Abstract
Objective:
Objective of this study was to analyze fifteen months after surgery the sensorimotor recovery of the first human double hand transplantation.
Summary Background Data:
As for any organ transplantation the success of composite tissue allografts such as a double hand allograft depends on prevention of rejection and its functional recovery.
Methods:
The recipient was a 33-year-old man with bilateral amputation. Surgery included procurement of the upper extremities from a multiorgan cadaveric donor, preparation of the graft and recipient’s stumps; then, bone fixation, arterial and venous anastomoses, nerve sutures, joining of tendons and muscles and skin closure. Rehabilitation program included physiotherapy, electrostimulation and occupational therapy. Immunosuppressive protocol included tacrolimus, prednisone and mycophenolate mofetil and, for induction, antithymocyte globulins and then CD25 monoclonal antibody were added. Sensorimotor recovery tests and functional magnetic resonance imaging (fMRI) were performed to assess functional return and cortical reorganization. All the results were classified according to Ipsen’s classification.
Results:
No surgical complications occurred. Two episodes of skin acute rejection characterized by maculopapular lesions were completely reversed increasing steroid dose within 10 days. By fifteen months the sensorimotor recovery was encouraging and the life quality improved. fMRI showed that cortical hand representation progressively shifted from lateral to medial region in the motor cortex.
Conclusion:
Even though at present this double hand allograft, treated using a conventional immunosuppression, allowed to obtain results at least as good as those achieved in replanted upper extremities, longer follow-up will be necessary to demonstrate the final functional restoration.
In any organ transplantation, the success of composite tissue allograft (CTA) depends on the prevention of rejection and also upon its functional recovery.
The introduction of new immunosuppressant agents like FK 506 and mycophenolate mofetil, especially in combination, as well as the improvement in detection and treatment of rejection have increased the degree of success in viability and function of the transplanted limbs in animal models.1,2,3 In addition, several studies reported an accelerated axonal regeneration in limb transplantation with the use of FK 506.4,5
The potential function of human limb allografts can be compared with that of upper extremity replantations. A study by Graham et al6 demonstrated that functional capacity in patients undergoing upper extremity replantation after traumatic amputation was significantly improved over those who received prostheses. Furthermore, when compared with autogenous reconstruction, alloreconstruction offers substantial technical benefits, such as the possibility of providing an intact and well preserved limb and a favorable amputation site.
Based on these findings, we performed the first human hand allotransplantation in September 1998.7,8 The results obtained in this first case confirmed the feasibility of the surgical procedure and demonstrated the efficacy and the limited adverse effects caused by a conventional nonspecific immunosuppressive protocol used in a CTA. Patient compliance and motivation critically affect the ultimate outcome after upper extremity transplantation, as shown by the first human hand transplantation. In this case, the right hand had to be removed 27 months posttransplantation because of patient’s noncompliance to immunosuppressive therapy. The positive results achieved in other single-hand transplants realized over the world9,10 encouraged us to perform the first double-hand transplantation in January 2000, which was then followed by 2 other cases in Austria and China.
Because in hand replantation as well as in hand transplantation, “survival without restoration of function is not a success” (Chen)11, the aim of this paper is to analyze the functional recovery 15 months after transplantation. We also document and discussed herein all the steps of this complex process.
METHODS
The recipient was a 33-year-old man who suffered an amputation of both hands in 1996 after a blast injury; the stump level was 3 cm above the wrist. After the accident, myoelectric prostheses were employed with bilateral pinch grip (Fig. 1). He experienced bilateral painless phantom limb sensation, which was very interesting because he felt he could “move” all digits easily and independently.
