liver metastasis, peritoneal carcinomatosis (dissemination) and pleural carcinomatosis (dissemination). Local recurrence was defined as when a tumor mass formation and/or lymph node enlargement was initially depicted in the pancreatic bed alone. Subsequently, they were confirmed to have gradually enlarged in parallel with the increases in the serum carcinoembryonic antigen and carbohydrate 19–9 levels until death. The latter 2 types of carcinomatosis were defined as when cancer cell-containing effusion was detected in the peritoneal or pleural cavities, which suggested a diffuse spread of cancer. No other type of cancer relapse was observed.
Statistical Analysis
For the statistical analysis, the Student’s t test (unmatched) or χ2 test was used. The cumulative survival rate and cumulative rate of cancer death were calculated by the life-table method and the difference was analyzed by the log-rank test. A P < 0.05 was considered statistically significant.
RESULTS
After pancreatectomy, none of the 12 patients with benign tumor showed any cancer cells in the drained fluid which had been collected from the pancreatic bed (Table 1). All patients received complete tumor resection and they have survived for 2.4 ±1.6 postoperative years (range, 0.8 to 5.4) without disease relapse. Among 17 patients with non-/minimally-invasive carcinoma, there were no patients (0%) with positive cytology. However, 21 (32%) out of 65 patients with invasive ductal carcinoma showed cancer cells in the drained fluid (Fig. 1). The number of detected cancer cells ranged from 2 to 30, and more than 10 cancer cells were seen in 6 of 21 positive patients. The incidence of positive cytology was 28% (16/58) in R0 resection, 75% (3/4) in R1 resection, and 67% (2/3) in R2- resection, respectively (P < 0.05 between R0 and R1 + R2). More than 10 cancer cells were seen in the 5 patients who had received R0 resection and in 1 patient who had undergone R2 resection. Both 3 positive patients and 5 negative patients also received cytology on the first postoperative day, but their results were all negative. Seven patients who had received R1 or R2 resection died within 1 year (mean, 0.5 ± 0.2 year), including 1 who died from postoperative complications.
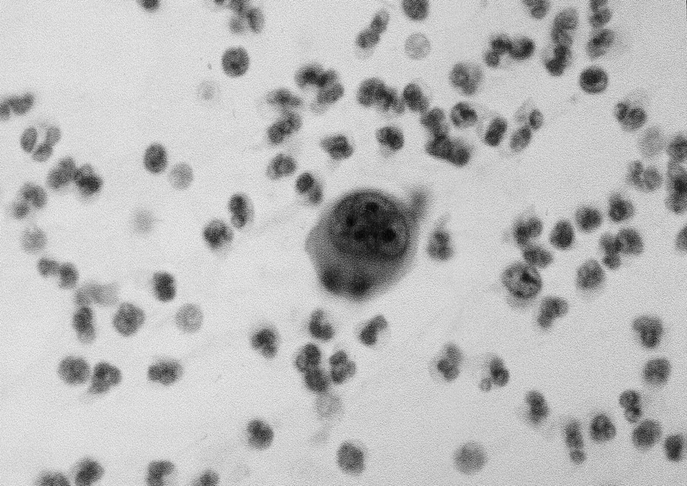
FIGURE 1. Cancer cells detected in the drained fluid from the pancreatic bed after a curative resection of pancreatic cancer (Papanicolaou stain).
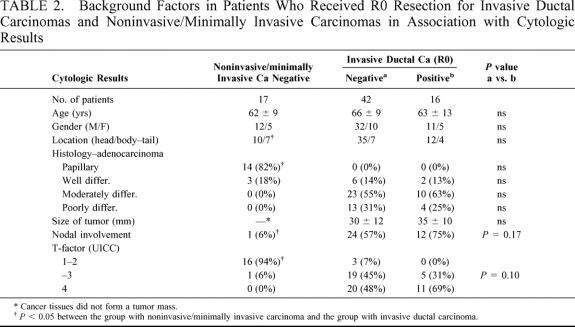
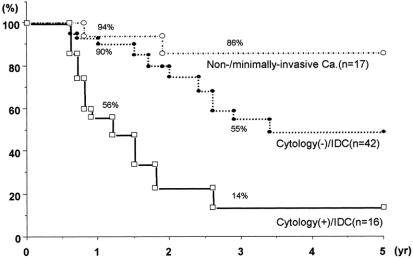
Table 2 compares both the clinical and pathologic factors among the 3 groups. The group with noninvasive/minimally invasive carcinoma (n = 17, negative in cytology) significantly differed from the group with invasive ductal carcinoma in tumor histology, status of nodal involvement, and the degree of direct invasion beyond the pancreatic confines (T factor in UICC classification10). Among 58 patients with invasive ductal carcinoma, the cytology-positive group (n = 16) was likely to have a larger tumor size and a higher incidence of both nodal involvement and T4 tumor than the cytology-negative group (n = 42). However, these differences did not reach statistical significance. The age, gender, location of tumor, and histologic types were similar. Figure 2 compares the cumulative survival rates among the 3 groups. In the group with noninvasive/minimally invasive carcinoma, the survival rate was 94% at 1 year and 86% at 3 years. The 1- and 3-year survival rates were 56% and 14% in the cytology-positive group and 90% and 55% in the cytology-negative group, respectively (P < 0.05). The 50% survival period in the cytology-positive group was 1.2 years and 3.3 years in the cytology-negative group.