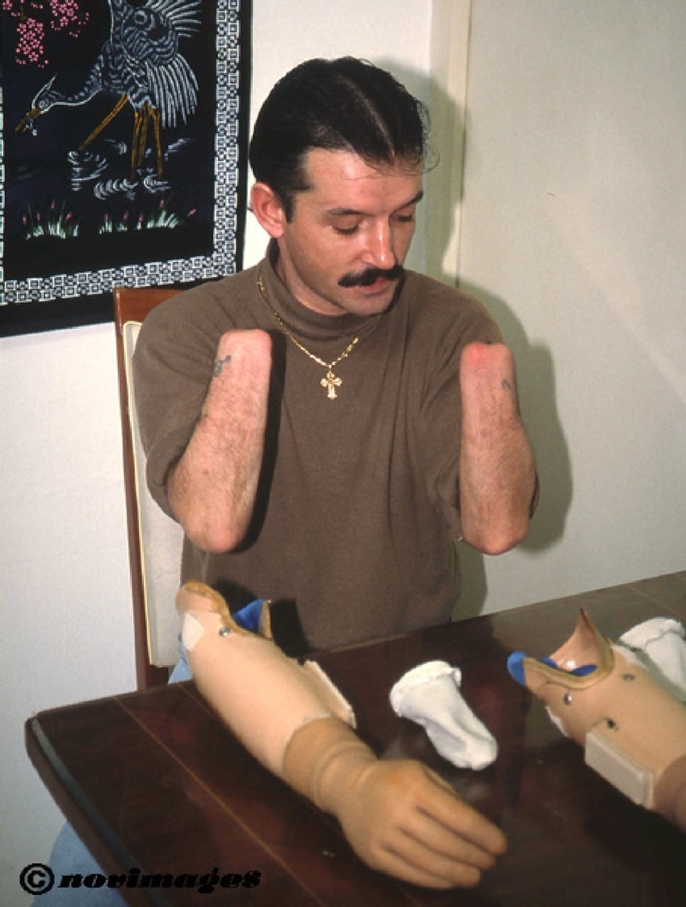
FIGURE 1. The recipient presented a bilateral amputation 4 years before the transplantation and used myoelectric prostheses.
The recipient underwent routine pretransplant investigations and specific morphologic and functional tests of forearm stumps such as angiogram, muscle and nerve charts, as well as brain functional magnetic resonance imaging (fMRI).
Angiogram showed normal vascularization of both stumps. MRI was performed to identify stump muscles, which were normal, and electromyography to test nerve stimulation; it was possible to evocate potentials on radial, ulnar and median nerve territories only at elbow level on both sides.
To evaluate the impact of a double-hand transplantation on the hand cortical motor representation, fMRI was performed 1 month before transplantation.
Procurement Procedure
The multiorgan cadaveric donor was an 18-year-old man, blood group compatible, 5 HLA A, B, DR mismatches; negative T and B cell cross match. Authorization for limb donation was obtained from donor family. Amputation was performed 3 cm above elbows. After brachial artery cannulation and perfusion with University of Wisconsin (UW) solution at 4°C, the upper extremities were packed in ice for the transport.
Graft Preparation and Transplantation
General anesthesia coupled with bilateral axillary block to achieve optimum postoperative analgesia and vasodilatation was used. A tourniquet (250 mm Hg) was applied for hemostasis. Skin was incised following a wide Z line on anterior and dorsal surfaces of both stumps. All anatomic structures were dissected, identified and tagged in both stumps. Preparation of anterior surface of radius and ulna was performed.
Concomitant benchwork was done on the procured upper extremities. Identification of arteries and nerves as well as of flexor and extensor tendons and muscles was performed, and their lengths were preserved to allow comfortable sutures.
On both side grafts, correct length was determined, and the bones were cut with an electric saw to achieve perfect matching. Osteosynthesis of ulna and radius was performed. Ulnar and radial artery were microsurgically sutured. On the right side, venous outflow was achieved by anastomosing a deep vein comitantes to the ulnar artery and the cephalic vein to a deep vein of the forearm and to the donor cephalic vein, respectively. On the left side, a venous graft (15 cm in length) was prepared and used to anastomose a large cubital vein to the isolated basilic vein. When the tourniquet was released, both hands rapidly presented a normal color. Total ischemia time did not exceed 12 hours for both upper extremities (9 hours and 20 minutes for the right hand and 8 hours and 40 minutes for the left hand). On both sides, median and ulnar nerves were microsurgically sutured by using an epi-perineural technique; on both sides, the superficial branch of the radial nerve was not identified and sutured. Based on anatomic conditions, donor flexor and extensor tendons were joined individually or in functional groups to the corresponding recipient tendons accordingly to Pulvertaft technique. The skin was sutured directly with the exception of a small dorsal area on the right side, where a split-thickness skin graft from the recipient’s right thigh was used.