TABLE 2. Background Factors in Patients Who Received R0 Resection for Invasive Ductal Carcinomas and Noninvasive/Minimally Invasive Carcinomas in Association with Cytologic Results
FIGURE 2. Cumulative survival after R0 resection (life-table method). The cytology-positive patients with invasive ductal carcinoma showed a 56% 1-year survival rate and 14% 3-year survival rate. These figures are significantly lower than those of cytology-negative patients with invasive ductal carcinoma or those with noninvasive/minimally invasive carcinoma (P < 0.05).
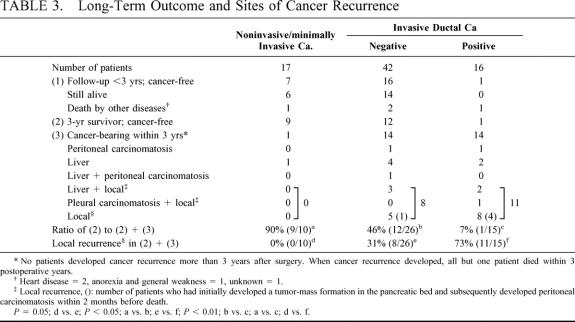
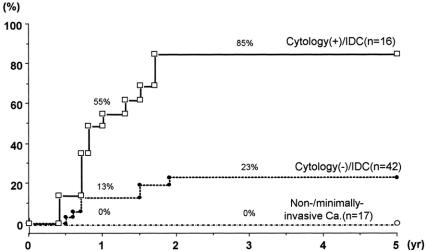
Table 3 compares the postoperative outcomes among the 3 groups. According to the follow-up data, patients were classified into the following 3 groups: (1) patients who are still alive or who died of other diseases, although their postoperative follow-up periods have not reached 3 years; (2) disease-free 3-year survivors; and (3) patients with a cancer relapse within 3 postoperative years. To date, cancer recurrence has not developed in any patient more than 3 years after surgery, and all but 1 died within 3 postoperative years. The ratio of (2) to (2) + (3), roughly corresponding to the 3-year cancer-free survival rate, was 90% (9/10) in the group with noninvasive/minimally invasive carcinoma, 46% (12/26) in the cytology-negative patients with invasive ductal carcinoma, and 7% (1/15) in the cytology-positive patients. A statistically significant difference was seen between each of the 2 groups. With regard to the sites of cancer recurrence, the pancreatic bed (local recurrence) was most common (19 patients), followed by liver metastasis (13 patients). Although both peritoneal and pleural carcinomatosis were less frequent, peritoneal carcinomatosis (seeding) was likely to develop subsequently in patients who had already developed local recurrence. Local recurrence has not developed in any patients with noninvasive/minimally invasive carcinoma, whereas it has occurred in 8 cytology-negative patients with invasive ductal carcinoma and in 11 cytology-positive patients. The ratio of patients with local recurrence to patients with (2) + (3) was 0%, 31%, and 73%, respectively (P = 0.05 between the former 2 groups; P < 0.01 between the latter 2 groups by χ2 test). Local recurrence developed within 2 postoperative years but not later. The cumulative rate of local recurrence was calculated according to the life-table method (Fig. 3), and the rate was 55% at year 1 and 85% at year 2 in the cytology-positive group. These figures were significantly higher than the corresponding 13% and 23% in the cytology-negative group (P < 0.05).
TABLE 3. Long-Term Outcome and Sites of Cancer Recurrence
FIGURE 3. Cumulative rate of local recurrence (limited area in the pancreatic bed; life-table method) Local recurrence developed within 2 postoperative years but not later. Between the cytology-positive and cytology-negative groups, the difference was statistically significant (P < 0.05).