Both forearms were supported by a volar splint with the elbow fixed at 45° and the wrist extended 30°. Both hands were uncovered to allow the postoperative monitoring.
Immunosuppressive protocol included an initial induction phase with polyclonal antibodies (Thymoglobulin, 1.25 mg/kg/day for 10 days), tacrolimus (Prograf, 0.2 mg/kg/day with blood levels between 15 and 20 μg/ml in the first month), prednisolone (Solu-Medrol, 250 mg on day 1; 1 mg/kg/day for 10 days, then slowly tapered to 20 mg/day), mycophenolate mofetil (Cell Cept, 2 g/day). Monoclonal CD25 antibodies (Simulect) were also used on day 12 and 20 posttransplant because the patient developed a serum sickness.
The maintenance treatment consisted of prednisone (Solupred, 10 mg/day), tacrolimus (blood levels between 5 and 10 μg/ml), mycophenolate mofetil (2 g/day).
Rehabilitation protocol included physiotherapy, electro-stimulation and occupational therapy. Different types of anticlaw splints were employed during the first year to protect hand allografts to avoid retractions and to facilitate rehabilitation process.
Physiotherapy started 12 hours after surgery twice daily for the first year posttransplantation. During the first 2 postoperative weeks, controlled-motion passive exercises were performed, then active exercises were added with respect of tenodesis effect between flexor and extensor muscles during the healing period. By the first month, exercises to improve forearm pronation, wrist and finger extension and pinch strength were performed. By 2 months, electro-stimulation and occupational therapy were started to improve development of extrinsic and intrinsic muscles. Electro-stimulation was used to improve extrinsic muscle power and to avoid intrinsic muscles fibrosis. Occupational therapy as well as physiotherapy focused on sensory, visual, motor and haptic stimulation of the hands (Table 1).
TABLE 1. Rehabilitation Program
Specific tests to assess sensitivity and motion recovery were performed each month for the first 7 months and then every 6 weeks thereafter.
RESULTS
Early and Late Complications
No early and late surgical complications occurred.
Skin, wound and bone healing were normal, as shown by clinical investigation (Fig. 2), x-rays and bone scintigraphy.
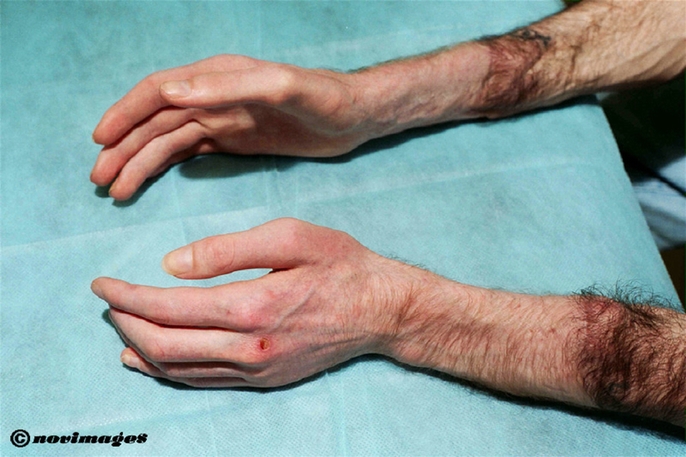
FIGURE 2. At 15 months posttransplantation, both upper extremities show normal skin, nails and hair growth. A moderate muscular atrophy is still present.
Blood supply was excellent in both hands, as shown initially by pO2 and later by capillaroscopy and scintigraphy. Venous and arterial Doppler were performed each month, and angiograms were performed at 3, 6 and 12 months posttransplant. By the third month, an occlusion (approximately a few centimeters after the origin) of both radial arteries was demonstrated by angiogram; they filled retrogradely from ulnar arteries with good palmar arch and digital arteries, whereas ulnar arteries were patent and predominant. This event occurred despite the fact that an infusion of Dextran was administered for 10 days and low molecular heparin was supplied for the first 30 postoperative days, followed by 100 mg aspirin as thrombosis prevention therapy.
Two episodes of skin rejection characterized by focal maculopapular asymptomatic lesions occurred on days 53 and 82 posttransplantation. Skin biopsies, which were performed systematically each month and at the time of skin lesion appearance, revealed an inflammatory infiltrate constituted of recipient’s mononuclear cells, as shown by the expression of HLA-A24 antigen. At the same time, no altered values of C-reactive protein, creatin-phosphokinase or white cell number were shown. An increase in oral prednisone (40 mg/day, tapered to 20 mg/day over a period of 8 days) and topical steroid cream (clobetasol, Dermoval) were employed as rejection treatment, with complete clinical and histologic resolution of the lesions within 10 days. The other skin biopsies showed a normal skin.
On day 8, the patient developed serum sickness that resulted in a complete clearance of circulating thymoglobulins. In the same period, hyperglycemia occurred, and it was treated with insulin therapy. By day 35, blood glucose and HbA1c were normal. No other metabolic or infectious complications related to the immunosuppressive protocol occurred during the follow-up period. Wide spectrum antibiotic therapy was administered for the first 10 postoperative days to prevent infectious complications, and sulfadoxine pyrimethamine (Fansidar) was administered to prevent Pneumocystis carinii pneumonia.
No signs of graft versus host disease and no donor lymphocytes in peripheral blood were detected.
Sensitivity Recovery
By 3 months, the presence of Tinel’s sign was noted 14 cm from the anastomosis of the left ulnar nerve, 12 cm from the anastomosis of the left radial nerve and 12 cm from the anastomosis of both right nerves.
By 6 months, the sensitivity to pain and thermal stimuli was present on dorsal and palmar face of both hands and of all fingertips except the left thumb. The patient felt needles and pins as well as hot and cold stimulation on the anterior side of both forearms.
To measure static and dynamic pressure, the Semmes-Weinstein test using 4.56 sensitivity monofilament was performed. By 3 months, it showed sensitivity recovery on the ulnar side of both wrists. By 6 months, the patient’s sensibility recovered (Semmes-Weinstein test, 4.56 monofilament) on palmar and dorsal side of both hands, especially on the right side. By 12 months, moving Semmes-Weinstein test using Wynn Parry chart demonstrated sensitivity recovery on right fingertips using 5.46-6.10 monofilaments that was not shown using all filaments on the left thumb, index and long finger. The static test showed a reduced sensitivity on the fingertips. By 15 months, sensitivity recovery was demonstrated on all fingertips of both hands using 4.56 monofilaments (Fig. 3).
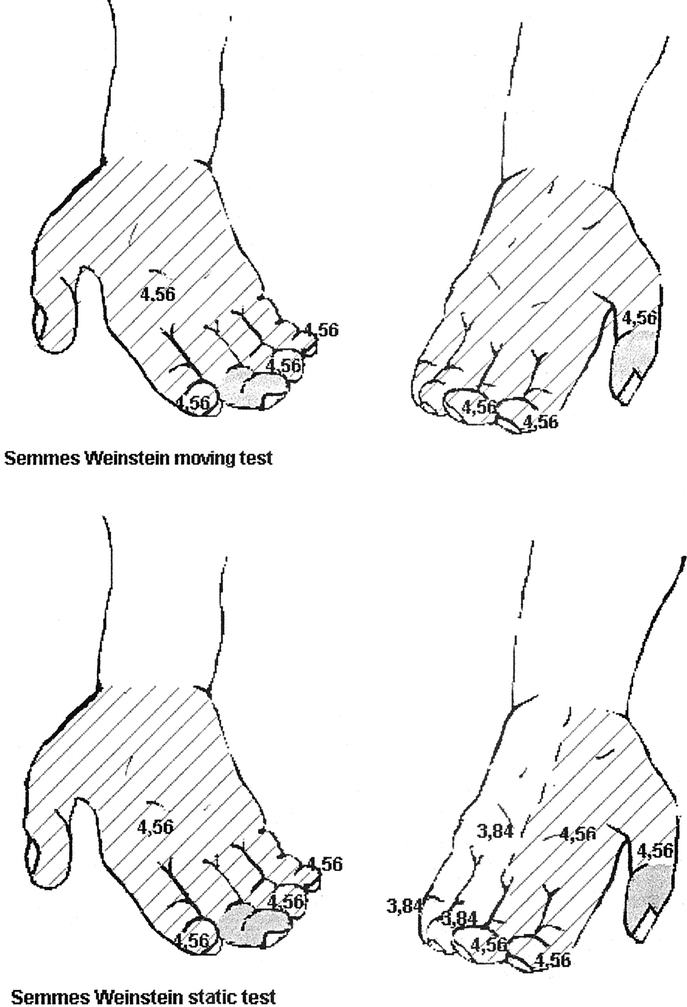
FIGURE 3. Semmes–Weinstein test shows hand sensitivity recovery to dynamic and static pressure. Gray area, negative recovery; striped area, positive recovery using 4.56 monofilament; white area, positive recovery using 3.48 monofilament.