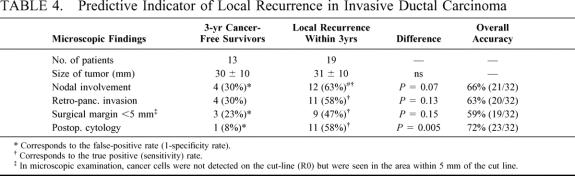
To determine which was the most potent indicator of local recurrence after curative (R0) resection of invasive ductal carcinoma, a comparison was made on the following 5 factors: size of tumor; nodal involvement; invasion beyond the posterior confine of the pancreas; the minimum distance (< or ≥5 mm) between the cancer and the cut line; and postoperative cytology (Table 4). For this comparison, patients with invasive ductal carcinoma were classified according to whether they have survived for 3 years without local recurrence (13 patients; (2) in Table 3) or died of local recurrence (19 patients; in Table 3). The postoperative cytology showed the lowest false-positive rate (1/13 = 8%) or the highest specificity rate (12/13 = 92%), and a 58% (11/19) sensitivity rate, resulting in a 72% (23/32) overall accuracy rate, whereas the other 4 indicators resulted in less than 70% of the overall accuracy rate.
TABLE 4. Predictive Indicator of Local Recurrence in Invasive Ductal Carcinoma
DISCUSSION
After pancreatectomy, most surgeons routinely place the drains in the pancreatic bed. This procedure was originally intended not only to remove the collected blood, chyle, debris, and digestive juice but also to obtain warning information about hemorrhage or anastomotic leakage. However, there have been no previous reports of cytology performed on the drained fluid after resecting cancers of the pancreas or other digestive organs. Only Higashiyama8 and Doki9 have performed a pleural washing cytology immediately after resecting lung or intrathoracic esophageal cancers, and they8,9 showed that cancer cells were newly detected in 8 to 9% of the postoperative patients. Compared with these figures, our result showed a higher positive rate (32%) in the group with invasive ductal carcinoma. Furthermore, the positive rate was as high as 71% in the patients who had received either R1 or R2 resection (cancer residual). However, no cancer cells were detected in any patients with a benign tumor (negative control) or noninvasive/minimally invasive carcinoma. Noninvasive/minimally invasive carcinomas, such as intraductal papillary mucinous, carcinoma in situ, and cystadenocarcinoma, differing from invasive ductal carcinoma, are well known to have a far better prognosis, if complete (R0) resection is performed.12–14 Based on such comparable data, our cytologic diagnosis seems to be suitable for the following discussion in relation to patient survival and sites of cancer recurrence among patients who received R0 resection for invasive ductal carcinomas.
The anterior surface of the pancreas is entirely covered by the peritoneal membrane whereas the posterior confine is not. It is well known that invasive ductal carcinomas are likely to microscopically infiltrate in a retroperitoneal direction involving the nerve plexuses15 and lymphatic tissues,16 whereas their primary tumors appear to be limited to the pancreas at the macroscopic level. Because the retroperitoneal spaces are widely revealed during pancreatectomy, it is not unreasonable to speculate that some cancer cells might have been exfoliated into the abdominal cavity. Simultaneously, a minute or occult level of cancer foci (residual) might have been left behind close to the cut lines. These 2 mechanisms could theoretically explain the high positive rate obtained in our drain-cytology. However, it remains suspicious whether the exfoliated cancer cells themselves resulted in shortening the patient’s survival via the development of the subsequent cancer spread. In most of our cytology-positive patients, cancer recurrence developed initially in the limited area of the pancreatic bed (local recurrence). Some subsequently developed peritoneal carcinomatosis, and peritoneal carcinomatosis alone was very rare. In addition, we had 3 patients who had negative results on the first postoperative day but positive on the second or third postoperative day. However, no patients converted from positive to negative results. Based on these findings, it was unlikely that exfoliated and thereby floating cancer cells had implanted in the pancreatic bed to form local recurrence. Instead, minute and occult foci of residual cancer (even after R0 resection) might have exfoliated cancer cells postoperatively in the drain and afterward manifested as a macroscopically obvious tumor in the pancreatic bed.
To date, microscopic observation of the resected specimens has long been the standard examination to determine the status of the surgical margin. However, as mentioned above, local recurrence is still common in patients whose surgical margin had been judged as negative (R0 resection). Compared with histologic diagnosis, our drain cytology seems to be more direct and specific in indicating the subsequent development of local recurrence. In addition, cytologic results could be obtained within 30 minutes after cell sampling, if rushed. These advantages of drain cytology are suitable for rapid planning of the subsequent adjuvant locoregional therapy. For instance, postoperative radiation therapy is supported by many authors. Willett4 suggested that the prognostic benefit of adjuvant radiation therapy was limited to patients who had received R0-resection but not for those who had obvious residual cancer. Future studies are needed to discover whether or not both patient survival and local control will be improved by adding postoperative radiation therapy for cytology-positive patients.