The recipient did not report paraesthesia, numbness or cold intolerance.
Movement Recovery
Finger passive range of movement was almost complete at 1 month posttransplant, whereas wrist passive movement was not yet complete at 15 months.
Active range of motion (ROM) of wrist, metacarpophalangeal and interphalangeal joints were registered every month. Although during the follow-up period, a progressive development of extrinsic muscles was demonstrated, active ROM of wrist on both sides was limited at 15 months after transplantation, possibly because of adherences. By 9 months, intrinsic muscles started to work, and this process was more evident 12 months after transplantation. Electromyography confirmed the reinnervation process. Indeed, at 12 months posttransplant, this examination showed potentials of 50% of amplitude compared with normal subjects on ulnar nerve and only the appearance of potentials on median nerve, particularly on the right side. By 12 months, neurovegetative innervation was also demonstrated by perspiration and electromyographic data.
The reinnervation process was also demonstrated by the presence of neurofilaments, which had been shown by histochemical studies since day 185 posttransplant in skin biopsies.
By 1 year, wrist active ROM was 50° on right side and 45° on left side. Mean total active ROM of fingers was 163 on the right hand and 158 on the left hand. Total active ROM thumb was 70° on both sides, whereas traces of opposition activity were demonstrated only on the right thumb by electromyography, which detected signs of reinnervation of the right opponens pollicis, while on the left side of the first and second lumbricales muscles. By 12 months, mean pinch grip was 250 g on the right hand and 200 g on the left hand, although it was impossible to evaluate grip strength with typical Jamar hand dynamometer. By 15 months, mean pinch grip was 300 g on both hands, and grip strength evaluated with typical Jamar hand dynamometer was 150 g. At present, several movements of fingers and thumbs are possible because the patient has partly started to dissociate single finger movements (Fig. 4).

FIGURE 4. At 15 months posttransplantation, the recipient is able to perform a pinch grip with both hands.
Cortical Reorganization
fMRI was performed at 2, 4, 6 and 12 months after transplantation.12 During the scans, the patient performed flexion/extension movements of the last 4 digits of both hands and flexion/extension of the left and right elbow. Directstatistical comparison between the presurgery and the examination performed posttransplantation showed that lateral motor cortex sites, which were active for hand movements in the pretransplant period, were not active after the graft, and that hand representation shifted from lateral to medial region in the motor cortex.12 Elbow movements produced a pattern of motor activations that evolved similarly to the hand motor representation (Fig. 5). In addition, parallel to the motor cortex modifications, changes were also recorded in the somatosensory cortex, with a displacement from lateral to medial regions.
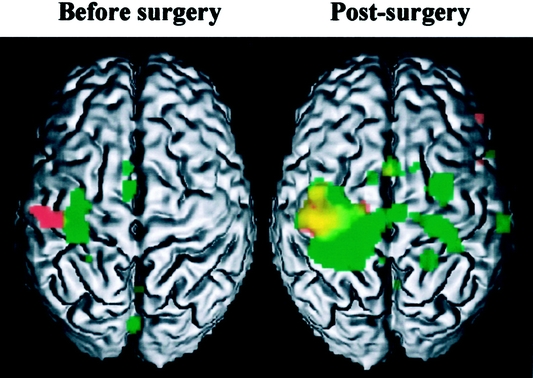
FIGURE 5. Results of MRI examination of right hand and elbow before surgery and 2 months after transplantation. Red, activation related to hand movement; green, activation related to elbow movement; yellow, overlap between hand and elbow.
Psychologic Aspects
A psychiatric team assessed the patient before the transplantation to evaluate his ability to understand the potential risks of the procedure, the absolute necessity of the immunosuppressive therapy, and his motivation to cope with the rehabilitation program. Furthermore, the same team regularly granted psychologic support during the follow-up. During the first 3 months after transplantation, the patient worried about the outcome of the transplantation procedure and, at the same time, there was the impact of “seeing” the transplanted extremities. The excellent healing and the progress obtained with physiotherapy and occupational therapy facilitated the psychic appropriation of the transplanted hands; by 3 months, he considered “the” hands as “his own” hands. The patient showed a good compliance to the immunosuppressive therapy as well as to the rehabilitation program. Although the rehabilitation program is long and the patient’s sensorimotor recovery is not yet complete, the patient could compare the possibilities offered by the double-hand transplantation to those offered by the myoelectric prostheses, and his self-report showed satisfaction.
Current Life
All the results were classified according to Ipsen’s classification (a modified version of the Tamai classification), which included ROM, sensory recovery, activities of daily living, subjective symptoms, patient’s cosmesis and ability to resume original work (reported in Table 2). The functional result, which was “fair” for both hands, was influenced by the limited joint movements, by the presence of moderate atrophy, and particularly by the absence of a job, because the recipient still follows physiotherapy and occupational therapy. There was a difference in the evaluation between the 2 hands, because the right hand was dominant and nerve regeneration was not completely symmetrical.
TABLE 2. Scoring for the Evaluation of Function After Upper Extremity Transplantation
By 1 year posttransplant, the patient was able to perform the same daily activities that were possible with the myoelectric prostheses before the transplantation. In addition, several activities such as holding a pen or a glass or a pair of scissors, shaving, taking care of his personal hygiene that were impossible before were then easily performed by the patient.
DISCUSSION
Functional recovery in upper extremity transplantation is of paramount importance. It is a long and complex process involving not only the preservation of the viability of neural, muscular and sensory end-organ components, but also appropriate and timely reinnervation of neural target and several degrees of cortical reorganization.
The reconstructive procedure in hand allotransplantation allows the grafting of uninjured, well-preserved extremities at the most favorable level, without donor-site limitations or morbidity.13 Consequently, compared with replantation, transplantation of hand allografts can be planned and executed electively with the selection of appropriate donors and recipients. In our experience, the healing process was always excellent and no early or late surgical complications occurred.
The advantages of limb transplantation are contingent upon an effective and safe immunosuppressive protocol.14,15 We decided to use the association FK 506/MMF/prednisone that is a combination therapy of agents which differ in their mechanism of action and toxicity. This successful immunosuppressive protocol was also used by the American,8 Chinese, Austrian and Italian teams demonstrating that long-term viability in hand allografts is possible with conventional therapeutic levels of immunosuppression. Only 2 episodes of skin acute rejection occurred in the first 3 posttransplant months, and they reversed completely by increasing steroid oral dose and applying topical immunosuppressant agents, thus demonstrating the complete reversibility of human skin rejection episodes. At 15 months after transplantation under this immunosuppressive protocol, the adverse effects were a serum sickness, which disappeared in a few days, and a transitory hyperglycemia. No other complications occurred.
At present, transplantation is routinely indicated for non-life-threatening situations such as dialysis-dependent renal failure or poorly controlled diabetes; immunosuppression risks are accepted because they allow a significant improvement in the patient’s quality of life.13 To apply the above rationale to double-hand transplantation, functional recovery and improvement in quality of life needs to be demonstrated.
Complete functional restoration is conditioned by nerve regeneration,16 and in the double-hand transplantation, this event has been demonstrated by the immunohistochemical studies of the skin, electromyography and sensitive recovery tests. Nerve regeneration was faster than in the autoreconstructions, as FK 506 seems17 to accelerate axonal regeneration, increasing the synthesis of axotomy-induced growth-associated protein (GAP-43) as shown by the appearance of protective sensitivity 3 months posttransplantation. Therefore, electromyographic data, passive and active ROM evaluation tests showed a relevant sensorimotor recovery with reinnervation of extrinsic muscles and the majority of intrinsic muscles.