Finally, our drain-cytology still has an unsolved problem because of its lower sensitivity rate (52%) compared with the high specificity rate (92%) in cases of R0 resection. Likewise, 2 of the 7 patients with R1/R2 resection showed negative cytology even though residual cancer had been diagnosed. We speculate that this sensitivity problem might have been attributed mainly to the small number of exfoliated cancer cells in the drained fluid. The pancreatic cancer is microscopically characterized by a small number of cancer cells packed in a large amount of fibrous stroma (desmoplasia), especially at the advancing (infiltrating) point of the tumor.11 Under such conditions, it is not strange for us to speculate that only a small number of cancer cells would have been exposed on the cut surface of the residual tumor. Therefore, to obtain a detectable number of exfoliated cancer cells, either repeated or prolonged fluid-sampling is helpful. In addition, the combination of immunocytochemical staining and/or genetic analysis17 are also promising. In the near future, as suggested in our previous study,18 we still also need a quick examination strategy which makes it possible to locate the sites of residual cancer at the microscopic level before closing the abdomen. Once these problems are solved, we will be able to far more precisely select both high-risk patients for local recurrence and the appropriate adjuvant therapy for each patient in a made-to-order fashion.
Footnotes
This study was supported in part by a grant from the Japan Foundation for Promotion of Cancer Research.
Reprints: Osamu Ishikawa, MD, Department of Surgery, Osaka Medical Center for Cancer and Cardiovascular diseases, 3-Nakamichi, 1-chome, Higashinari-ku, Osaka, 537–8511, Japan. Tel: 6-6972-1181, Fax: 6-6981-08055.
REFERENCES
- 1.Yeo CJ, Cameron JL, Lillemore KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. Ann Surg. 1995;221:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa O, Ohigashi H, Sasaki Y, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma oaf the pancreas head. Ann Surg. 1988;208:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. [DOI] [PubMed] [Google Scholar]
- 4.Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg. 1993;217:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. [DOI] [PubMed] [Google Scholar]
- 6.Leach SD, Rose JA, Lowy AM, et al. Significance of peritoneal cytology in patients with potentially resectable adenocarcinoma of the pancreatic head. Surgery. 1995;118:472–478. [DOI] [PubMed] [Google Scholar]
- 7.Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26–30. [DOI] [PubMed] [Google Scholar]
- 8.Higashiyama M, Doi O, Kodama K, et al. Pleural lavage cytology immediately after thoracotomy and before closure of the thoracic cavity for lung cancer without pleural effusion and dissemination: clinicopathologic and prognostic analysis. Ann Surg Oncol. 1997;4:409–415. [DOI] [PubMed] [Google Scholar]
- 9.Doki Y, Kabuto T, Ishikawa O, et al. Does pleural lavage cytology before thoracic closure predict both patient’s prognosis and site of cancer recurrence after resection of esophageal cancer? Surgery. 2001;130:792–797. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind Ch (International Union Against Cancer). TNM Classification of Malignant Tumours. 5th ed. New York: Wiley-Liss; 1997:53. [Google Scholar]
- 11.Salcia E, Capella C, Kloppel G: Diagnosis of pancreatic tumors. In: Rosai J, ed. Atlas of Tumor Pathology. Third series, Fasc. 20. Washington, DC: Armed Forces Institute of Pathology; 1997:247–257. [Google Scholar]
- 12.Warshaw AL, Compton CC, Lemodrowski K, et al. Cystic tumors of the pancreas. New clinical, radiologic and pathologic observations in 67 patients. Ann Surg. 1990;212:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada M, Kozuka S, Yamano K, et al. Mucin-producing tumor of the pancreas. Cancer. 1991;68:159–168. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa O, Ohigashi H, Imaoka S, et al. Surgical techniques of cytology-guided pancreatectomy for occult neoplasm of the pancreas. Hepato-Gastroenterology. 1995;42:724–729. [PubMed] [Google Scholar]
- 15.Ohigashi H, Ishikawa O, Sasaki Y, et al. K-ras point mutation in the nerve plexuses around the superior mesenteric artery in resectable adenocarcinoma of the pancreatic head. Arch Surg. 2000;135:1450–1455. [DOI] [PubMed] [Google Scholar]
- 16.Demeuure MJ, Doffek KM, Komorowski RA, et al. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328–1334. [PubMed] [Google Scholar]
- 17.Nomoto S, Nakao A, Kasai Y, et al. Peritoneal washing cytology combined with immunocytochemical staining and detecting mutant K-ras in pancreatic cancer: comparison of the sensitivity and availability of various methods. Pancreas. 1997;14:126–132. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa O, Ohigashi H, Sasaki Y, et al. Intraoperative cytodiagnosis for detecting a minute invasion of the portal vein during pancreatoduodenectomy for adenocarcinoma of the pancreatic head. Am J Surg. 1998;175:477–481. [DOI] [PubMed] [Google Scholar]