Furthermore, fMRI results12 have demonstrated that peripheral input can modify cortical hand organization in sensorimotor regions. It is very interesting that in a 4-year bilateral amputated patient, 6 months after the double-hand transplantation, the hand representation regained the cortical site which corresponds to the hand knob area in normal subjects. Nerve regeneration as well as all the information arising from grafted joint, muscle and skin receptors may probably influence the cortical hand reorganization. These findings demonstrated an impressive human brain plasticity and, particularly, the importance of the rehabilitation program in the future of the upper extremity transplantation. Consequently, we recommend performing a twice-daily physiotherapy during the first year posttransplant. Our rehabilitation program was complex and intensive, including physiotherapy, electro-stimulation and occupational therapy, as its final goal was to cooperate with the process of cortical reorganization, which seems to condition functional recovery of transplanted hands. The compliance to physiotherapy appeared to be better in this patient than in single-hand transplanted patients; consequently, the best indication for upper extremity transplantation could be bilateral amputated patients.
To evaluate functional recovery of double-hand transplantation, we have studied the various classification systems used in extremity replantations. The method of Chen11 is probably too unspecific for description of upper extremity function, and the Tamai method18 is very well adapted for the distal part of the upper extremities but is not very easily used for evaluation of bilateral major upper extremity reconstruction in daily clinical situations. The main goal of double-hand transplantation is to improve the activities of daily living. Consequently, we decided to adopt a modified version of the Tamai classification employed by Ipsen et al19 to evaluate the results of major upper extremity replantations and the patient’s progress in function of the rehabilitation protocol. At 15 months after transplantation, we considered the result achieved to be encouraging, as the recovery of sensibility was good and muscular power with the range of joint motion was going to improve because intrinsic function recovery had started. Intrinsic muscle recovery is particularly important as it contributes markedly to the overall score by conditioning activities of daily living. At present, the recipient can perform all the activities which were possible with the use of sophisticated myoelectric prostheses and other several activities that were impossible before the transplantation. Therefore, at 15 months, the results achieved with the double-hand transplantation are at least as good as those obtained in replanted upper extremities, but further evaluation will be necessary to compare these 2 techniques 2 or 3 years after transplantation.
We recognize that the ultimate goal of hand allotransplantation and replantation is the functional recovery (Chen11). However, it may be more important to estimate “what really happened to the patient” rather than performing a complex series of physical measurements. In terms of realistic life, the recipient was satisfied with his recovered sensibility as well as his body image and the social aspect, which must not be underestimated. The concept of function must be expanded to embrace the total performance of the patient, taking into consideration all related socioeconomic, esthetic and psychologic factors.
At present, the results achieved in the first case of double-hand transplantation are very encouraging, and the immunosuppressive protocol as well as the rehabilitation program were demonstrated to be efficient.
Since the manuscript was submitted (April 2002), the patient has not shown any rejection episodes nor adverse effects caused by the immunosuppressive treatment, which includes prednisone (5 mg/day), tacrolimus (4 mg/day) and mycophenolate mofetil (2 g/day). The patient’s sensitivity and motion recovery has been improving, as well as his ability to perform daily activities. Indeed, the Semmes-Weinstein test using 3.8 monofilaments demonstrates sensitivity recovery with few localization errors. Dallon and Weber tests show a useful moving and static 2-point discrimination. Recognition of small objects is possible by using shape, contour and temperature criteria. The majority of extrinsic muscles are present at a level between M4 and M5. Intrinsic thenar, hypothenar and interosseous muscles are present at a level between M3 and M4. Pinch strength is 1500 g on the right side and 1000 g on the left side. Moreover, the patient started to work in March 2003; consequently, Chen’s score is “good”.
ACKNOWLEDGMENTS
We thank Dominique Vincent-Semard for her invaluable help. We also thank Dr. Vallet and the physiotherapy team from Val Rosay, and Dr. Vial and the physiotherapy team from La Villa Richelieu for their support in the rehabilitation program; Drs. Henri and Haouet for their collaboration during the follow-up period; and the psychiatric team (D. Bachman and G. Burloux) for their help before and after the transplantation. We thank Dr. A. Sirigu for her precious suggestions and M. P. Auboyer, Procedure Coordinator, for her support.
Footnotes
Reprints: Jean Michel Dubernard, Service d’Urologie et de Transplantation, Hopital Edouard Herriot, Place d’Arsonval, 69347 Lyon, France. E-mail: jean-michel.dubernard@chu-lyon.fr.
REFERENCES
- 1.Jensen JN, Mackinnon SE. Composite tissue allotransplantation: A comprehensive review of the literature. Part II. J Reconstr Microsurg. 2000;16(3):235–251. [PubMed] [Google Scholar]
- 2.Üstüner ET, Zdichavsky M, Ren X, et al. Long-term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation. 1998;66:1581–1587. [DOI] [PubMed] [Google Scholar]
- 3.Jones JW, Ustuner ET, Zdichavsky M, et al. Long-term survival of an extremity composite tissue allograft with FK 506-mycophenolate mofetil therapy. Surgery. 1999;126:384–388. [PubMed] [Google Scholar]
- 4.Gold BG, Storm-Dickerson T, Austin DR. The immunosuppressant FK 506 increases functional recovery and nerve regeneration following peripheral nerve injury. Rest Neurol Neurosci. 1994;6:298–301. [DOI] [PubMed] [Google Scholar]
- 5.Doolabh VB, Mackinnon SE. FK 506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103:1928–1936. [DOI] [PubMed] [Google Scholar]
- 6.Graham B, Adkins P, Tsai T-M, et al. Major replantation versus revision amputation and prosthetic fitting in the upper extremity: Alate functional outcome study. J Hand Surg. 1998:23A:783. [DOI] [PubMed] [Google Scholar]
- 7.Dubernard JM, Owen E, Herzberg G, et al. Human hand allograft: Report on first 6 months. Lancet. 1999;353:1315–1320. [DOI] [PubMed] [Google Scholar]
- 8.Dubernard JM, Owen E, Lefrancois N, et al. First human hand transplantation. Transpl Int. 2000;13:521–524. [DOI] [PubMed] [Google Scholar]
- 9.Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation: One year follow-up. New Engl J Med. 2000;343:468–473. [DOI] [PubMed] [Google Scholar]
- 10.Francois CG, Bredeinbach WC, Maldonado C, et al. Hand transplantation: Comparison and observations of the first four clinical cases. Microsurgery. 2000;20(8):360–371. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z-W, Meyer VE, Kleinert HE, Beasley RW. Present indications and controindications for replantation as reflected by long-term functional results. Orthop Clin North Am. 1981;12:849–870. [PubMed] [Google Scholar]
- 12.Giraux P, Sirigu A, Schneider F, Dubernard JM. Functional cortical reorganization after transplantation of both hands as revealed by fMRI. Nature Neurosci. 2001;4:691–692. [DOI] [PubMed] [Google Scholar]
- 13.Llull R. An open proposal for clinical composite tissue allotransplantation. Transplant Proc. 1998;30:2692–2696. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt CW. Update and outline of the experimental problems facing clinical composite tissue transplantation. Transplant Proc. 1998;30:2704–2707. [DOI] [PubMed] [Google Scholar]
- 15.Lee WPA, Mathes DW. Hand transplantation: pertinent data and future outlook. J Hand Surg. 1999;24A:906–913. [DOI] [PubMed] [Google Scholar]
- 16.Jensen JN, Mackinnon SE. Composite tissue allotransplantation: a comprehensive review of the literature–Part III. J Reconstr Microsurgery. 2000;16(3):235–251. [PubMed] [Google Scholar]
- 17.Gold BG, Yew JY, Zeleny-Pooley M. The immunosuppressant FK 506 increases GAP-43 mRNA levels in axotomized sensory neurons. Neurosci Lett. 1998;241:25–28. [DOI] [PubMed] [Google Scholar]
- 18.Tamai S. Twenty years’ experience of limb replantation- review of 293 upper extremity replants. J Hand Surg. 1982;7:549–556. [DOI] [PubMed] [Google Scholar]
- 19.Ipsen T, Lundkvist L, Barfred T, et al. Principles of evaluation and results in microsurgical treatment of major limb amputations: A follow-up study of 26 consecutive cases 1978–1987. Scand J Plast Reconstr Hand Surg. 1990;24:775–780. [DOI] [PubMed] [Google Scholar]